Chapter 8 Key Concepts Introduction to Metabolism Examples

Chapter 8 Key Concepts Introduction to Metabolism: Examples of endergonic and exergonic reactions Role of ATP in energy coupling Enzymes: lower the activation energy. Catalytic cycle of an enzyme Factors that affect enzyme activity
An organism’s metabolism transforms matter and energy, subject to the laws of thermodynamics Metabolism Catabolism: “energy releasing” Anabolism: “energy consuming” © 2011 Pearson Education, Inc.

Figure 8. 3 a Chemical energy (a) First law of thermodynamics = conservation of energy
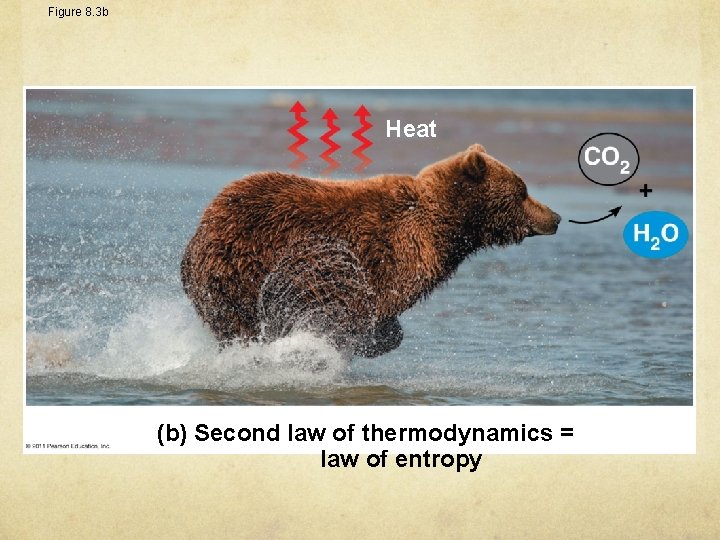
Figure 8. 3 b Heat (b) Second law of thermodynamics = law of entropy
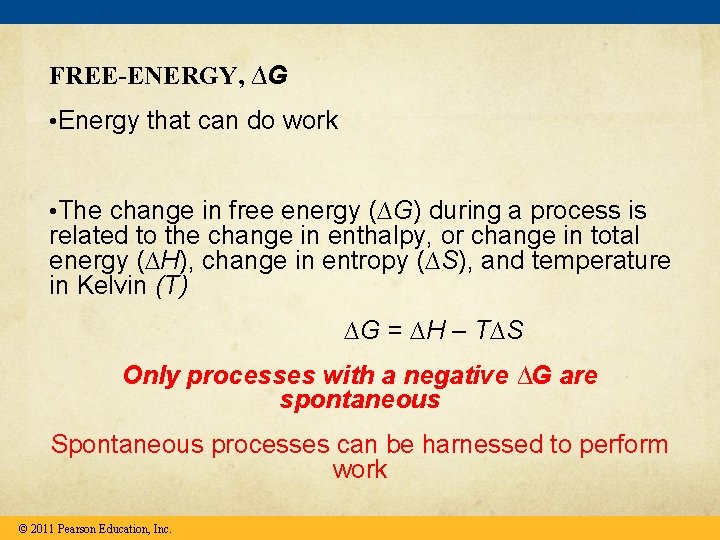
FREE-ENERGY, ∆G • Energy that can do work • The change in free energy (∆G) during a process is related to the change in enthalpy, or change in total energy (∆H), change in entropy (∆S), and temperature in Kelvin (T) ∆G = ∆H – T∆S Only processes with a negative ∆G are spontaneous Spontaneous processes can be harnessed to perform work © 2011 Pearson Education, Inc.
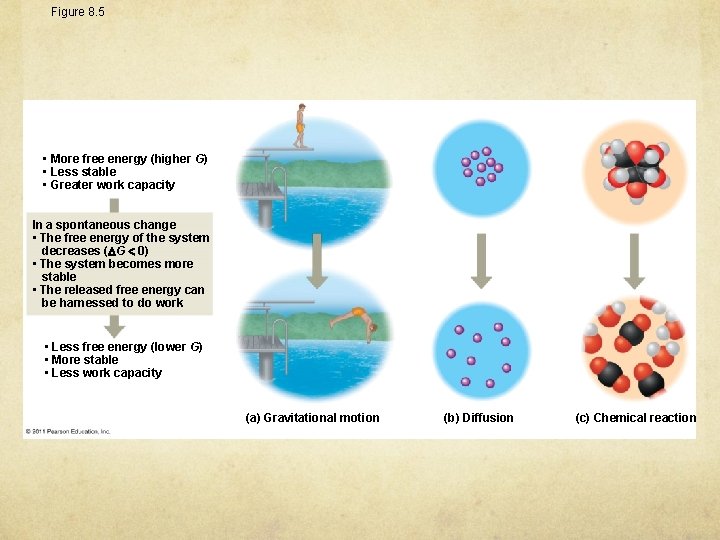
Figure 8. 5 • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases ( G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity (a) Gravitational motion (b) Diffusion (c) Chemical reaction
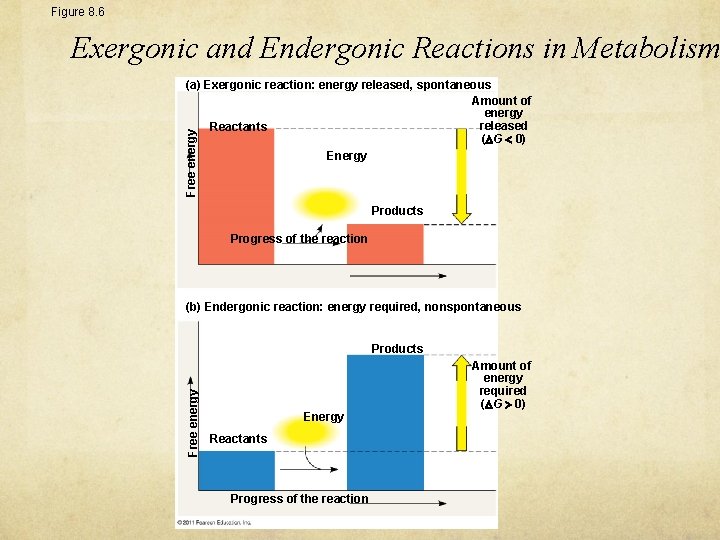
Figure 8. 6 Exergonic and Endergonic Reactions in Metabolism Free energy (a) Exergonic reaction: energy released, spontaneous Amount of energy released Reactants ( G 0) Energy Products Progress of the reaction (b) Endergonic reaction: energy required, nonspontaneous Free energy Products Energy Reactants Progress of the reaction Amount of energy required ( G 0)

Living Organisms and Order How do living organisms create macromolecules, organelles, cells, tissues, and complex higher-order structures? The laws of thermodynamics do not apply to living organisms. b) Living organisms create order by using energy from the sun. c) Living organisms create order locally, but the energy transformations generate waste heat that increases the entropy of the universe. a)

Life and Chemical Equilibrium Are most chemical reactions at equilibrium in living cells? yes b) no c) only the exergonic reactions d) all reactions except those powered by ATP hydrolysis a)
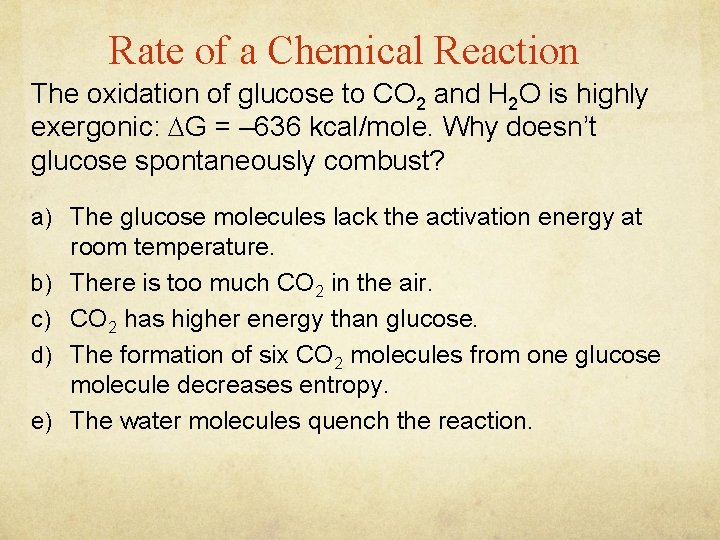
Rate of a Chemical Reaction The oxidation of glucose to CO 2 and H 2 O is highly exergonic: G = – 636 kcal/mole. Why doesn’t glucose spontaneously combust? a) The glucose molecules lack the activation energy at b) c) d) e) room temperature. There is too much CO 2 in the air. CO 2 has higher energy than glucose. The formation of six CO 2 molecules from one glucose molecule decreases entropy. The water molecules quench the reaction.
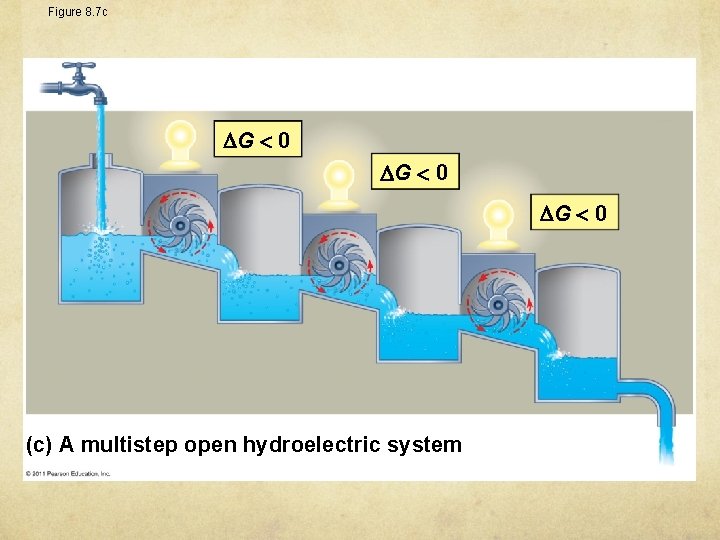
Figure 8. 7 c G 0 (c) A multistep open hydroelectric system
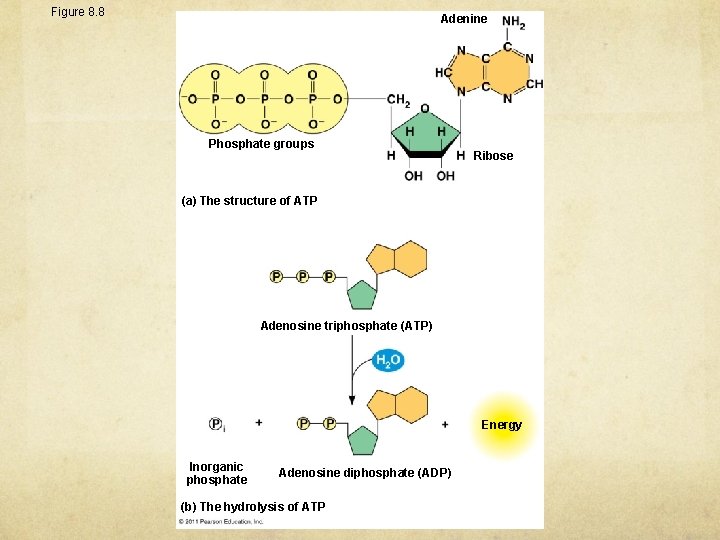
Figure 8. 8 Adenine Phosphate groups Ribose (a) The structure of ATP Adenosine triphosphate (ATP) Energy Inorganic phosphate Adenosine diphosphate (ADP) (b) The hydrolysis of ATP
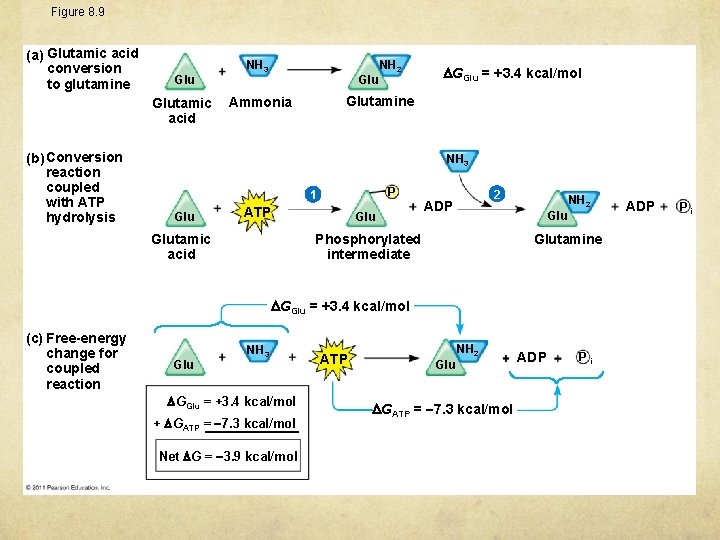
Figure 8. 9 (a) Glutamic acid conversion to glutamine Glutamic acid (b) Conversion reaction coupled with ATP hydrolysis NH 3 Glu NH 2 GGlu = +3. 4 kcal/mol Glutamine Ammonia NH 3 P 1 Glu ATP Glu 2 ADP Glu Phosphorylated intermediate Glutamic acid NH 2 Glutamine GGlu = +3. 4 kcal/mol (c) Free-energy change for coupled reaction Glu NH 3 GGlu = +3. 4 kcal/mol + GATP = 7. 3 kcal/mol Net G = 3. 9 kcal/mol ATP NH 2 Glu GATP = 7. 3 kcal/mol ADP Pi
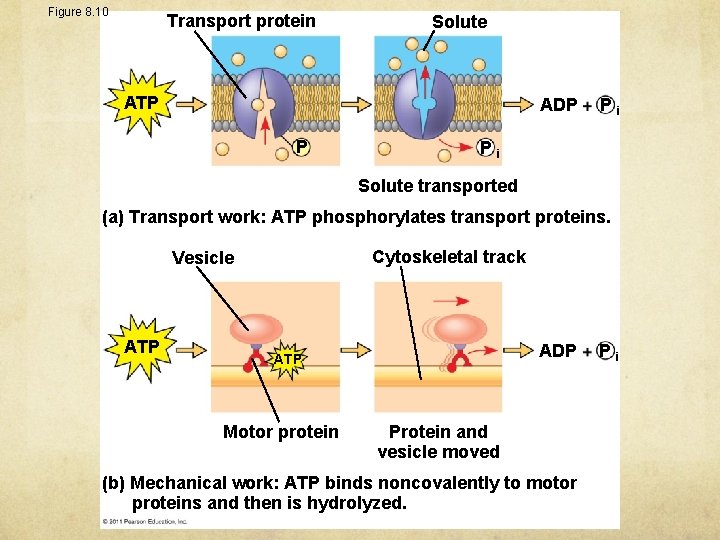
Figure 8. 10 Transport protein Solute ATP ADP P Pi Pi Solute transported (a) Transport work: ATP phosphorylates transport proteins. Cytoskeletal track Vesicle ATP ADP ATP Motor protein Protein and vesicle moved (b) Mechanical work: ATP binds noncovalently to motor proteins and then is hydrolyzed. Pi
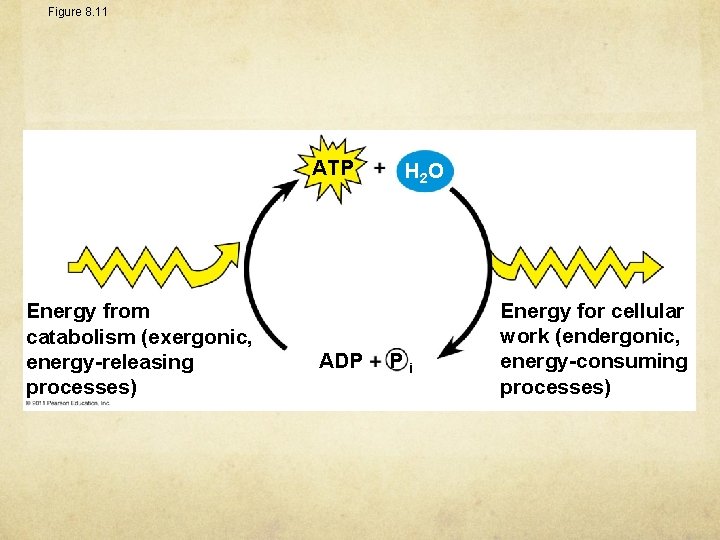
Figure 8. 11 ATP Energy from catabolism (exergonic, energy-releasing processes) ADP H 2 O Pi Energy for cellular work (endergonic, energy-consuming processes)
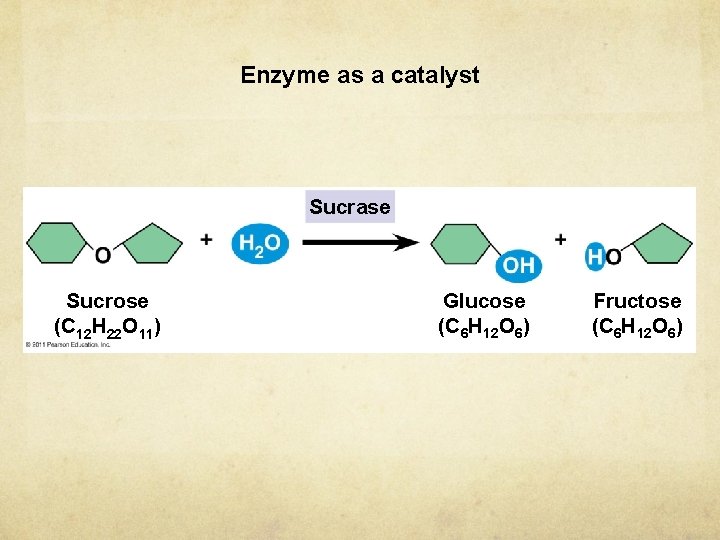
Enzyme as a catalyst Sucrase Sucrose (C 12 H 22 O 11) Glucose (C 6 H 12 O 6) Fructose (C 6 H 12 O 6)
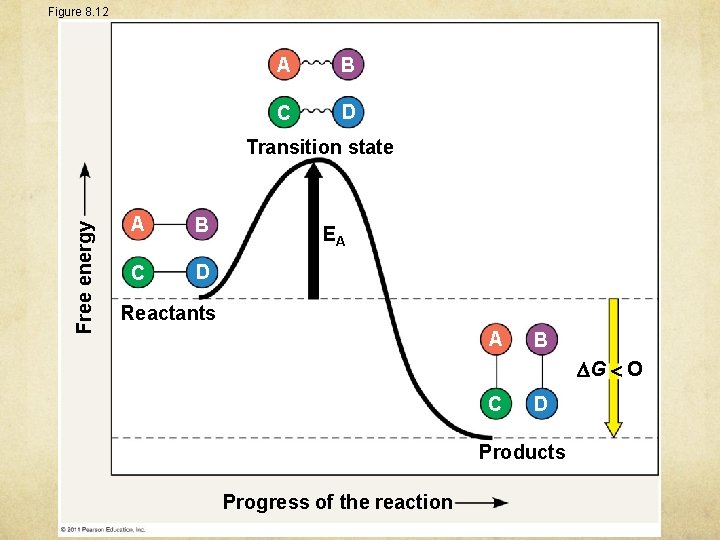
Figure 8. 12 A B C D Free energy Transition state A B C D EA Reactants A B G O C D Products Progress of the reaction
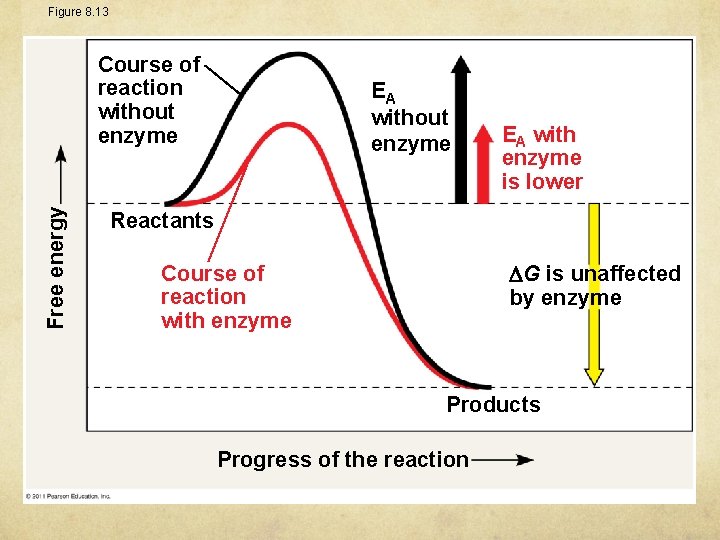
Figure 8. 13 Free energy Course of reaction without enzyme EA with enzyme is lower Reactants G is unaffected by enzyme Course of reaction with enzyme Products Progress of the reaction
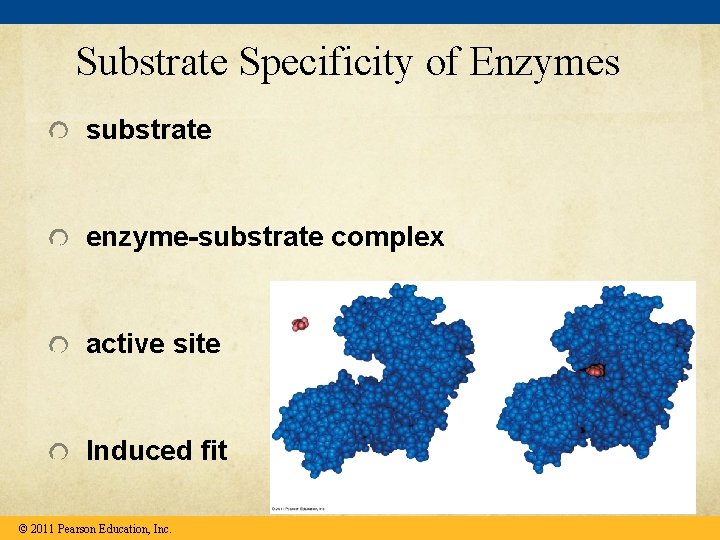
Substrate Specificity of Enzymes substrate enzyme-substrate complex active site Induced fit © 2011 Pearson Education, Inc.

Catalysis in the Enzyme’s Active Site In an enzymatic reaction, the substrate binds to the active site of the enzyme The active site can lower an EA barrier by Orienting substrates correctly Straining substrate bonds Providing a favorable microenvironment Covalently bonding to the substrate © 2011 Pearson Education, Inc.
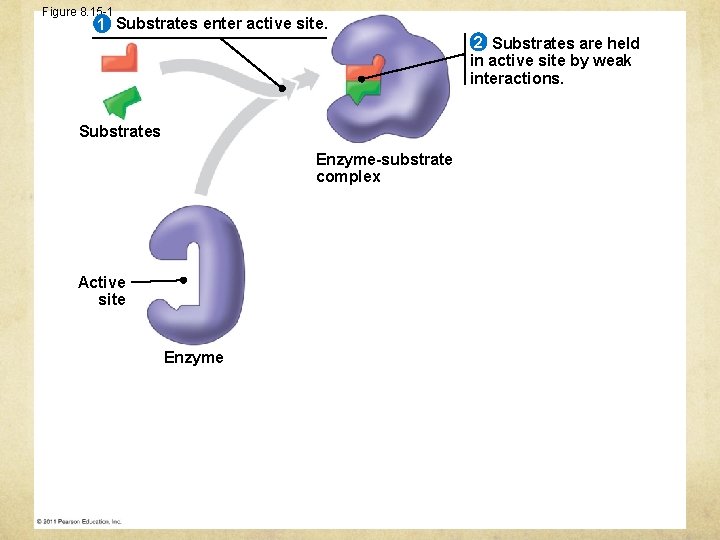
Figure 8. 15 -1 1 Substrates enter active site. 2 Substrates are held in active site by weak interactions. Substrates Enzyme-substrate complex Active site Enzyme
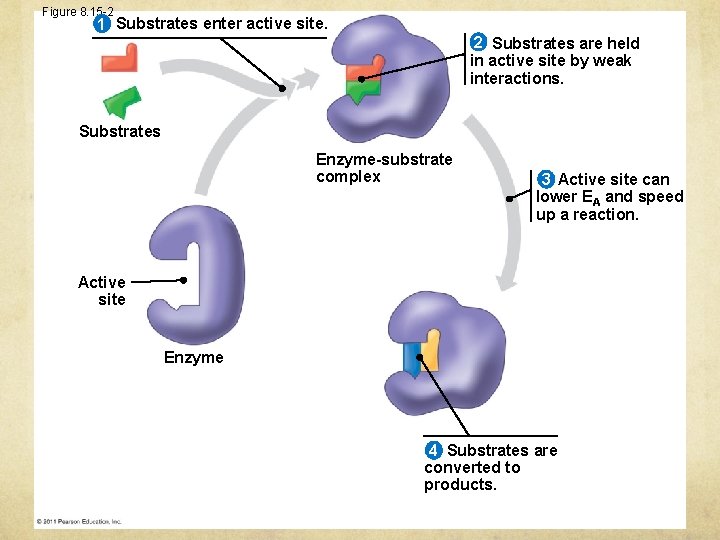
Figure 8. 15 -2 1 Substrates enter active site. 2 Substrates are held in active site by weak interactions. Substrates Enzyme-substrate complex 3 Active site can lower EA and speed up a reaction. Active site Enzyme 4 Substrates are converted to products.
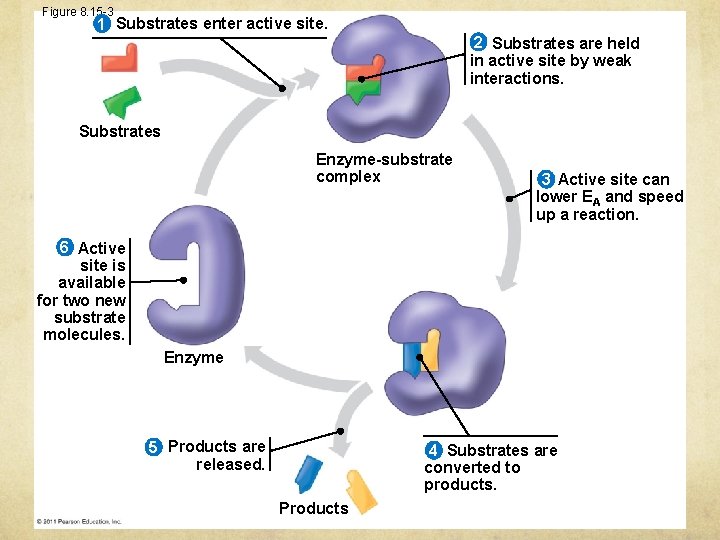
Figure 8. 15 -3 1 Substrates enter active site. 2 Substrates are held in active site by weak interactions. Substrates Enzyme-substrate complex 3 Active site can lower EA and speed up a reaction. 6 Active site is available for two new substrate molecules. Enzyme 5 Products are released. 4 Substrates are converted to products. Products
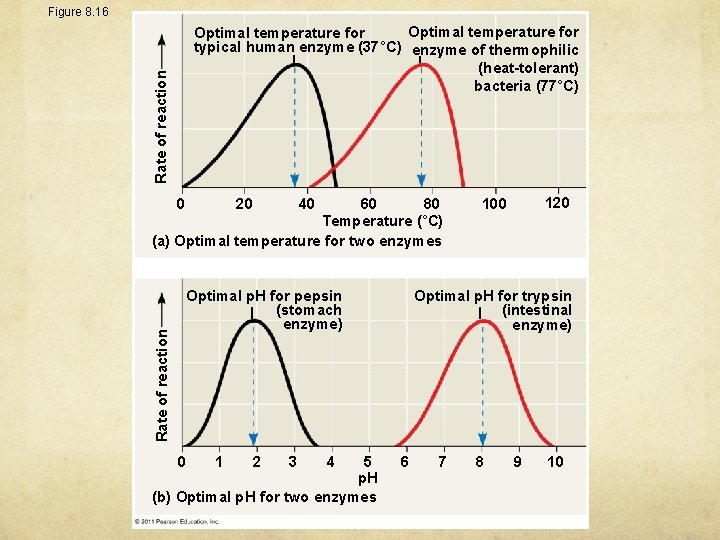
Figure 8. 16 Rate of reaction Optimal temperature for typical human enzyme (37°C) enzyme of thermophilic (heat-tolerant) bacteria (77°C) 60 80 Temperature (°C) (a) Optimal temperature for two enzymes 0 20 40 Rate of reaction Optimal p. H for pepsin (stomach enzyme) 0 5 p. H (b) Optimal p. H for two enzymes 1 2 3 4 120 100 Optimal p. H for trypsin (intestinal enzyme) 6 7 8 9 10

Cofactors Non-protein enzyme helpers = Cofactors organic cofactor = coenzyme vitamins © 2011 Pearson Education, Inc.
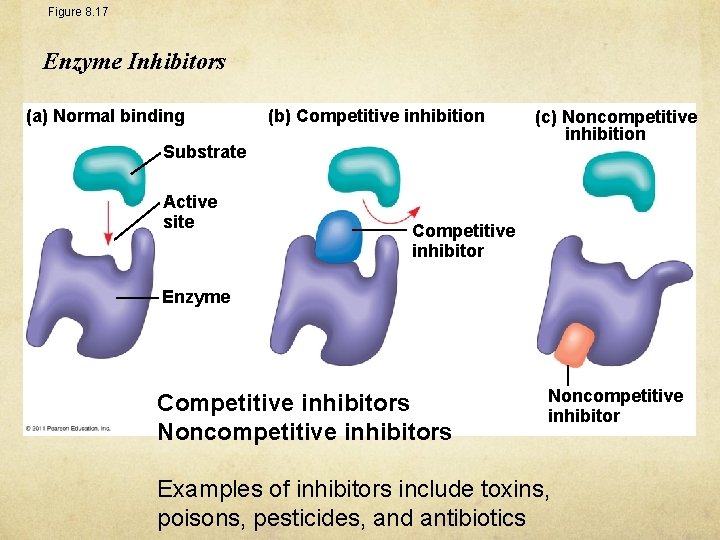
Figure 8. 17 Enzyme Inhibitors (a) Normal binding (b) Competitive inhibition Substrate Active site (c) Noncompetitive inhibition Competitive inhibitor Enzyme Competitive inhibitors Noncompetitive inhibitor Examples of inhibitors include toxins, poisons, pesticides, and antibiotics

Enzyme Inhibitors Vioxx and other prescription nonsteroidal antiinflammatory drugs (NSAIDs) are potent inhibitors of the cycloxygenase-2 (COX-2) enzyme. High substrate concentrations reduce the efficacy of inhibition by these drugs. These drugs are competitive inhibitors. b) noncompetitive inhibitors. c) allosteric regulators. d) prosthetic groups. e) feedback inhibitors. a)
The Evolution of Enzymes = proteins encoded by genes Changes (mutations) in genes lead to changes in amino acid composition of an enzyme Altered amino acids in enzymes may alter their substrate specificity Under new environmental conditions a novel form of an enzyme might be favored © 2011 Pearson Education, Inc.

Allosteric Activation and Inhibition Most allosterically regulated enzymes are made from polypeptide subunits Each enzyme has active and inactive forms The binding of an activator stabilizes the active form of the enzyme The binding of an inhibitor stabilizes the inactive form of the enzyme
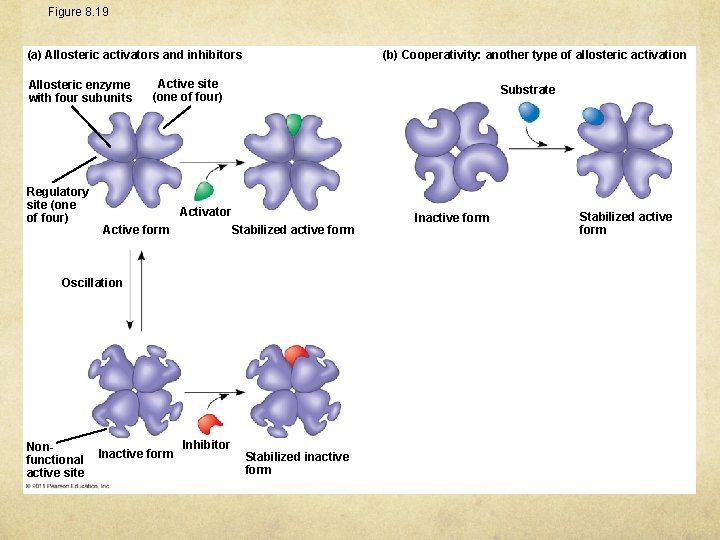
Figure 8. 19 (b) Cooperativity: another type of allosteric activation (a) Allosteric activators and inhibitors Allosteric enzyme with four subunits Regulatory site (one of four) Active site (one of four) Substrate Activator Stabilized active form Active form Oscillation Nonfunctional active site Inactive form Inhibitor Stabilized inactive form Inactive form Stabilized active form

Figure 8. 21 Feedback Inhibition Active site available Isoleucine used up by cell Active site of Feedback enzyme 1 is inhibition no longer able to catalyze the conversion of threonine to intermediate A; pathway is switched off. Isoleucine binds to allosteric site. Initial substrate (threonine) Threonine in active site Enzyme 1 (threonine deaminase) Intermediate A Enzyme 2 Intermediate B Enzyme 3 Intermediate C Enzyme 4 Intermediate D Enzyme 5 End product (isoleucine)
- Slides: 31