Chapter 8 COVALENT BONDING The Covalent Bond Stability

Chapter 8 COVALENT BONDING

The Covalent Bond Stability of an atom, ion, or compound is related to its energy Lower Ions energy => more stable became stable by gaining/losing electrons Covalent bonds become stable by sharing electrons
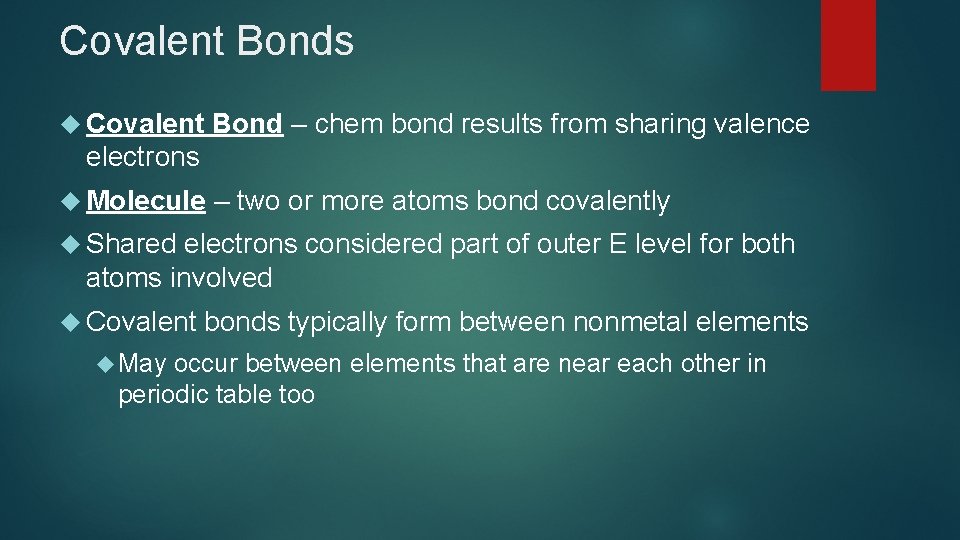
Covalent Bonds Covalent Bond – chem bond results from sharing valence electrons Molecule – two or more atoms bond covalently Shared electrons considered part of outer E level for both atoms involved Covalent May bonds typically form between nonmetal elements occur between elements that are near each other in periodic table too
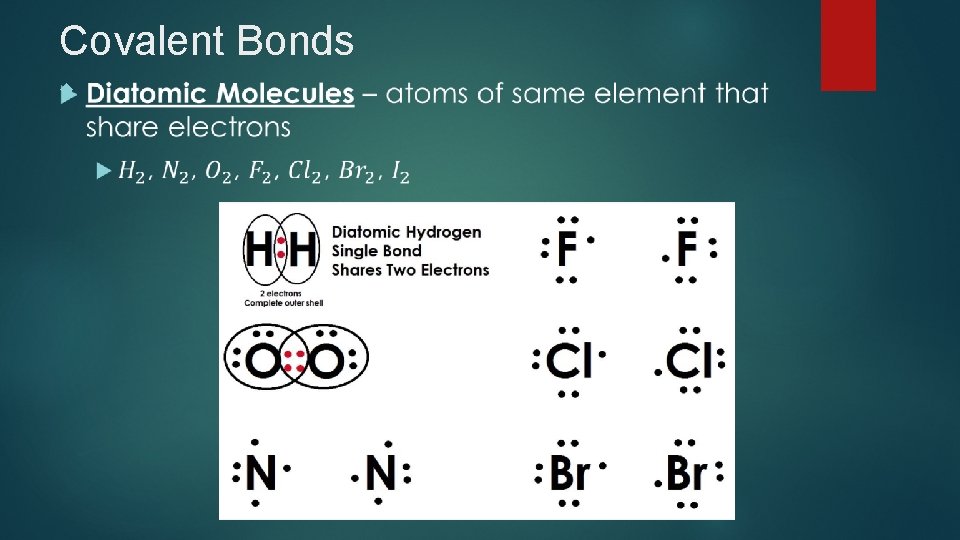
Covalent Bonds

Covalent Bonds Several 2 forces involved: repulsive forces 1 from each atoms like-charged electrons 1 from each atoms like-charged protons Attraction between protons of 1 element and electrons of the other element Atoms will move closer together until point of maximum net attraction => covalent bond If nuclei move too close together, repulsion occurs This exceeds the attraction forces
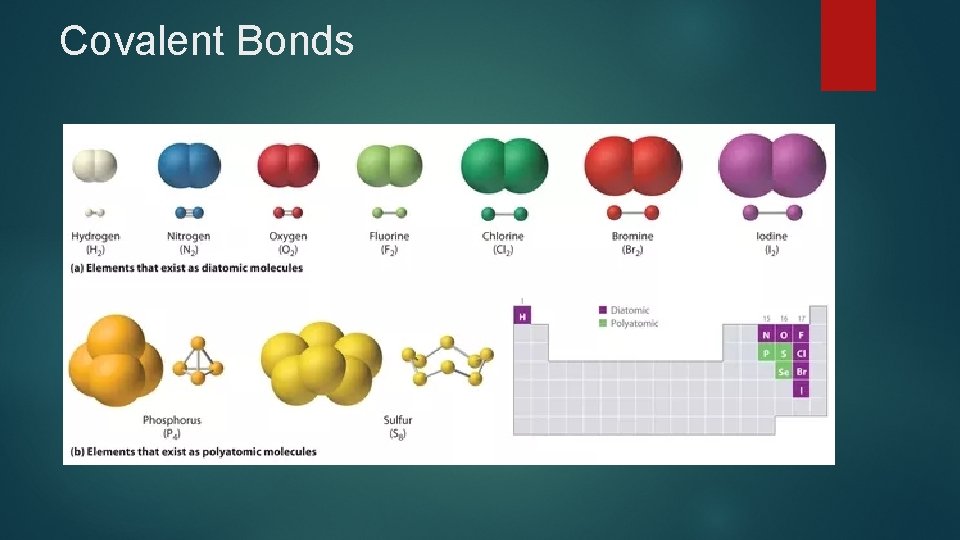
Covalent Bonds
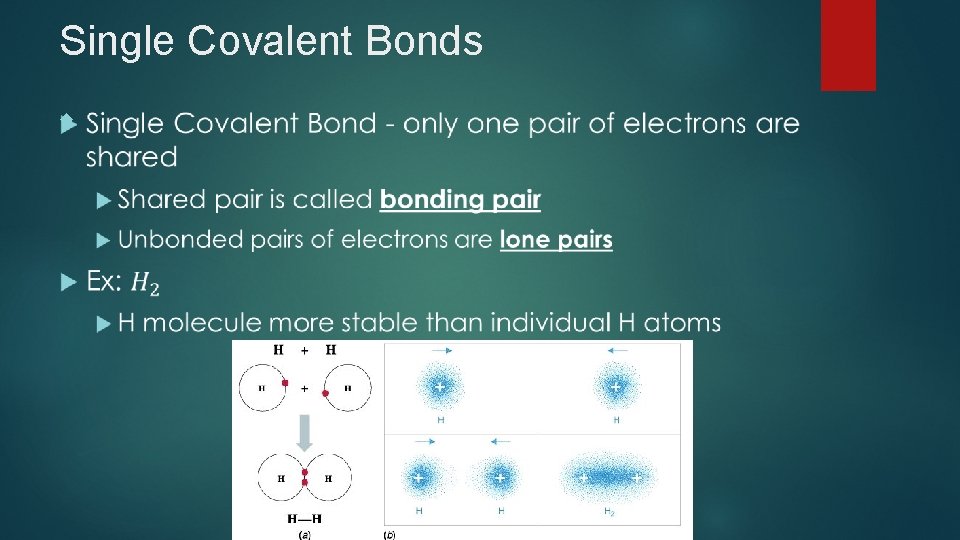
Single Covalent Bonds
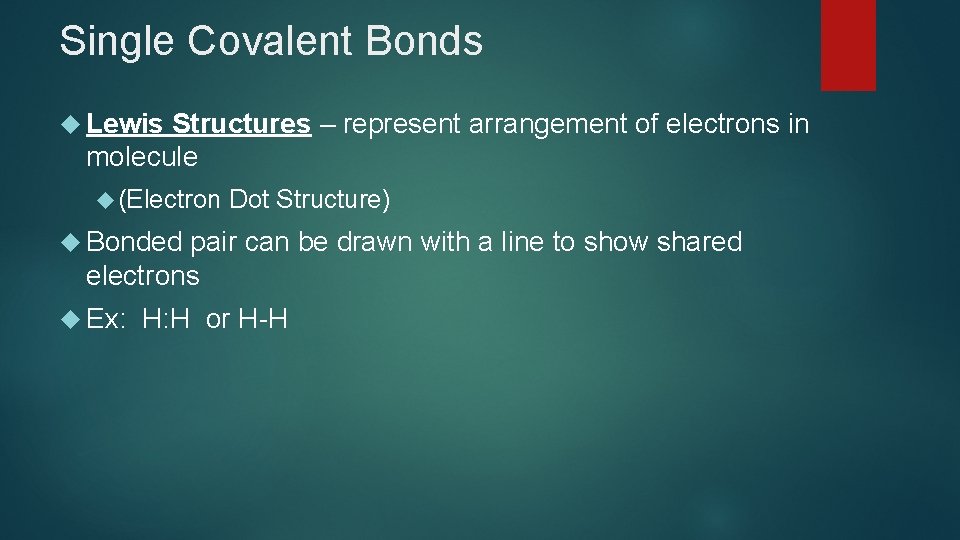
Single Covalent Bonds Lewis Structures – represent arrangement of electrons in molecule (Electron Dot Structure) Bonded pair can be drawn with a line to show shared electrons Ex: H: H or H-H
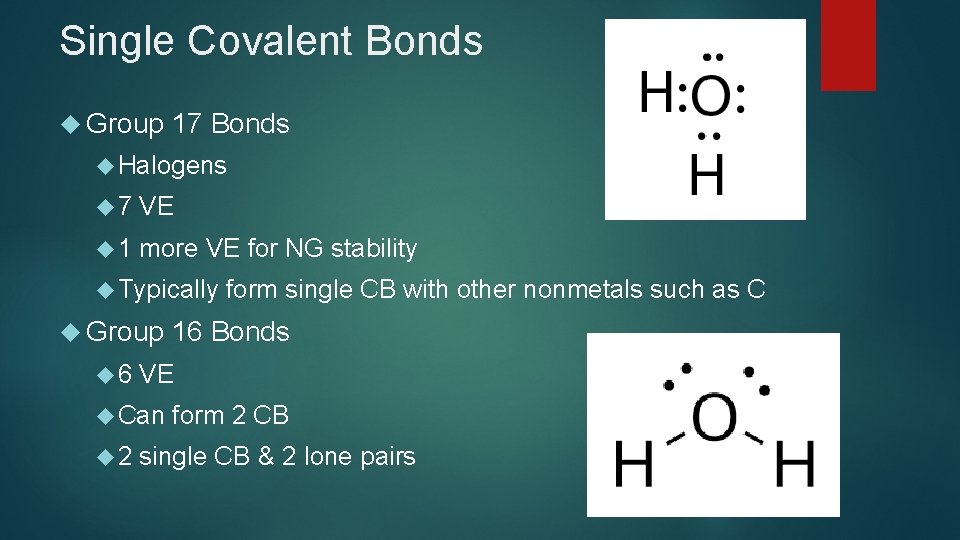
Single Covalent Bonds Group 17 Bonds Halogens 7 VE 1 more VE for NG stability Typically Group 6 16 Bonds VE Can 2 form single CB with other nonmetals such as C form 2 CB single CB & 2 lone pairs
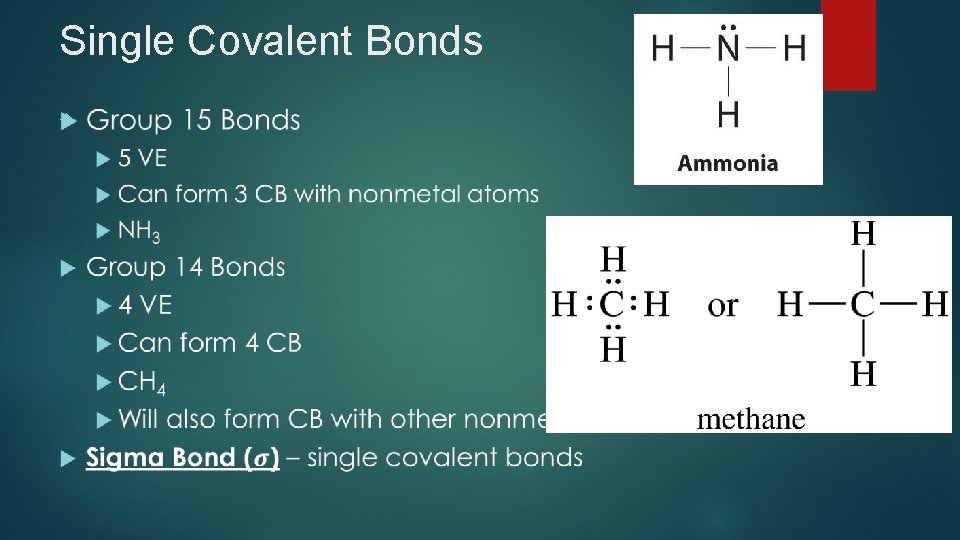
Single Covalent Bonds

Example 1 Draw Lewis structures for each molecule 1. PH 3 2. H 2 S 3. HCl 4. CCl 4 5. Si. H 4
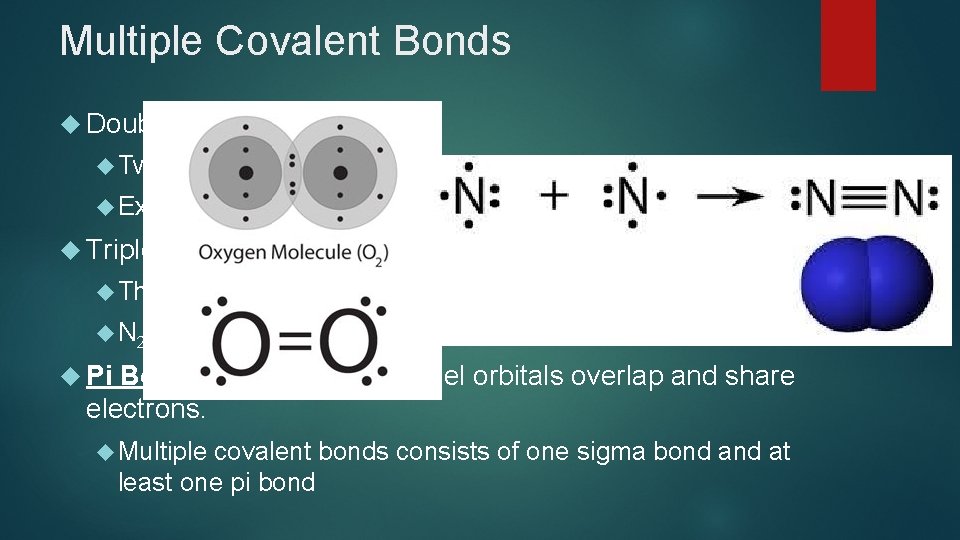
Multiple Covalent Bonds Double Two Ex: Triple Bonds: pairs of electrons are shared between 2 atoms O 2 Bonds: Three pairs of electrons are shared between 2 atoms N 2 Pi Bond – forms when parallel orbitals overlap and share electrons. Multiple covalent bonds consists of one sigma bond at least one pi bond

Strength of Covalent Bonds CB involve attractive/repulsive forces CB differ in strength due to these varying forces Bond Length: Strength is dependent upon length between bonded nuclei Determined Shorter by size of two bonding atoms bonds are stronger Triple bond is shortest Double Single bond is shorter than single and longer than triple bond is longest
Bonds and Energy changes occur when a bond between atoms in a molecule form or break Energy is released when bond forms Energy is required to break a bond Bond Dissociation Energy - energy required to break a covalent bond BDE indicate strength of chemical bond Small the bond length, the greater the BDE Endothermic Rxn – greater amount of energy is required to break existing bond in reactants than is released when new bonds form in products Exothermic Rxn – more energy is released during product formation that is required to break bonds in reactants

8. 2 – Naming Molecules
Naming Binary Molecular Compounds 1. The first element in the formula always uses the name of the entire element 2. The second element in the formula is named using its root and adding the suffix –ide 3. Prefixes are used to indicate the # of atoms of each element that are present in the compound.
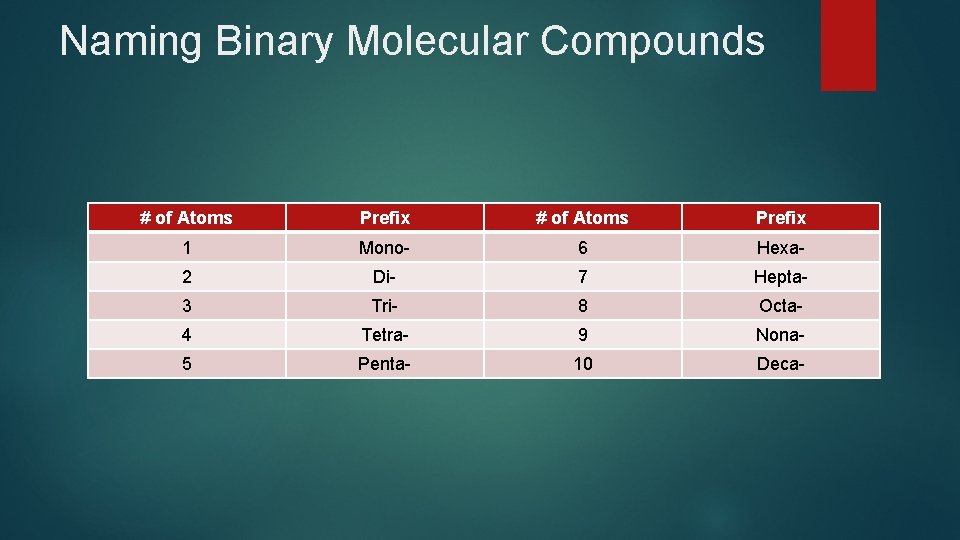
Naming Binary Molecular Compounds # of Atoms Prefix 1 Mono- 6 Hexa- 2 Di- 7 Hepta- 3 Tri- 8 Octa- 4 Tetra- 9 Nona- 5 Penta- 10 Deca-

Naming Binary Molecular Compounds Exceptions: The first element in a compound never uses the mono- prefix If the prefix results in 2 consecutive vowels, drop one of the vowels Ex: CO Monocarbon Carbon monooxide monoxide

Example 2 Name each of the binary covalent compounds 1. CO 2 2. SO 2 3. NF 3 4. CCl 4 5. P 2 O 5

Common Names H 2 O Water NH 3 Ammonia N 2 H 4 Hydrazine NO Nitric Oxide

Naming Acids Water solns of some molecules are acidic and named as acids If compound produces H+ ions in soln, it’s an acid Two common types: Binary acids oxyacids

Naming Acids Naming Binary Acids: Contains a H and one other element 1. The first word has the prefix hydro- to name the H part. The rest of the first word comes from the root of the second element plus the suffix –ic 2. The second word is always acid Ex: HCl

Naming Acids Naming Oxyacids: Contains both H and O First, identify the oxyanion present. The first word will consist of the root of the oxyanion and the prefix per- or hypo- if it’s part of the name and a suffix. If name ends with –ate, replace the suffix with –ic. If the name of oxyanion ends with –ite, replace suffix with –ous. 2. The second word is always acid 1. Ex: HNO 3
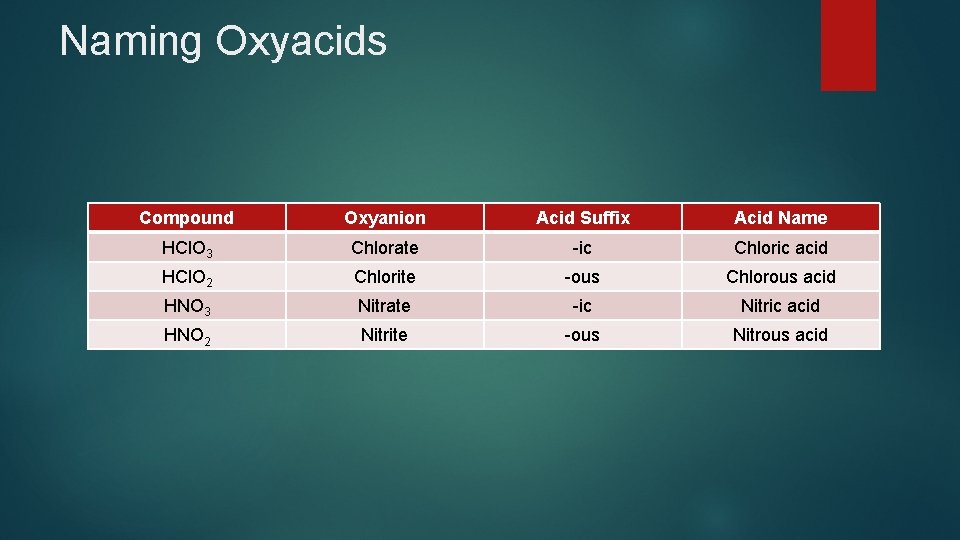
Naming Oxyacids Compound Oxyanion Acid Suffix Acid Name HCl. O 3 Chlorate -ic Chloric acid HCl. O 2 Chlorite -ous Chlorous acid HNO 3 Nitrate -ic Nitric acid HNO 2 Nitrite -ous Nitrous acid

Example 3 Name each of the binary covalent compounds HI Hydroiodic acid 2. HCl. O 3 Chloric acid 3. HCl. O 2 1. Chlorous acid 4. H 2 SO 4 Sulfuric acid 5. H 2 S Hydrosulfuric acid

Example 4 Give the formula for each compound: 1. Silver chloride 2. Dihydrogen monoxide 3. Chlorine tetrafluoride 4. Diphosphorus trioxide 5. Disulfur decafluoride
8. 3 –Molecular Structures
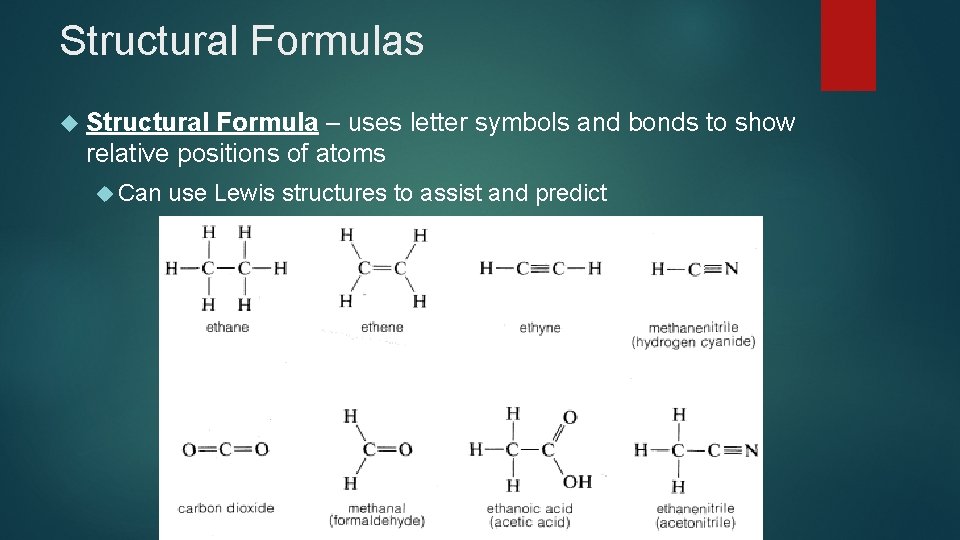
Structural Formulas Structural Formula – uses letter symbols and bonds to show relative positions of atoms Can use Lewis structures to assist and predict

Drawing Lewis Structures 1. 2. Predict location of certain atoms Atom w/ least attraction for shared electrons is central atom of molecule (usually the element closer to left side of periodic table!) All other atoms become terminal atoms Hydrogen is always a terminal/end atom Determine # of electrons available for bonding 3. # is equal to total # of VE in atom Determine # bonding pairs Divide # of electrons available for bonding by 2
Drawing Lewis Structures 4. Place bonding pairs 5. 6. Place 1 pair (single bond) between central atom and each terminal atom Determine # of bonding pairs remaining Subtract # of pairs used (step 4) from total bonding pairs determined in step 3. Remaining pairs are lone pairs or may be used in double/triple bonds Place lone pairs around terminal atoms (except H) Determine whether central atom satisfies octet rule Will double/triple bonds need to be added?

Example 5
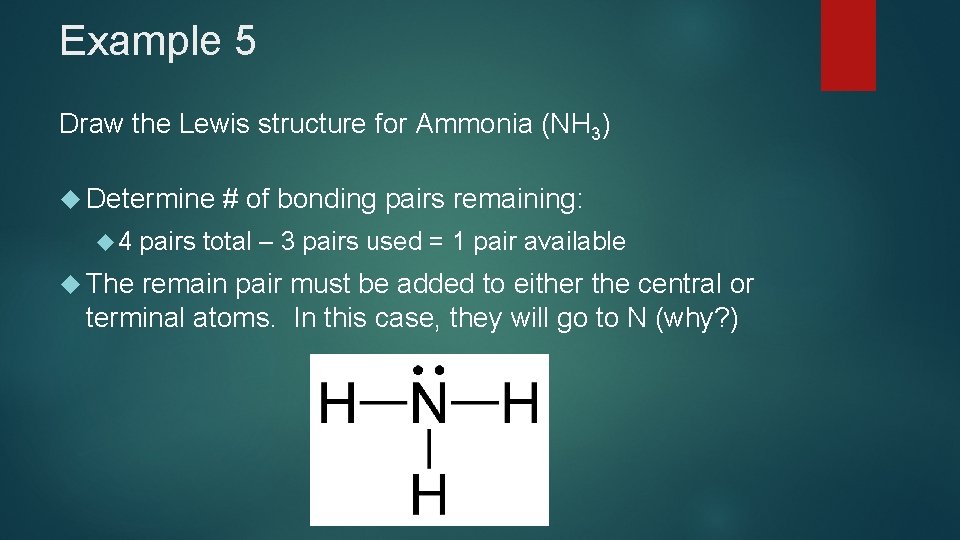
Example 5 Draw the Lewis structure for Ammonia (NH 3) Determine 4 The # of bonding pairs remaining: pairs total – 3 pairs used = 1 pair available remain pair must be added to either the central or terminal atoms. In this case, they will go to N (why? )

Example 6
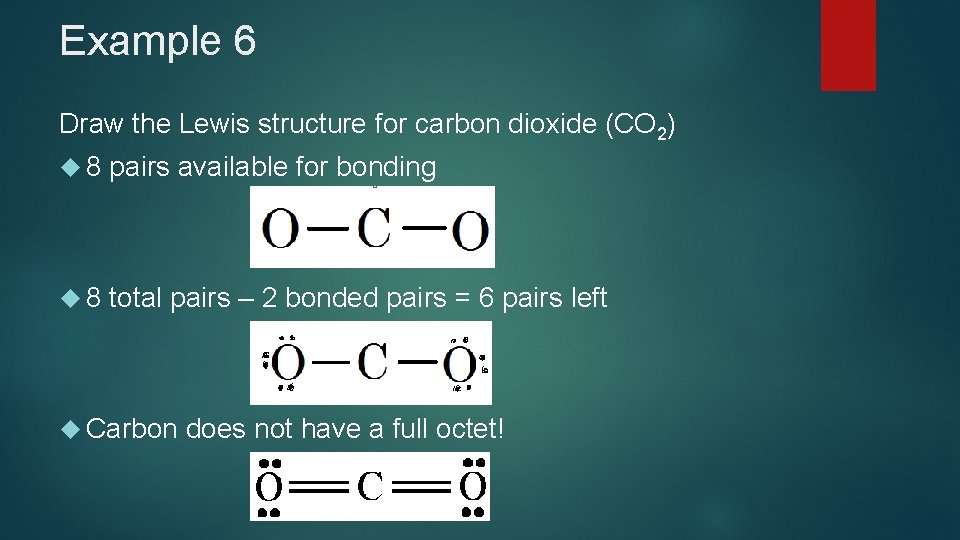
Example 6 Draw the Lewis structure for carbon dioxide (CO 2) 8 pairs available for bonding 8 total pairs – 2 bonded pairs = 6 pairs left Carbon does not have a full octet!

Lewis Structures for Polyatomic Ions Polyatomic ions act as an ionic unit, however, they are held together covalently! Only difference for creating Lewis structure of PA ion is that we have to keep track of electrons available for bonding. For negatively charged ions, more electrons will be added For positively charged ions, fewer electrons will be available

Example 7
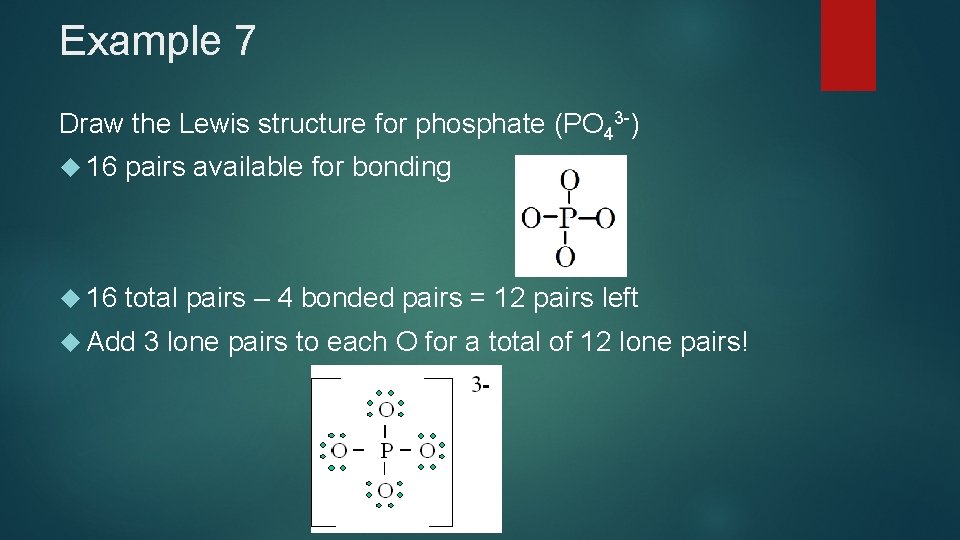
Example 7 Draw the Lewis structure for phosphate (PO 43 -) 16 pairs available for bonding 16 total pairs – 4 bonded pairs = 12 pairs left Add 3 lone pairs to each O for a total of 12 lone pairs!
- Slides: 37