CHAPTER 8 Fluids are Affected by Forces Pressure

CHAPTER 8 Fluids are Affected by Forces, Pressure and Heat
8. 1 Forces � Force= push or pull that acts on an object 2 Groups of Forces Contact Forces = only have an effect on objects they touch Action-at-a-Distance Forces = can apply forces to an object without touching it
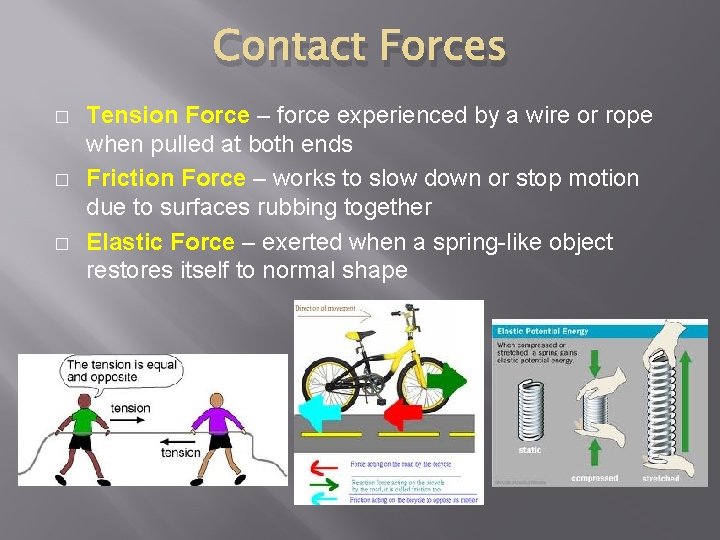
Contact Forces � � � Tension Force – force experienced by a wire or rope when pulled at both ends Friction Force – works to slow down or stop motion due to surfaces rubbing together Elastic Force – exerted when a spring-like object restores itself to normal shape

Action-at-a-Distance Forces � � � Gravitational Force – force of attraction between objects due to mass (depends on mass and distance of objects) Electrostatic Force – static electricity = the excess of positive or negative charge, causes pushing and pulling forces (Like charges repel Opposite charges attract) Magnetic Forces – Natural magnets, electromagnets, and the Earth exert pushing or pulling forces on certain metals (iron, nickel, cobalt)
Measuring Mass � � � Mass is measured in metric units (grams, kilograms) Measured using a balance scale Mass is the same anywhere in the universe

Weight Depends on Gravity � � Weight is the amount of force on an object due to gravity Bathroom scales actually measure force not mass

The Newton � � � Force is measured in Newtons (N) (Sir Isaac Newton) It is a small amount of force about enough to hold up an apple Weight is measured in Newtons because weight is a force

Measuring Force � � � Use a force meter to measure (the most common type = spring scale) Mass and weight are directly proportional but not the same thing! If you change one it affects the other. To convert mass to weight multiply by 9. 8 meter/second squared
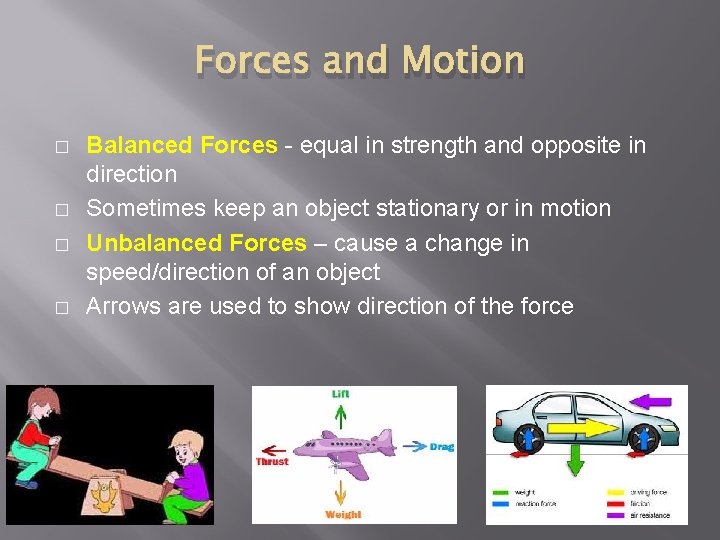
Forces and Motion � � Balanced Forces - equal in strength and opposite in direction Sometimes keep an object stationary or in motion Unbalanced Forces – cause a change in speed/direction of an object Arrows are used to show direction of the force

Homework � Textbook Questions! Page 289 Questions # 2 -4, 6 � Complete your Ch. 8 vocab. �

8. 2 Pressure � � Pressure = the amount of force acting over a given area on an object Compression = decrease in volume produced by a force
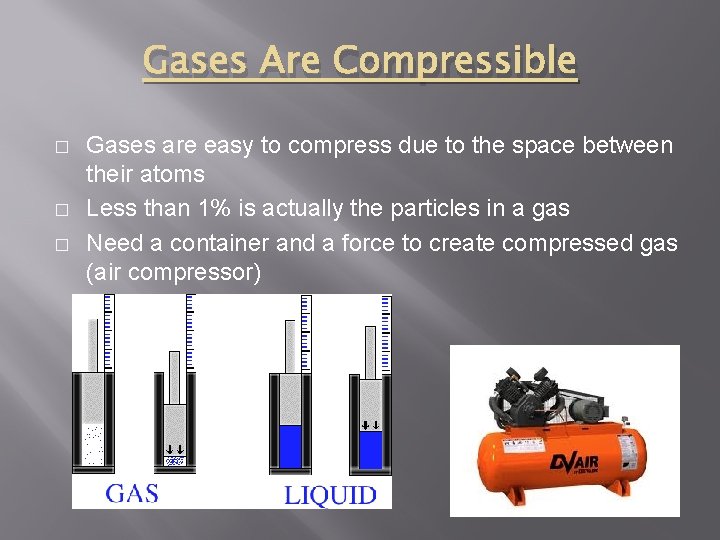
Gases Are Compressible � � � Gases are easy to compress due to the space between their atoms Less than 1% is actually the particles in a gas Need a container and a force to create compressed gas (air compressor)

Pressure and Kinetic Activity in Gases � � Pressure is a good indicator of kinetic energy of a gas Heat added increases the pressure can cause explosions or implosions
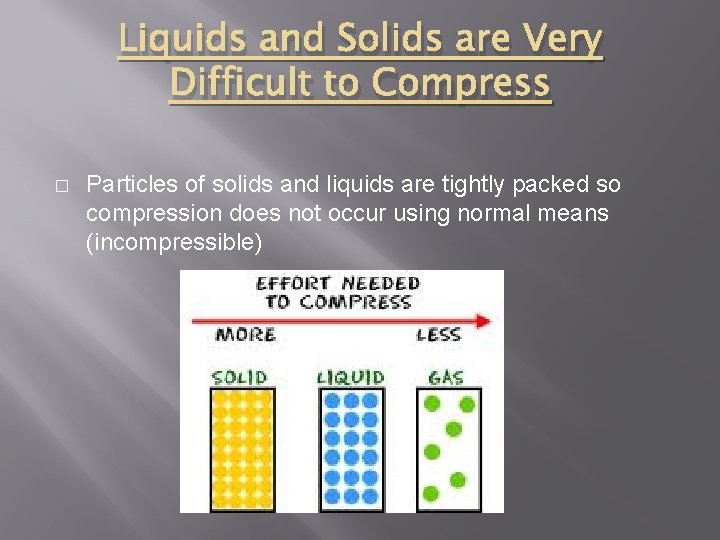
Liquids and Solids are Very Difficult to Compress � Particles of solids and liquids are tightly packed so compression does not occur using normal means (incompressible)

Compression and Deformation � � � Deformation = change in shape without being forced into a smaller volume Examples - Styrofoam cups, bouncing a ball The air inside is being compressed

Comparing Pressure Spreading force over a greater area means the pressure can be reduced (bed of nails) We use the formula: � Force (measured in Newtons (N) Area (measured in m 2)

Calculating Pressure � A BMX rider exerts a force of 1200 N on a sheet of plywood that measures 1 m x 2 m. How much pressure is exerted? � P= F/A P = 1200 N/(1 mx 2 m) P = 600 N/m 2 = 600 Pa � �

Homework Questions Textbook Questions � Page 296 Practice Problems 1 -3 � Page 299 Questions # 2, 3, 5, 6

8. 3 Viscosity, Adhesion, and Cohesion � � � Viscosity = the resistance of a fluid to flow, the slower it flows the more viscose the liquid is Due to the: Particles in some liquids flow past each other better than others Some particles have greater attraction to each other Oil = complicated molecule ; water = simple molecule

Flow Rate of Various Liquids � You can compare liquids by measuring their flow rates = speed at which a fluid flows from one point to another
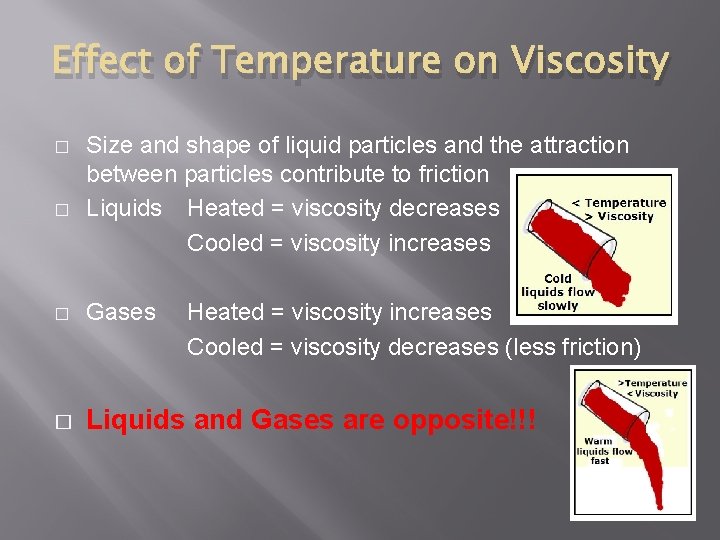
Effect of Temperature on Viscosity � � Size and shape of liquid particles and the attraction between particles contribute to friction Liquids Heated = viscosity decreases Cooled = viscosity increases � Gases Heated = viscosity increases Cooled = viscosity decreases (less friction) � Liquids and Gases are opposite!!!

Adhesion � � � Another property of fluids that can affect their flow is adhesion Adhesion = attraction or joining of two different objects or fluids to each other Fluids are sometimes attracted to surfaces Example : water molecules have polarity which means one end of the molecule is positive and the other is negative – makes the molecule sticky It is hard to get all water out of a cup

Cohesion � � � Cohesion = strength with which the particles of an object or fluid attract each other • Water molecules attract one another at the surface creating surface tension • Example: water strider insects

Homework Questions � � Textbook Questions Page 309 Questions# 1, 3, 4, 5, 6
- Slides: 24