Chapter 8 Covalent Bonding Section 1 Molecular Compounds

Chapter 8 Covalent Bonding

Section 1 Molecular Compounds

Section 1 Learning Targets 8. 1. 1 – I can distinguish between the melting points and boiling points of molecular compounds and ionic compounds. 8. 1. 2 – I can describe the information provided by a molecular formula.
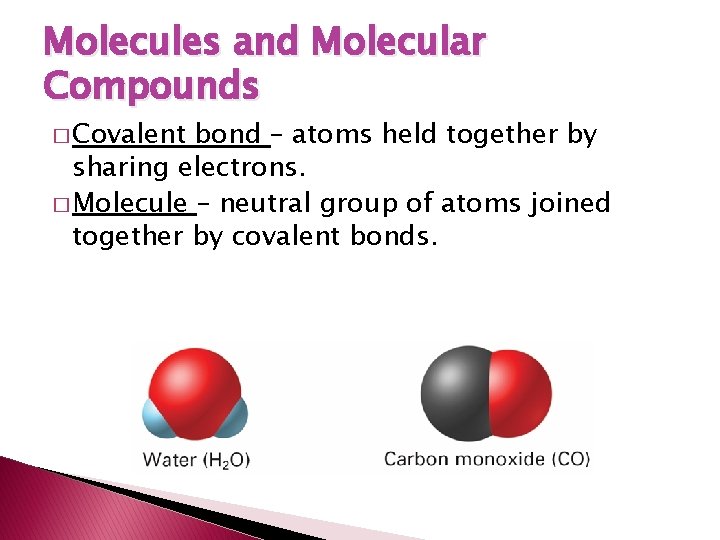
Molecules and Molecular Compounds � Covalent bond – atoms held together by sharing electrons. � Molecule – neutral group of atoms joined together by covalent bonds.
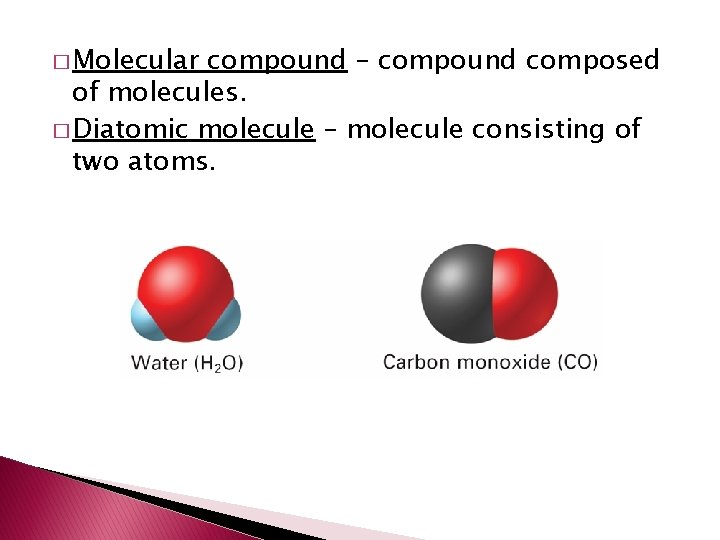
� Molecular compound – compound composed of molecules. � Diatomic molecule – molecule consisting of two atoms.
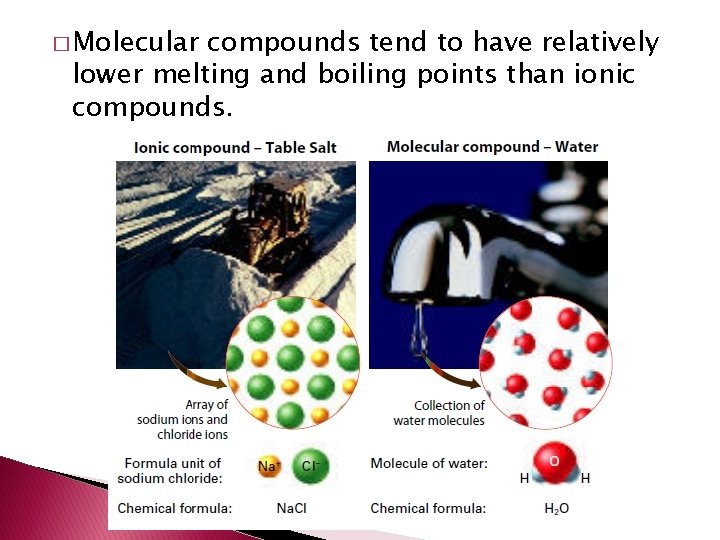
� Molecular compounds tend to have relatively lower melting and boiling points than ionic compounds.
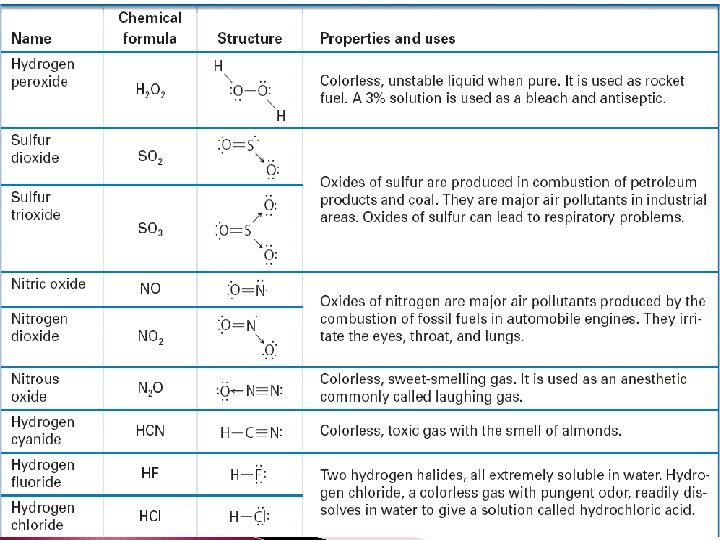
Molecular Formulas � Molecular formula – chemical formula of a molecular compound. � A molecular formula shows how many atoms of each element a molecule contains.
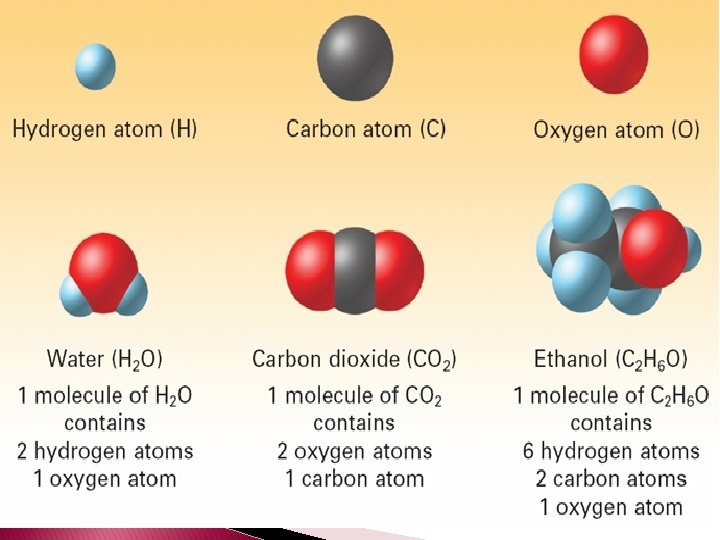
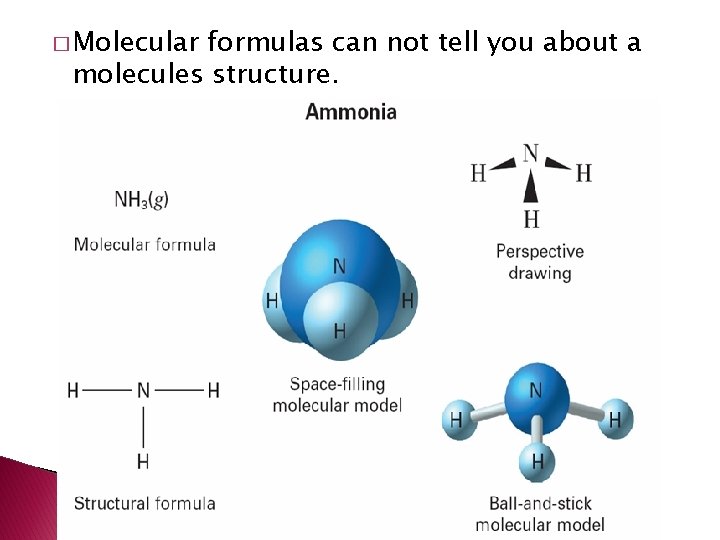
� Molecular formulas can not tell you about a molecules structure.

Section 2 The Nature of Covalent Bonding Part 1

Section 2 Learning Targets 8. 2. 1 – I can describe how electrons are shared to form covalent bonds and identify exceptions to the octet rule. 8. 2. 2 – I can demonstrate how electron dot structures represent shared electrons. 8. 2. 3 – I can describe how atoms form double or triple bonds.

Section 2 Learning Targets 8. 2. 4 – I can distinguish between a covalent bond a coordinate covalent bond and describe how the strength of a covalent bond is related to its bond dissociation energy. 8. 2. 5 – I can describe how oxygen atoms are bonded in ozone.
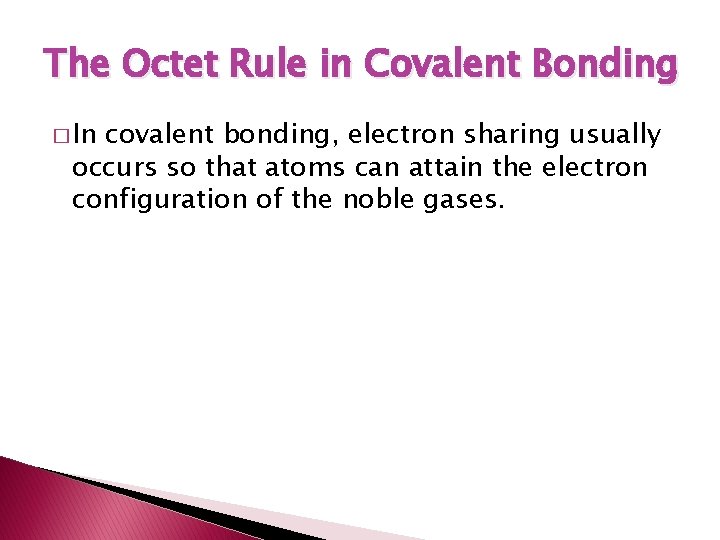
The Octet Rule in Covalent Bonding � In covalent bonding, electron sharing usually occurs so that atoms can attain the electron configuration of the noble gases.
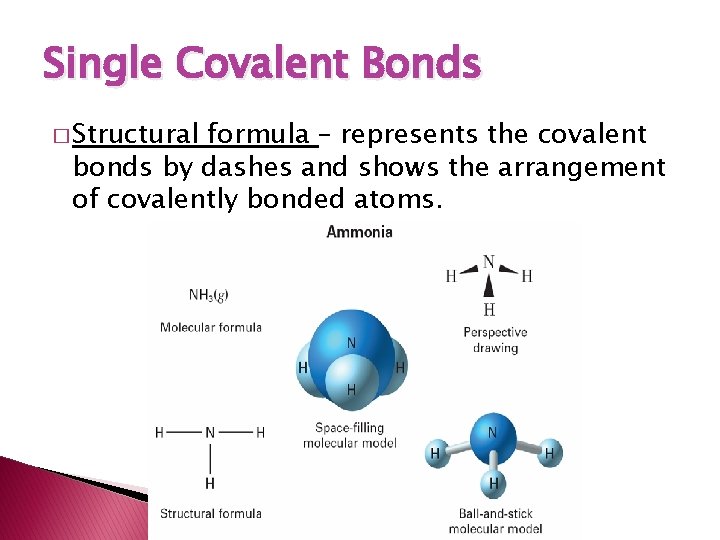
Single Covalent Bonds � Structural formula – represents the covalent bonds by dashes and shows the arrangement of covalently bonded atoms.
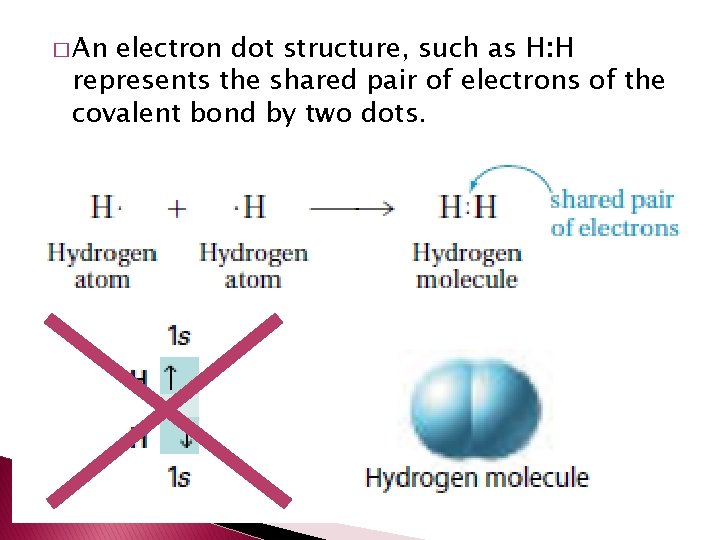
� An electron dot structure, such as H: H represents the shared pair of electrons of the covalent bond by two dots.
� Single covalent bond – two atoms held together by sharing a pair of electrons. � Unshared pair – (lone pair) or nonbonding pair of electrons.
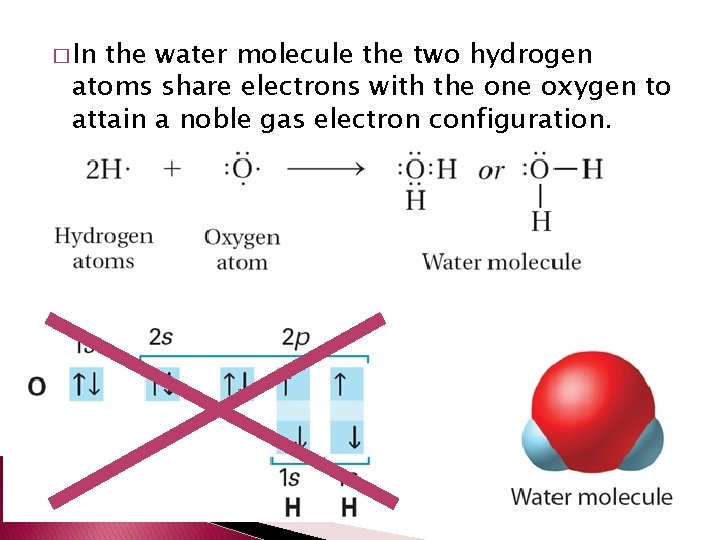
� In the water molecule the two hydrogen atoms share electrons with the one oxygen to attain a noble gas electron configuration.
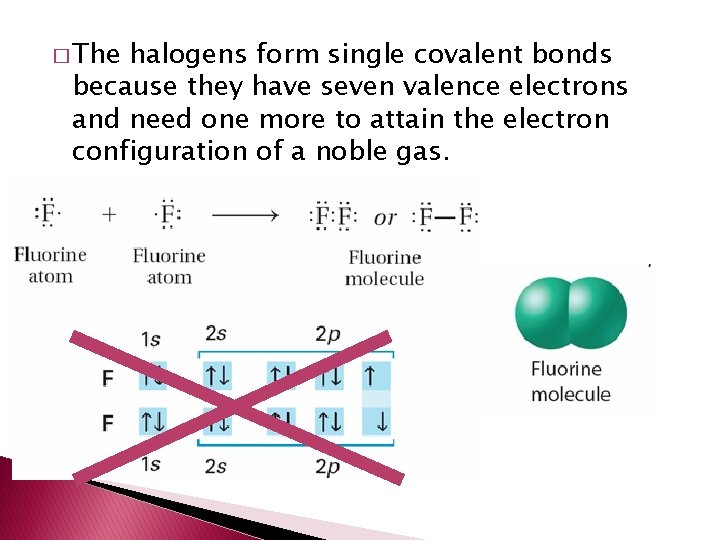
� The halogens form single covalent bonds because they have seven valence electrons and need one more to attain the electron configuration of a noble gas.
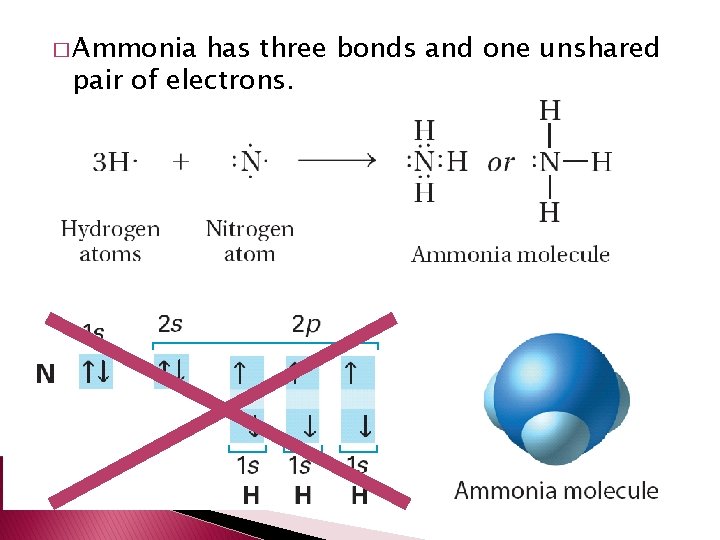
� Ammonia has three bonds and one unshared pair of electrons.
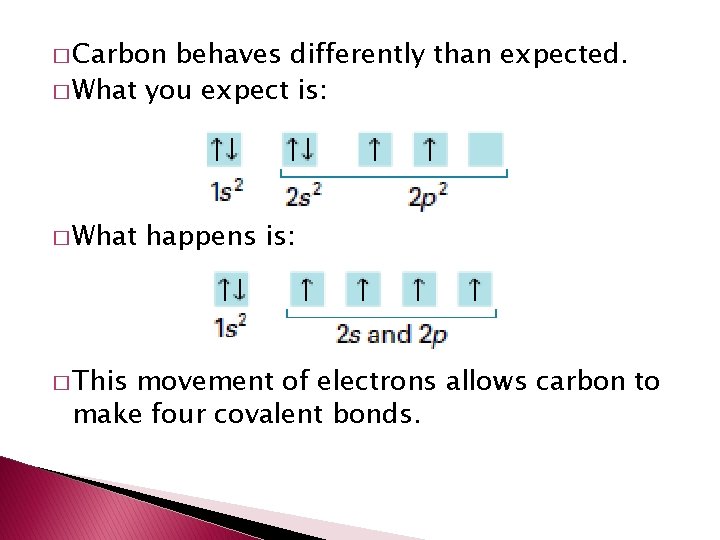
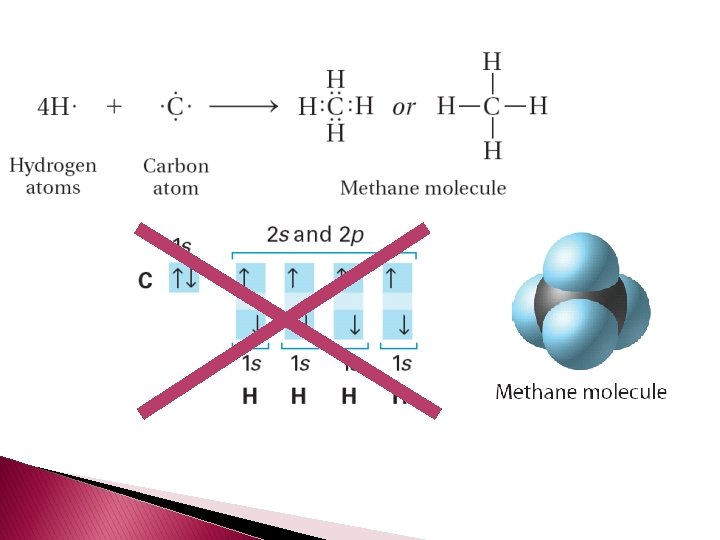
� Carbon behaves differently than expected. � What you expect is: � What � This happens is: movement of electrons allows carbon to make four covalent bonds.
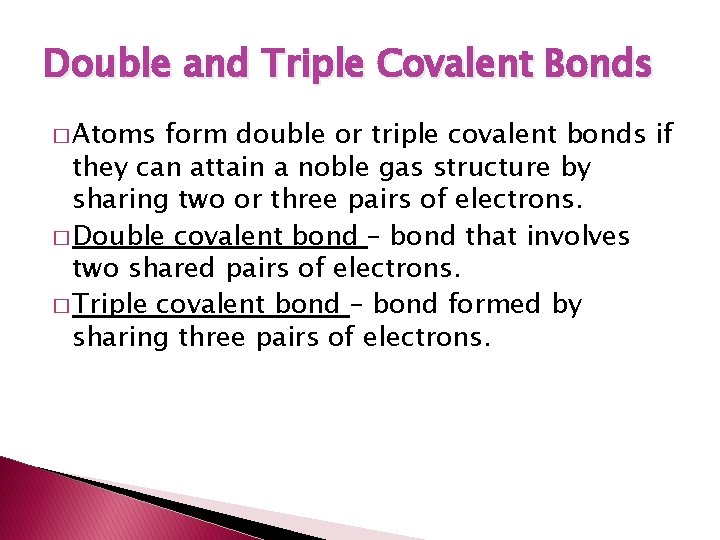
Double and Triple Covalent Bonds � Atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two or three pairs of electrons. � Double covalent bond – bond that involves two shared pairs of electrons. � Triple covalent bond – bond formed by sharing three pairs of electrons.
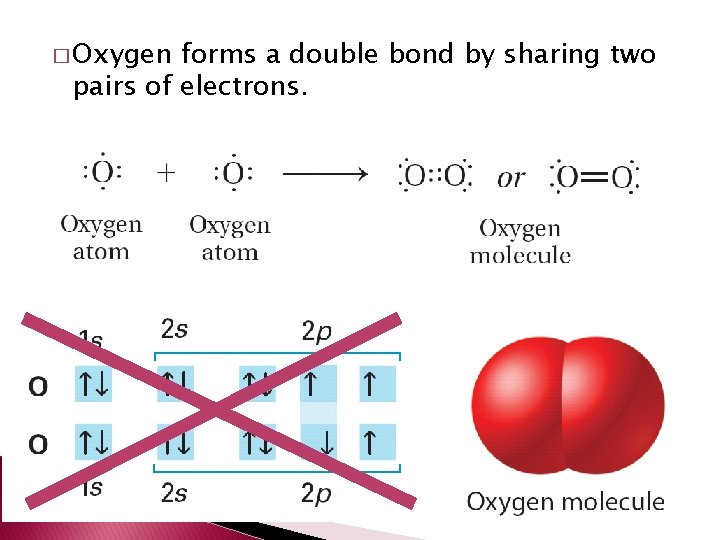
� Oxygen forms a double bond by sharing two pairs of electrons.
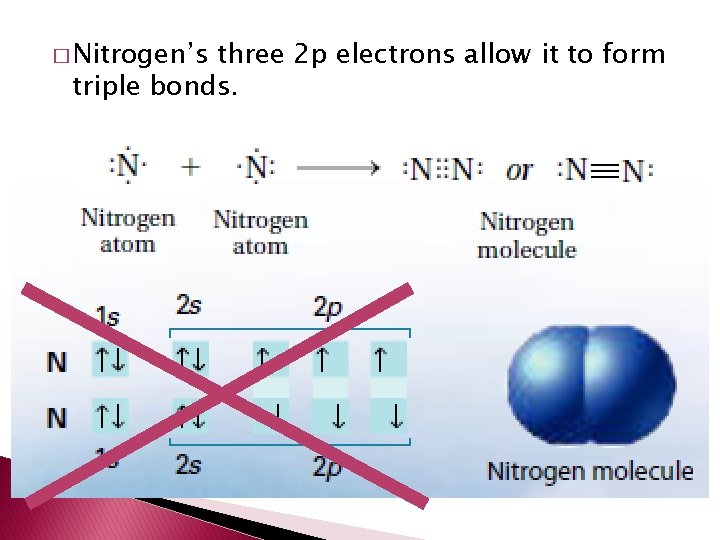
� Nitrogen’s three 2 p electrons allow it to form triple bonds.
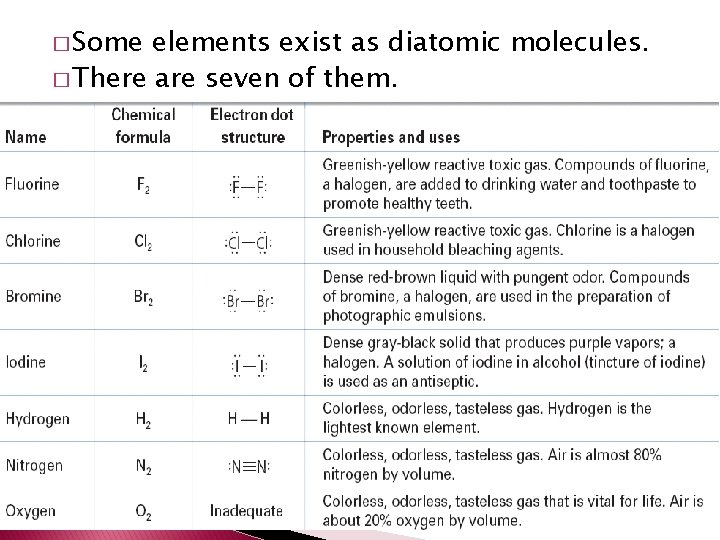
� Some elements exist as diatomic molecules. � There are seven of them.
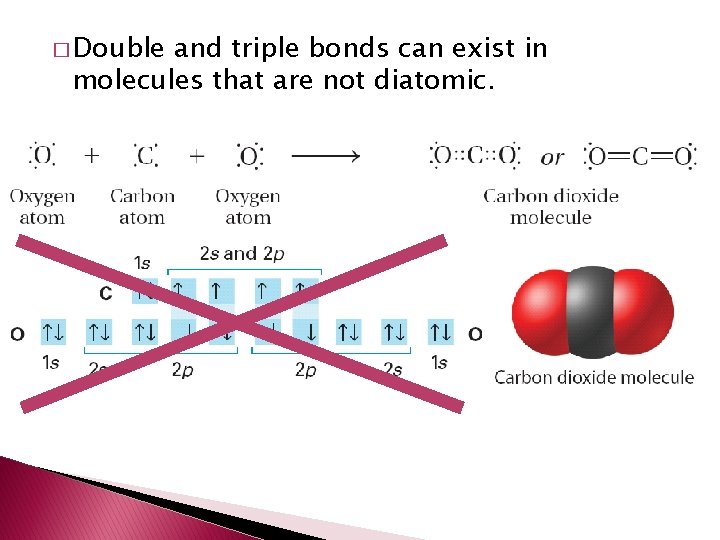
� Double and triple bonds can exist in molecules that are not diatomic.

Section 2 The Nature of Covalent Bonding Part 2
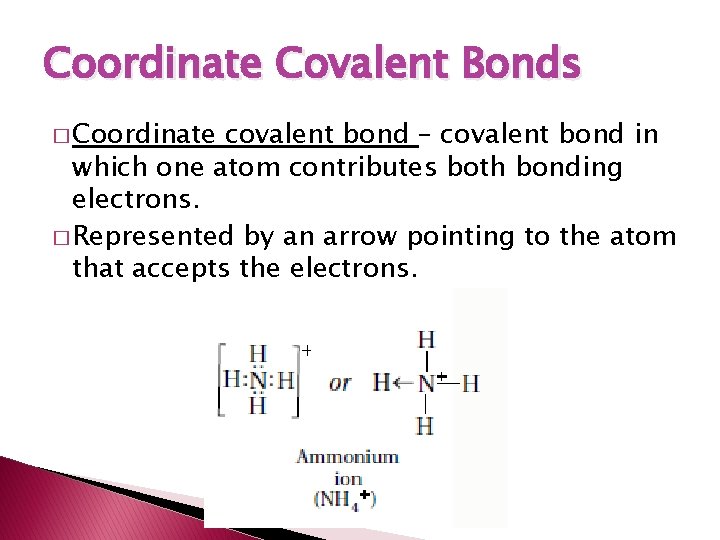
Coordinate Covalent Bonds � Coordinate covalent bond – covalent bond in which one atom contributes both bonding electrons. � Represented by an arrow pointing to the atom that accepts the electrons.
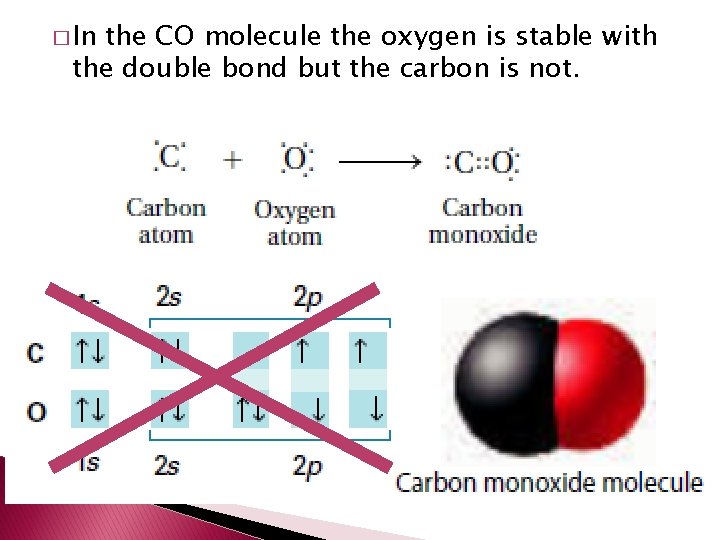
� In the CO molecule the oxygen is stable with the double bond but the carbon is not.
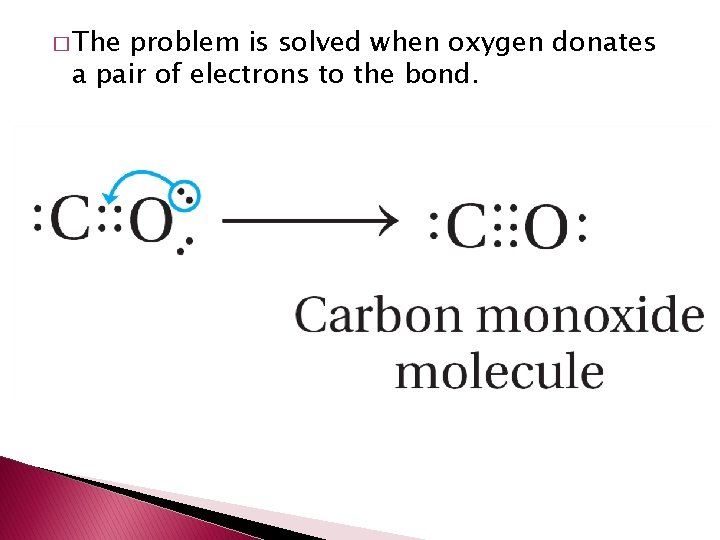
� The problem is solved when oxygen donates a pair of electrons to the bond.
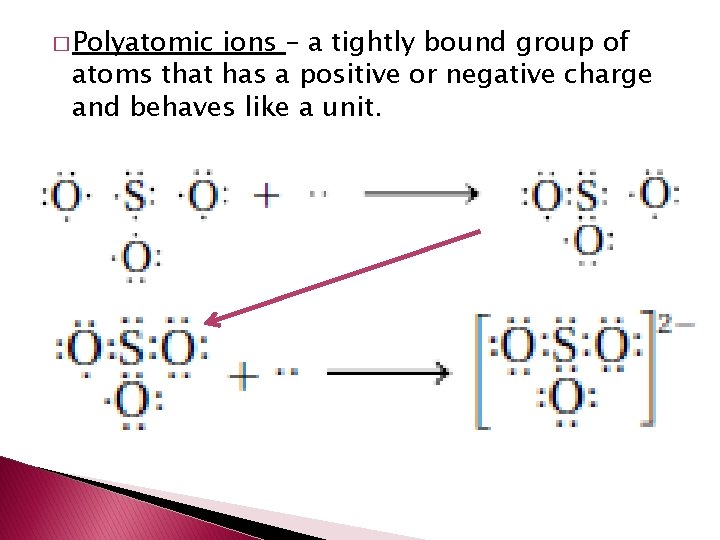
� Polyatomic ions – a tightly bound group of atoms that has a positive or negative charge and behaves like a unit.
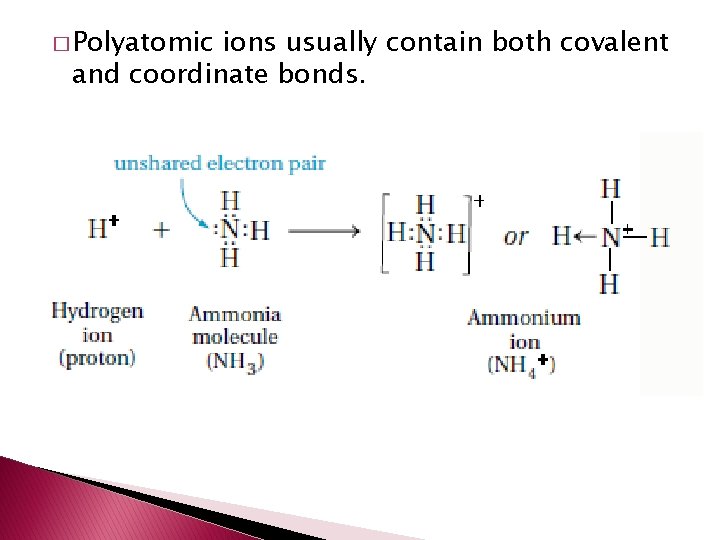
� Polyatomic ions usually contain both covalent and coordinate bonds.
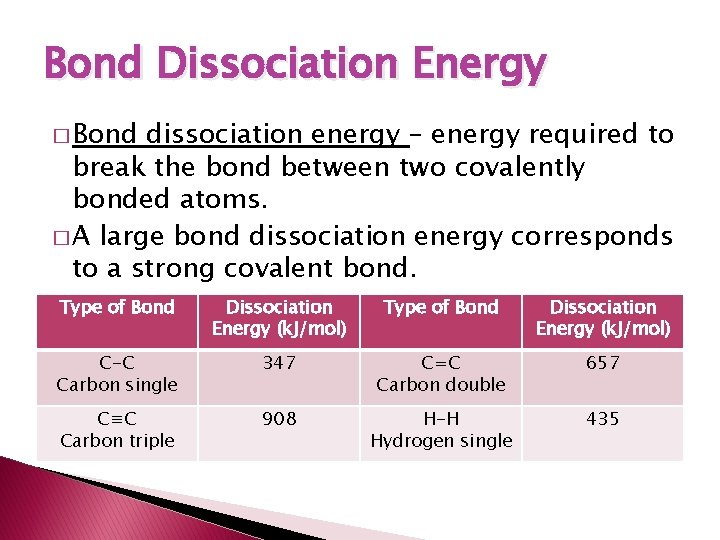
Bond Dissociation Energy � Bond dissociation energy – energy required to break the bond between two covalently bonded atoms. � A large bond dissociation energy corresponds to a strong covalent bond. Type of Bond Dissociation Energy (k. J/mol) C-C Carbon single 347 C=C Carbon double 657 C≡C Carbon triple 908 H-H Hydrogen single 435
Resonance � Resonance structures – structure that occurs when it is possible to draw two or more valid electron dot structures. � Usually seen with double bonds – where they could shift around.
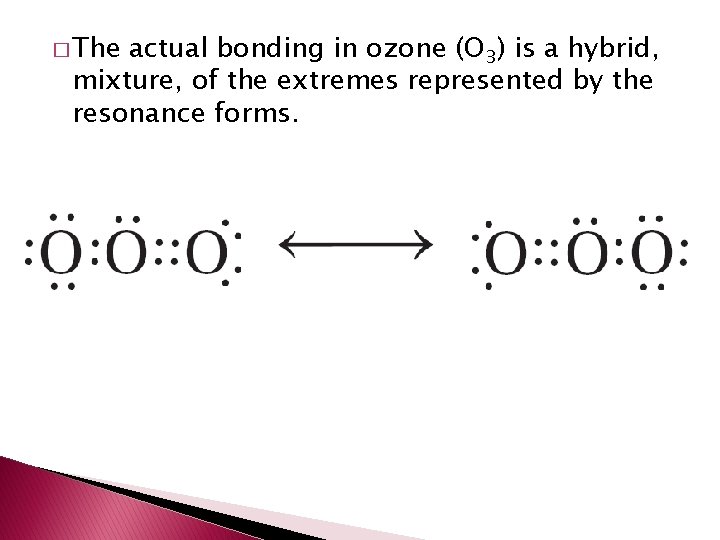
� The actual bonding in ozone (O 3) is a hybrid, mixture, of the extremes represented by the resonance forms.
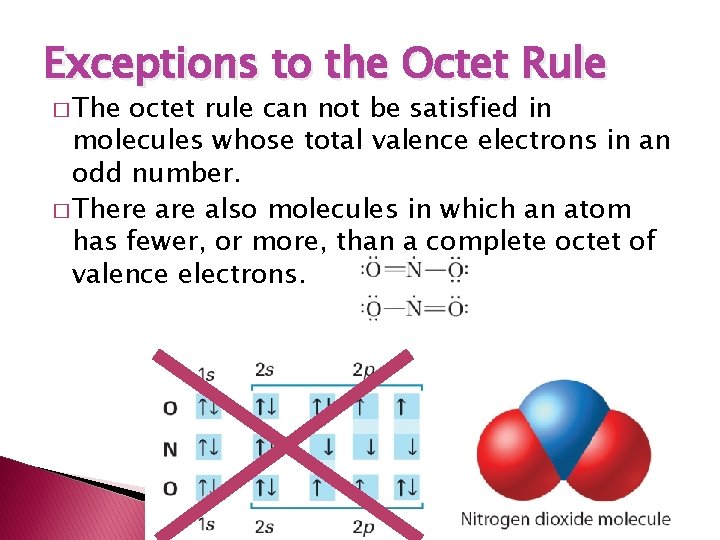
Exceptions to the Octet Rule � The octet rule can not be satisfied in molecules whose total valence electrons in an odd number. � There also molecules in which an atom has fewer, or more, than a complete octet of valence electrons.
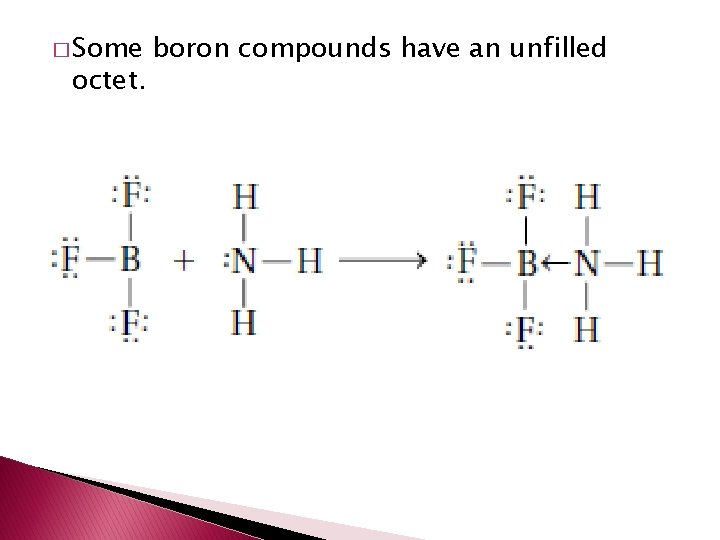
� Some octet. boron compounds have an unfilled
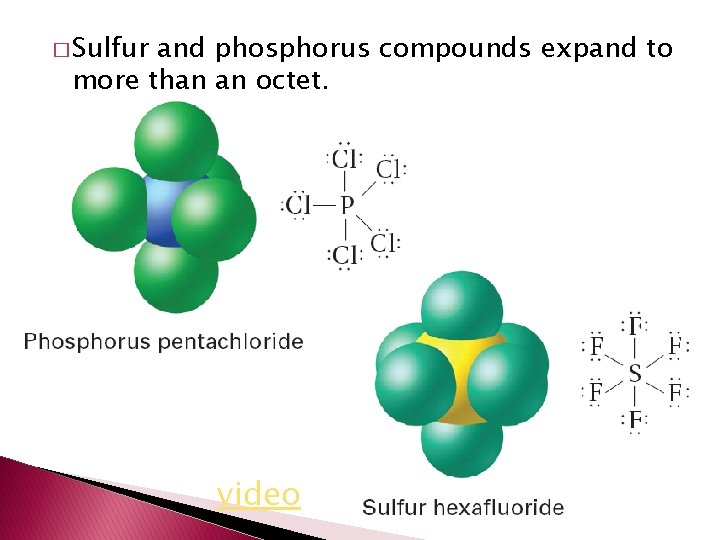
� Sulfur and phosphorus compounds expand to more than an octet. video

Section 3 Bonding Theories
Section 3 Learning Targets 8. 3. 2 – I can describe how VSEPR theory helps predict the shapes of molecules.
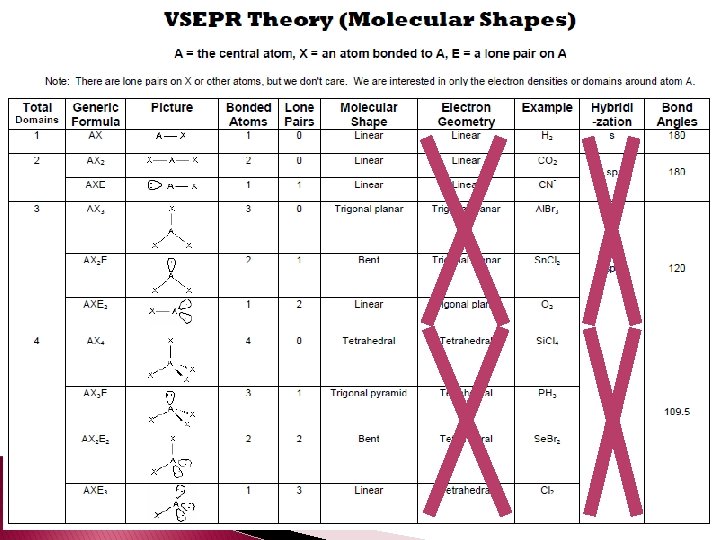
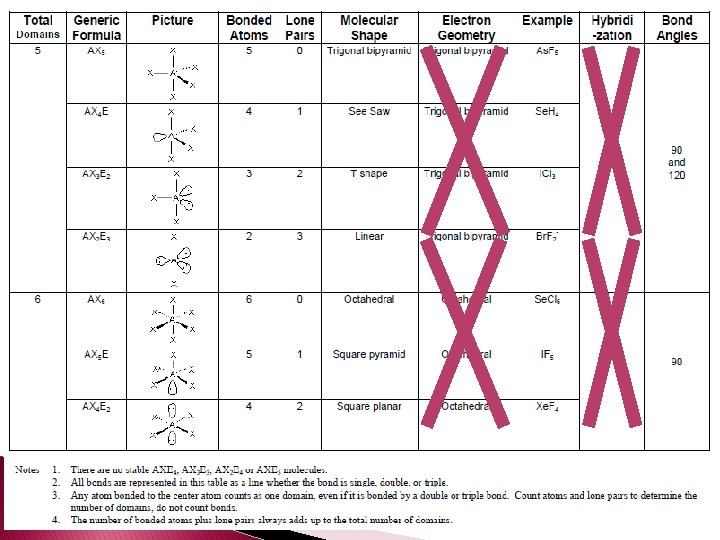
VSEPR Theory � VSEPR (Valence Shell Electron Pair Repulsion Theory � VSEPR video � According to VSEPR the repulsion between electron pairs causes molecular shapes to adjust so that the valence-electron pairs stay as far apart as possible.
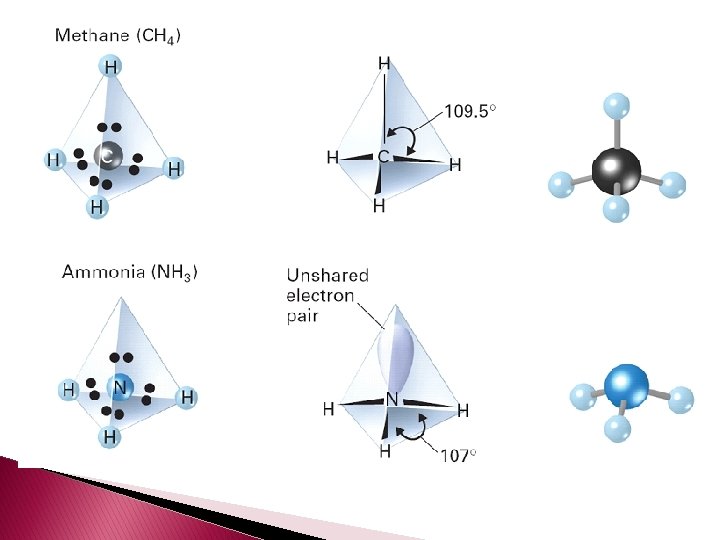
� Unshared pairs are just as important as bonds because they help determine the shapes of the molecules. � Unshared pairs of electrons are held closer to the nucleus and push bonded atoms out of their way. � Tetrahedral angle – a bond angle of 109. 5° that results when a central atom forms four bonds.
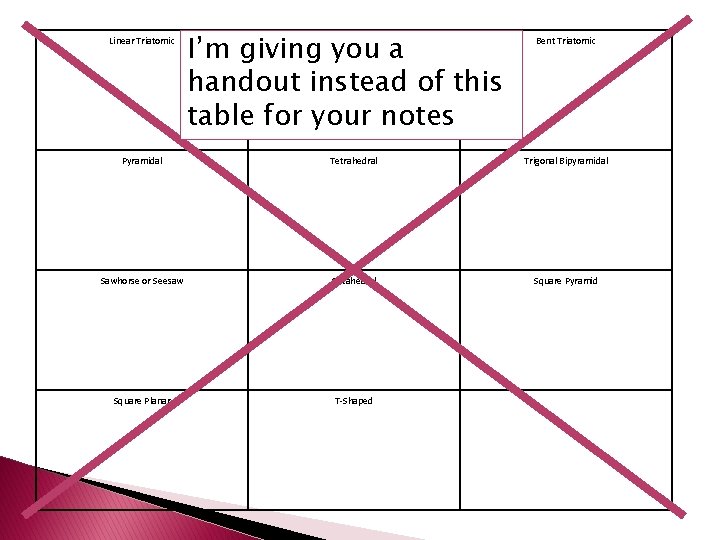
Linear Triatomic I’m giving you a handout instead of this table for your notes Trigonal Planar Bent Triatomic Pyramidal Tetrahedral Trigonal Bipyramidal Sawhorse or Seesaw Octahedral Square Pyramid Square Planar T-Shaped

Let’s Practice � On the back of your yellow paper answer the following: How many bonds do each of these make? Carbon Nitrogen Oxygen Fluorine Phosphorus Sulfur Chlorine Bromine Iodine Hydrogen Group 5 Group 6 Group 7
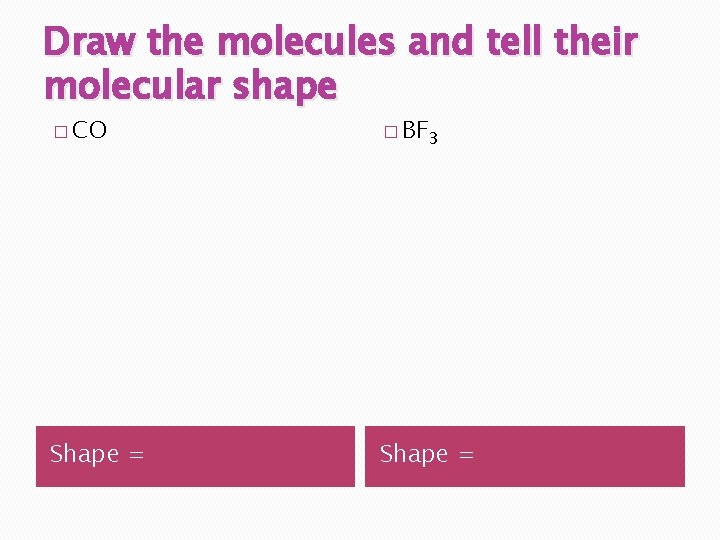
Draw the molecules and tell their molecular shape � CO � BF 3 Shape =
Draw the molecules and tell their molecular shape � SO 2 � CH 4 Shape =
Draw the molecules and tell their molecular shape � NH 3 � H 2 O Shape =
Draw the molecules and tell their molecular shape � PF 5 � SF 4 Shape =
Draw the molecules and tell their molecular shape � Br. F 3 � Xe. F 2 Shape =
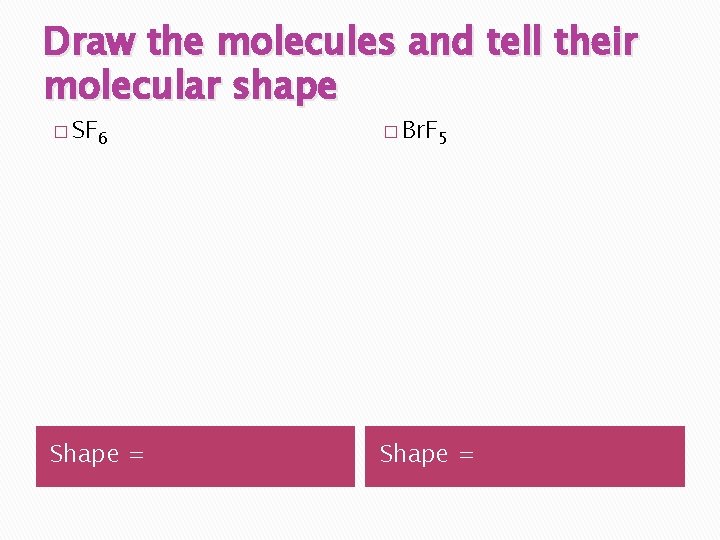
Draw the molecules and tell their molecular shape � SF 6 � Br. F 5 Shape =
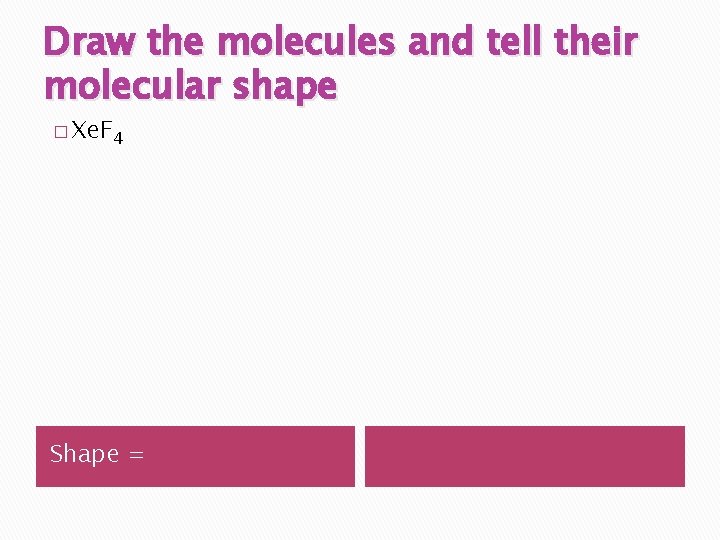
Draw the molecules and tell their molecular shape � Xe. F 4 Shape =

� VSEPR Video Page 233 in your text book may help with the matching on your daily work if you’re struggling.
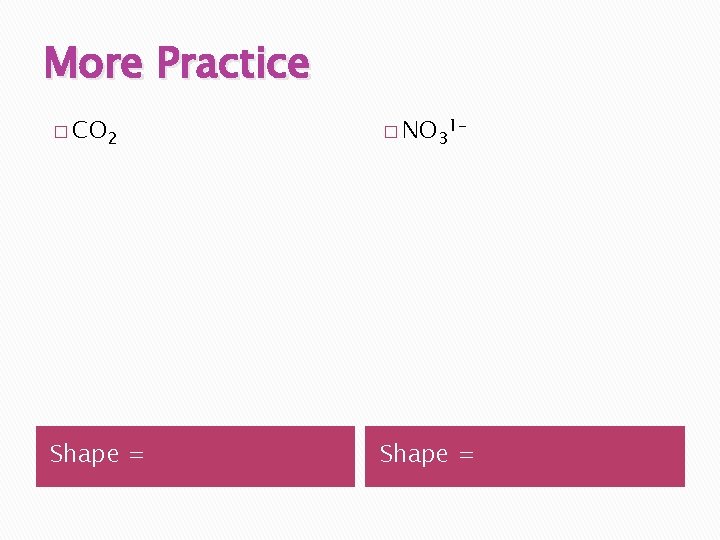
More Practice � CO 2 � NO 31 - Shape =
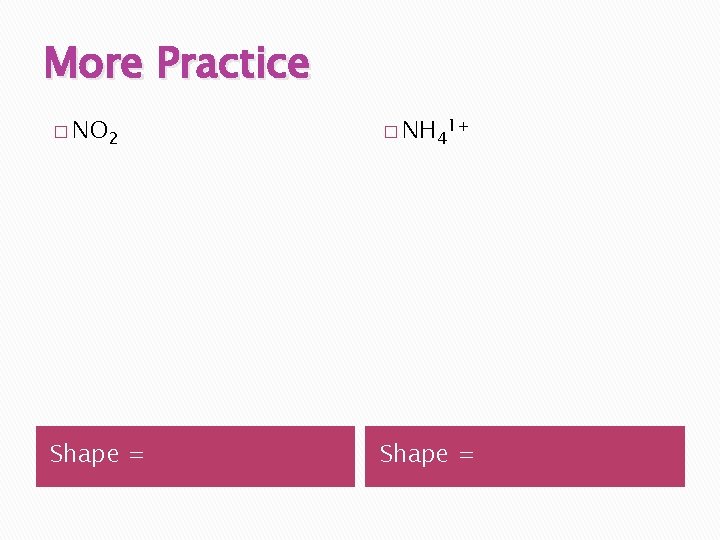
More Practice � NO 2 � NH 41+ Shape =

More Practice � ICl 21 - � ICl 41+ Shape =

Section 4 Polar Bonds and Molecules

Section 4 Learning Targets 8. 4. 1 – I can describe how electronegativity values determine the distribution of charge in a polar molecule. 8. 4. 2 – I can describe what happens to polar molecules when they are placed between oppositely charged metal plates.

Section 4 Learning Targets 8. 4. 3 – I can evaluate the strength of intermolecular attractions compared with the strength of ionic and covalent bonds. 8. 4. 4 – I can identify the reason why network solids have high melting points.
Bond Polarity � Electrons are not always shared equally in compounds. � Nonpolar covalent bond – when atoms in the bond pull equally the bonding electrons are shared equally.
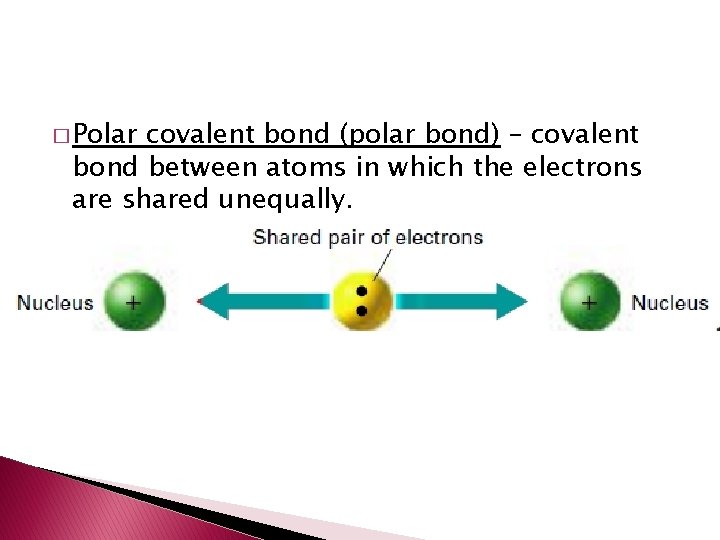
� Polar covalent bond (polar bond) – covalent bond between atoms in which the electrons are shared unequally.
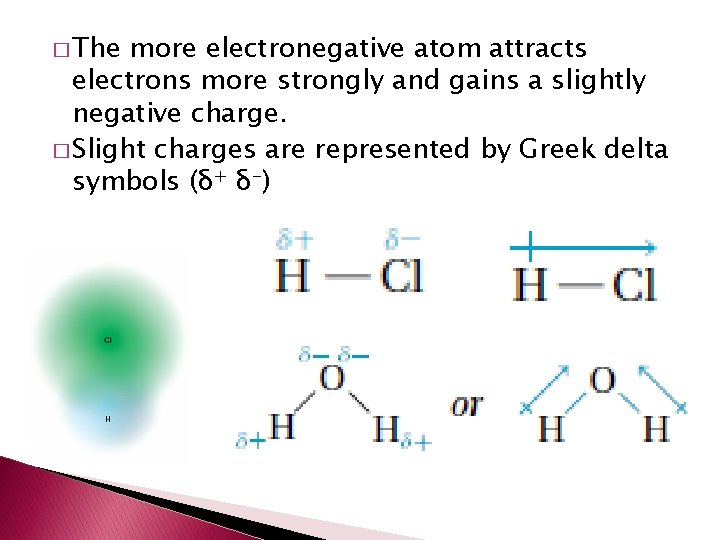
� The more electronegative atom attracts electrons more strongly and gains a slightly negative charge. � Slight charges are represented by Greek delta symbols (δ+ δ-)
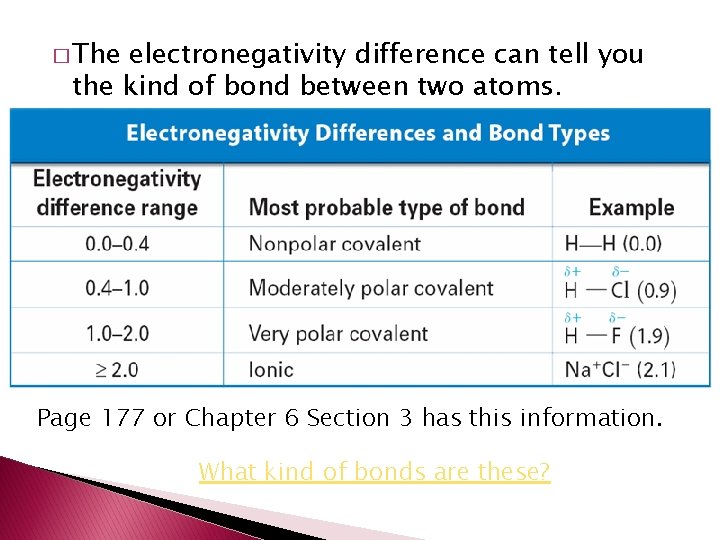
� The electronegativity difference can tell you the kind of bond between two atoms. Page 177 or Chapter 6 Section 3 has this information. What kind of bonds are these?
Polar Molecules � Polar molecule – one end of the molecule is slightly negative and the other end is slightly positive. � Dipole – a molecule that has two poles.
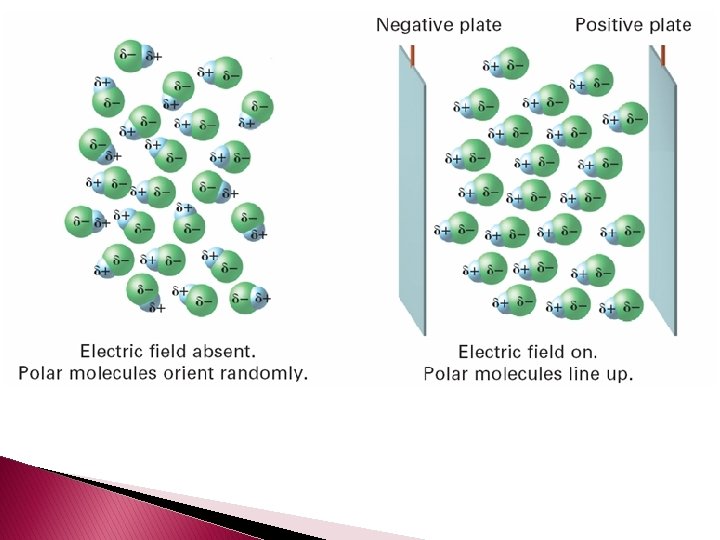
� When polar molecules are placed between oppositely charged plates, they tend to become oriented with respect to the positive and negative plates.
Attractions Between Molecules � Intermolecular attractions are weaker than either ionic or covalent bonds.

Van der Waals Forces � Van der Waals forces – the two weakest attractions between molecules. � After Dutch chemist Johannes van der Waals (1837 – 1923). � Two kinds of van der Waals forces: ◦ Dipole interactions ◦ Dispersions forces
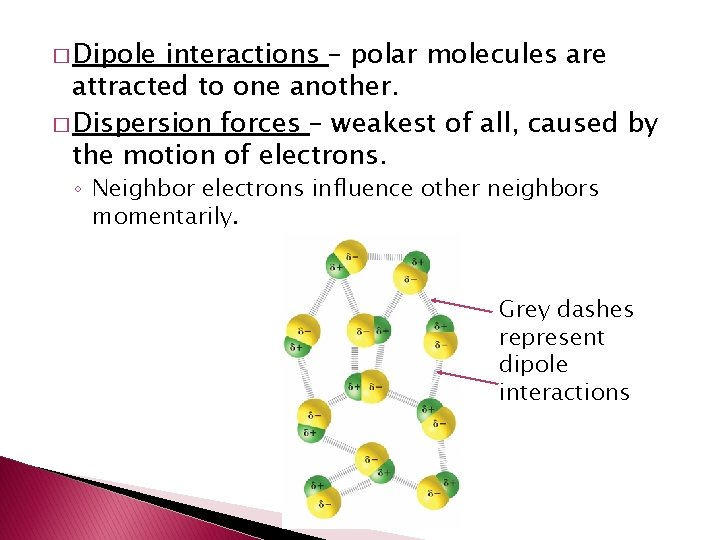
� Dipole interactions – polar molecules are attracted to one another. � Dispersion forces – weakest of all, caused by the motion of electrons. ◦ Neighbor electrons influence other neighbors momentarily. Grey dashes represent dipole interactions
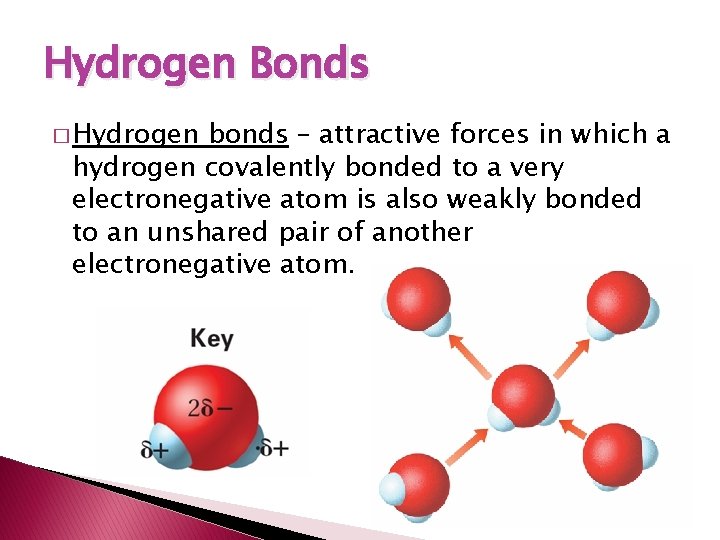
Hydrogen Bonds � Hydrogen bonds – attractive forces in which a hydrogen covalently bonded to a very electronegative atom is also weakly bonded to an unshared pair of another electronegative atom.
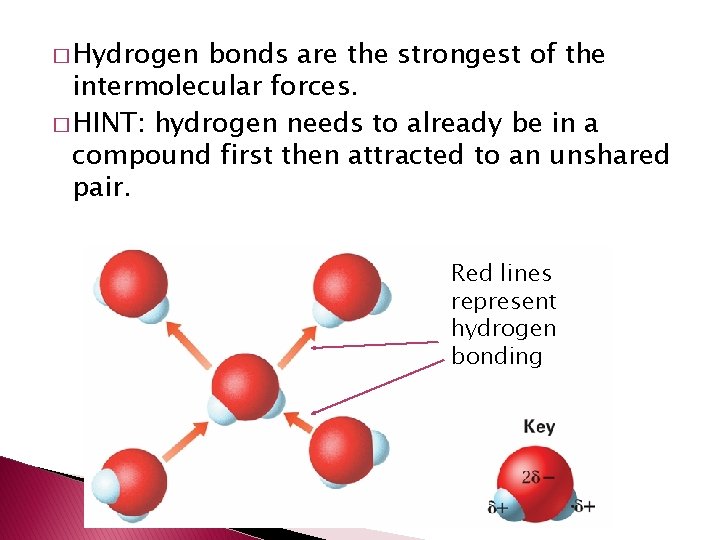
� Hydrogen bonds are the strongest of the intermolecular forces. � HINT: hydrogen needs to already be in a compound first then attracted to an unshared pair. Red lines represent hydrogen bonding
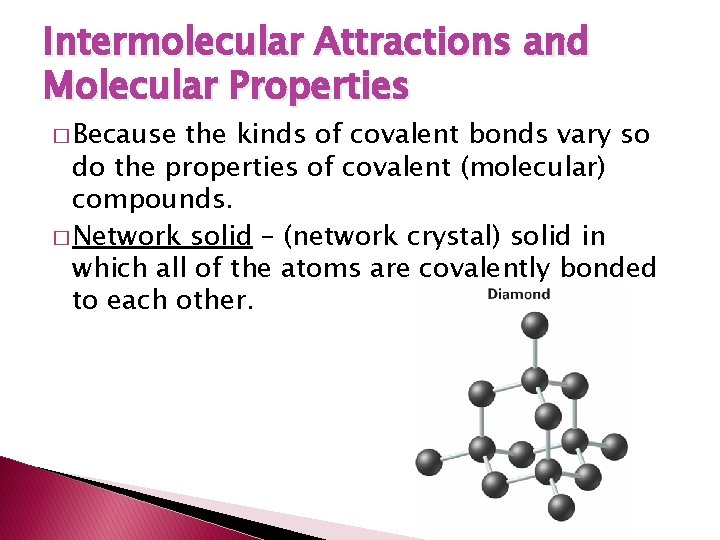
Intermolecular Attractions and Molecular Properties � Because the kinds of covalent bonds vary so do the properties of covalent (molecular) compounds. � Network solid – (network crystal) solid in which all of the atoms are covalently bonded to each other.
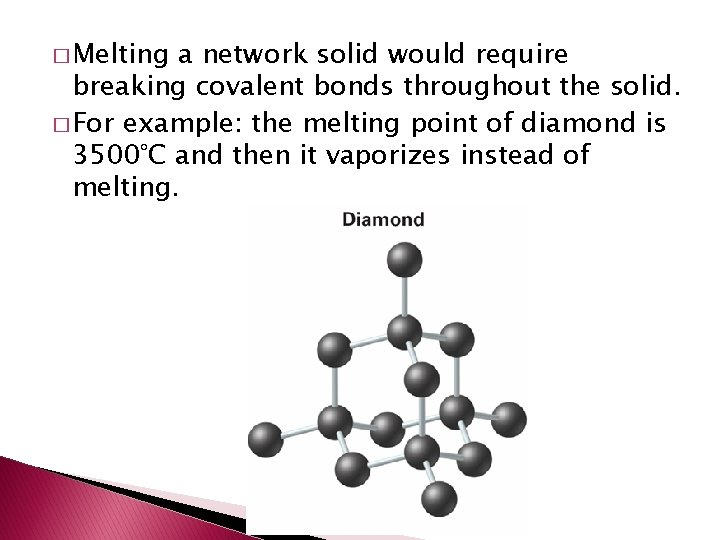
� Melting a network solid would require breaking covalent bonds throughout the solid. � For example: the melting point of diamond is 3500°C and then it vaporizes instead of melting.
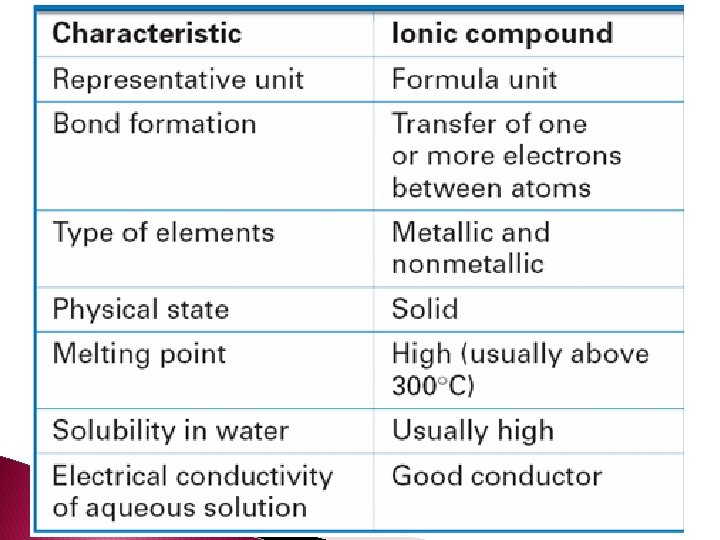
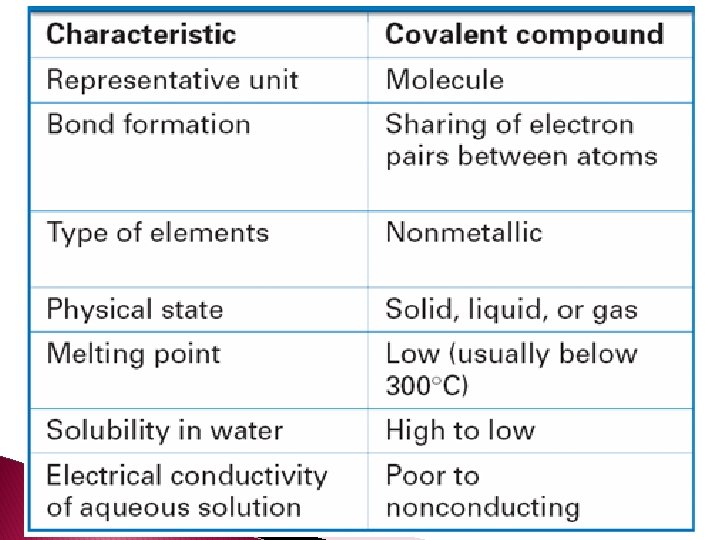
- Slides: 78