Chapter 8 Lesson 2 THE PERIODIC TABLE Vocabulary

Chapter 8 Lesson 2 THE PERIODIC TABLE
Vocabulary Element: a substance that cannot be broken apart into other substances Compound: a pure substance made of two or more elements that are chemically combined Periodic Table: a table in which the elements are arranged by their properties Chemical Symbol: an abbreviation of the element’s name

Vocabulary Metal: usually shiny, can be bent or stretched, and conducts electricity Nonmetal: an element that is usually dull in color, does not conduct electricity, does not bend or stretch very much, and breaks easily Semimetal: a metal that is like metals in some ways and like nonmetals in other ways Noble Gas: a substance that cannot be broken apart into other substance

Organizing the Elements §Around 450 B. C. Greek philosopher Empedocles, suggested that all matter is made of four elements: earth, air, fire, and water. §By the 1600 s, people knew there were many more elements §By the 1800 s, scientists had begun to identify and list many elements. They knew these elements had things in common but they did not know how to organize them. §In 1869, Russian chemist Dimitri Mendeleyev developed a table to arrange the elements. §Today, scientists use the Periodic Table of Elements.
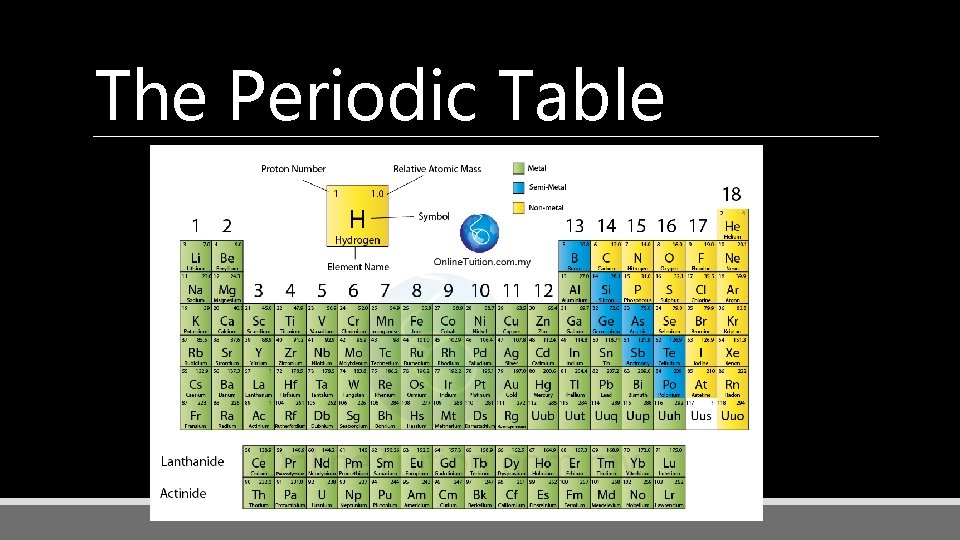
The Periodic Table

Metals

Nonmetals

Semimetals

Elements Alone and Joined §All matter is made of elements §Elements are made up of atoms §Elements join to form compounds §Noble gases hardly ever combine to form compounds

Quiz Chapter 8 Lesson 2 §How is the Periodic Table a useful tool? (1 point) §What are three classifications used in the periodic table? (3 points) §What are the elements organized by? (1 point)
- Slides: 10