Chapter 8 Covalent Bonding Resources Chapter 8 of

Chapter 8: Covalent Bonding Resources: Chapter 8 of our TB and unit 6 of workbook Ch. 8 reading and study guide (in part) Powerpoint used in class Lab practical: Molecular models Handouts: practice problems and naming of covalent/molecular compounds Animations and simulation Simulations and animations: Ionic and covalent bonding animation: http: //www. youtube. com/watch? v=Qqj c. Cvz. Wwww Animation and song: what kinds of bonds are these: http: //www. youtube. com/watch? v=o. N Bzy. M 6 Tc. K 8 Mark Rosengarten video tutorials on polarity of molecules: http: //www. youtube. com/watch? v=mt Rge. BSe 1 o 8&feature=related glencoe animations and interactive lesson: http: //glencoe. mcgrawhill. com/sites/0078807239/student_vie w 0/chapter 9/concepts_in_motion. html # Animation: Ionic compounds versus nonpolar and polar molecules Properties of ionic and covalent compounds:

8. 1 Molecular Compounds > Molecules and Molecular Compounds In nature, matter takes many forms. The noble gases, including helium and neon, are monatomic. That means they exist as single atoms. • Represented by symbols: He, Ne, etc) • the noble gases are not molecules Slide 2 of 18 © Copyright Pearson Prentice Hall End Show
8. 1 Molecular Compounds > Molecules and Molecular Compounds A molecule is a neutral group of atoms joined together by covalent bonds. Air contains oxygen molecules. A diatomic molecule is a molecule consisting of two atoms. An oxygen molecule is a diatomic molecule. Slide 3 of 18 © Copyright Pearson Prentice Hall End Show
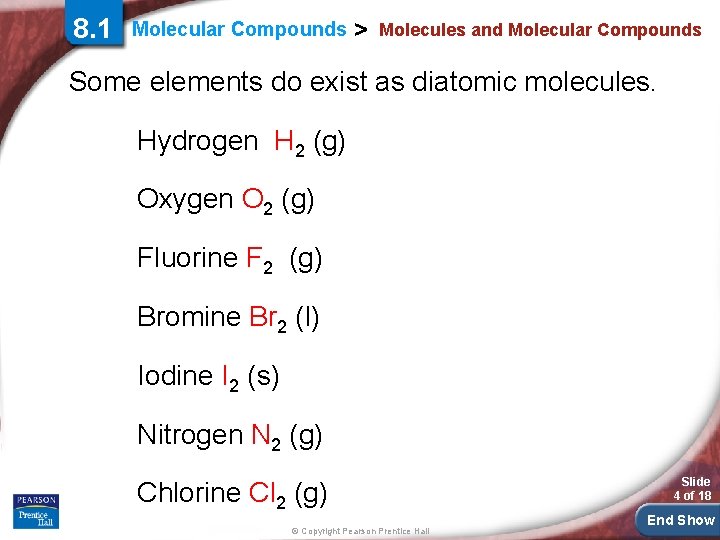
8. 1 Molecular Compounds > Molecules and Molecular Compounds Some elements do exist as diatomic molecules. Hydrogen H 2 (g) Oxygen O 2 (g) Fluorine F 2 (g) Bromine Br 2 (l) Iodine I 2 (s) Nitrogen N 2 (g) Chlorine Cl 2 (g) © Copyright Pearson Prentice Hall Slide 4 of 18 End Show
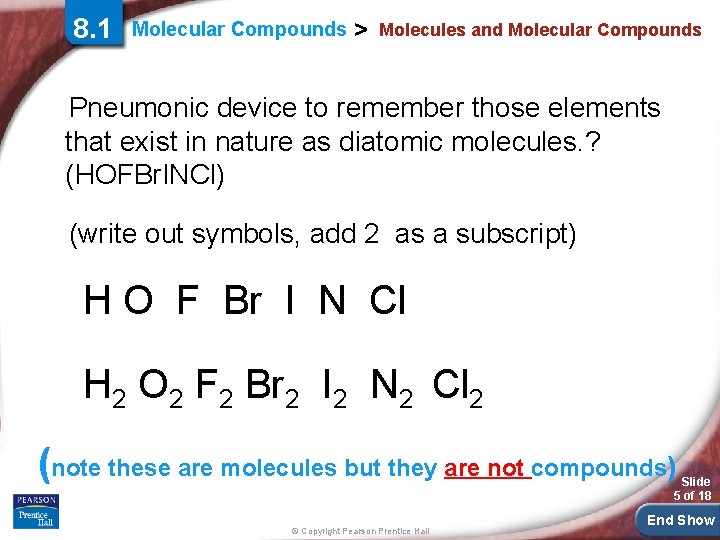
8. 1 Molecular Compounds > Molecules and Molecular Compounds Pneumonic device to remember those elements that exist in nature as diatomic molecules. ? (HOFBr. INCl) (write out symbols, add 2 as a subscript) H O F Br I N Cl H 2 O 2 F 2 Br 2 I 2 N 2 Cl 2 (note these are molecules but they are not compounds) Slide 5 of 18 © Copyright Pearson Prentice Hall End Show

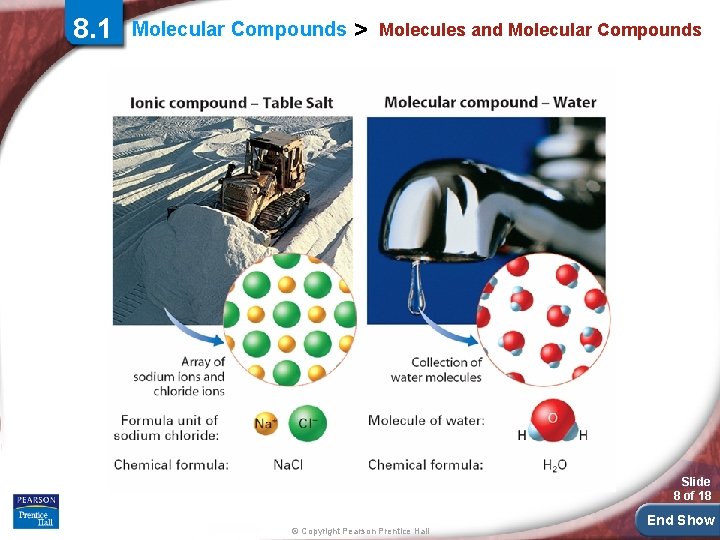
8. 1 Molecular Compounds > Molecules and Molecular Compounds A compound composed of molecules is called a molecular compound. Water and carbon monoxide are molecular compounds. Slide 6 of 18 © Copyright Pearson Prentice Hall End Show

8. 1 Molecular Compounds > Molecular Formulas of Some Molecular Compounds Slide 7 of 18 © Copyright Pearson Prentice Hall End Show
8. 1 Molecular Compounds > Molecules and Molecular Compounds Slide 8 of 18 © Copyright Pearson Prentice Hall End Show
8. 1 Molecular Compounds > Molecular Formulas A molecular formula is the chemical formula of a molecular compound. A molecular formula shows how many atoms of each element a molecule contains. Slide 9 of 18 © Copyright Pearson Prentice Hall End Show
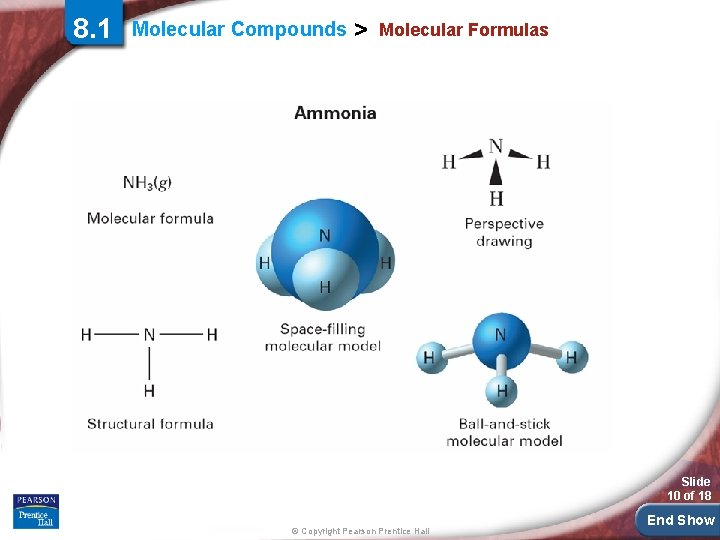
8. 1 Molecular Compounds > Molecular Formulas Slide 10 of 18 © Copyright Pearson Prentice Hall End Show
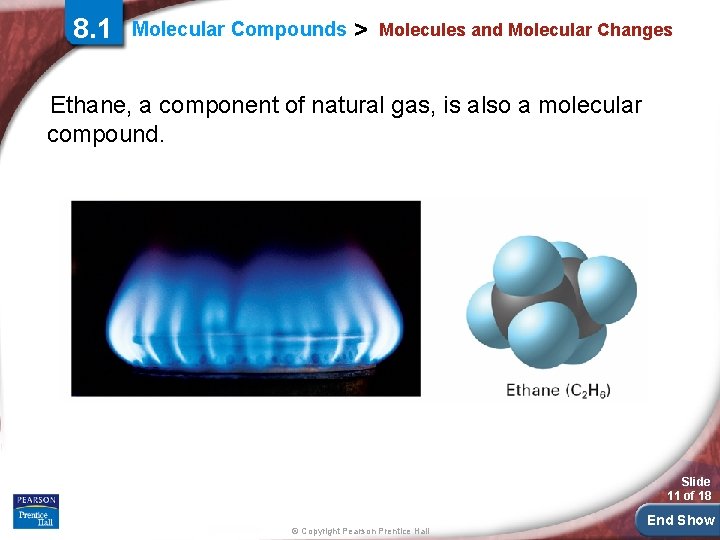
8. 1 Molecular Compounds > Molecules and Molecular Changes Ethane, a component of natural gas, is also a molecular compound. Slide 11 of 18 © Copyright Pearson Prentice Hall End Show
Chapter 8: Covalent Bonding Types of Bonding ? (review)
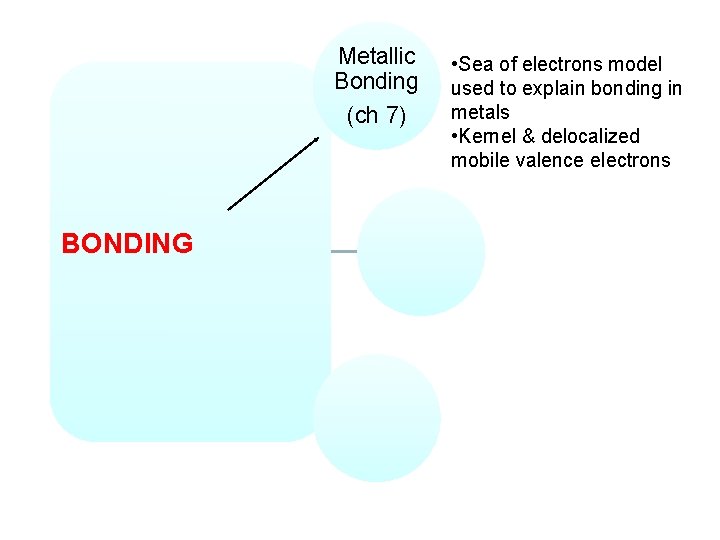
Metallic Bonding (ch 7) BONDING • Sea of electrons model used to explain bonding in metals • Kernel & delocalized mobile valence electrons
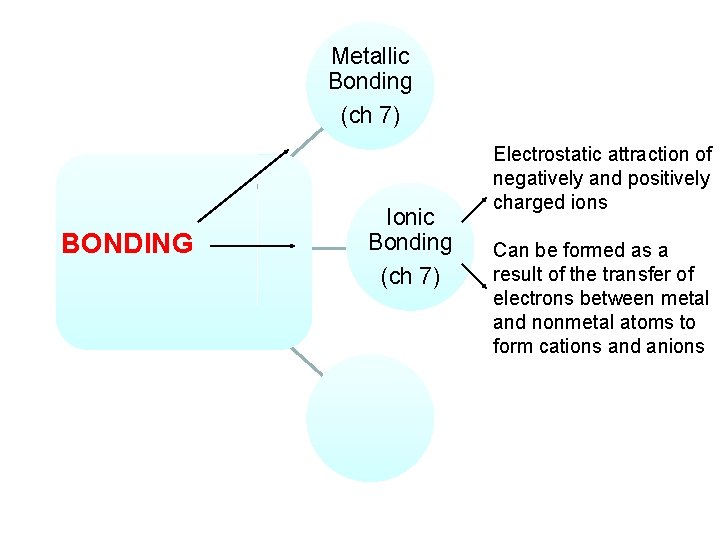
Metallic Bonding (ch 7) BONDING Ionic Bonding (ch 7) Electrostatic attraction of negatively and positively charged ions Can be formed as a result of the transfer of electrons between metal and nonmetal atoms to form cations and anions
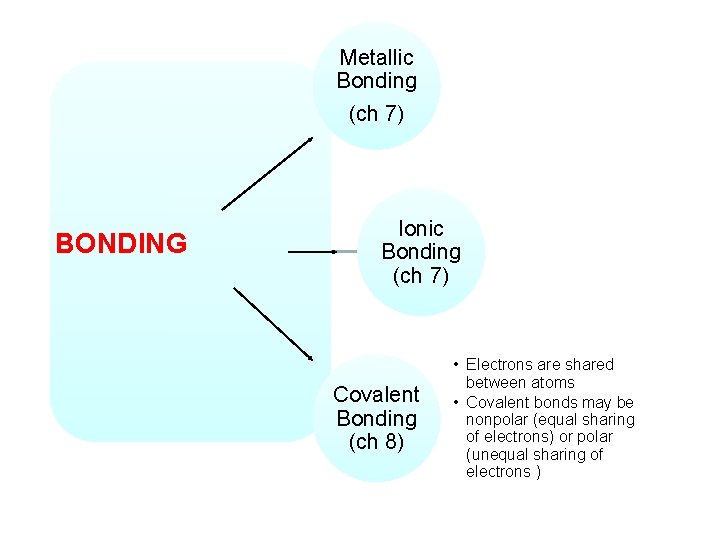
Metallic Bonding (ch 7) BONDING Ionic Bonding (ch 7) Covalent Bonding (ch 8) • Electrons are shared between atoms • Covalent bonds may be nonpolar (equal sharing of electrons) or polar (unequal sharing of electrons )
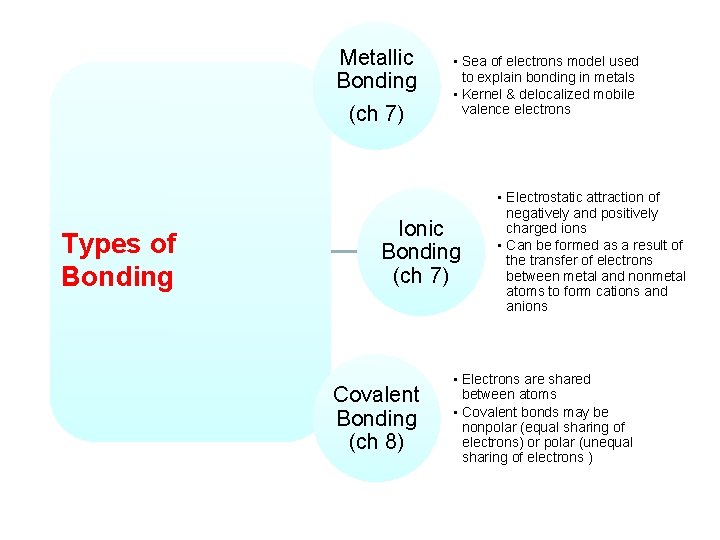
Metallic Bonding (ch 7) Types of Bonding • Sea of electrons model used to explain bonding in metals • Kernel & delocalized mobile valence electrons Ionic Bonding (ch 7) Covalent Bonding (ch 8) • Electrostatic attraction of negatively and positively charged ions • Can be formed as a result of the transfer of electrons between metal and nonmetal atoms to form cations and anions • Electrons are shared between atoms • Covalent bonds may be nonpolar (equal sharing of electrons) or polar (unequal sharing of electrons )
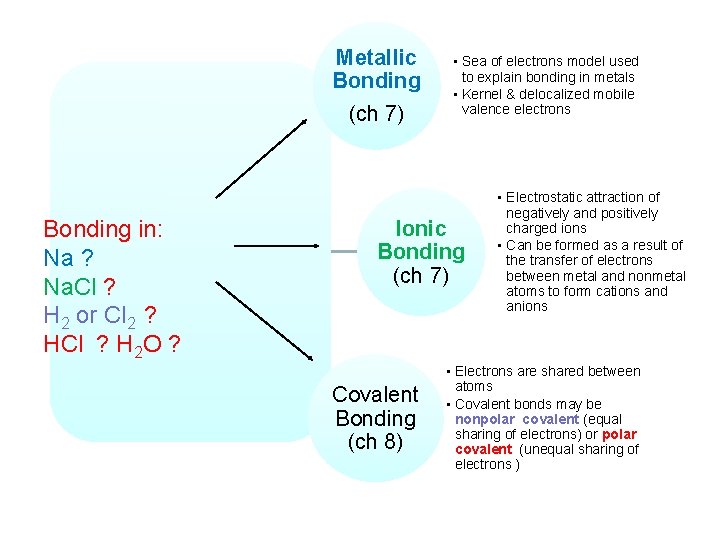
Metallic Bonding (ch 7) Bonding in: Na ? Na. Cl ? H 2 or Cl 2 ? HCl ? H 2 O ? • Sea of electrons model used to explain bonding in metals • Kernel & delocalized mobile valence electrons Ionic Bonding (ch 7) Covalent Bonding (ch 8) • Electrostatic attraction of negatively and positively charged ions • Can be formed as a result of the transfer of electrons between metal and nonmetal atoms to form cations and anions • Electrons are shared between atoms • Covalent bonds may be nonpolar covalent (equal sharing of electrons) or polar covalent (unequal sharing of electrons )
8. 1 Molecular Compounds > Molecules and Molecular Compounds How are the melting points and boiling points of molecular compounds different from those of ionic compounds? Slide 18 of 18 © Copyright Pearson Prentice Hall End Show
8. 1 Molecular Compounds > Molecules and Molecular Changes Molecular compounds tend to have relatively lower melting and boiling points than ionic compounds. Slide 19 of 18 © Copyright Pearson Prentice Hall End Show

Practice – properties of ionic and covalent compounds Go to interactive table on Glencoe site: Physical Properties of ionic and covalent compounds http: //glencoe. mcgrawhill. com/sites/0078807239/student_view 0/chapter 9/concepts_in_motion. html#
8. 1 Section Quiz. Assess students’ understanding of the concepts in Section 8. 1. Continue to: -or- Launch: Section Quiz Slide 21 of 18 © Copyright Pearson Prentice Hall End Show

8. 1 Section Quiz. 1. Compared to ionic compounds, molecular compounds tend to have relatively a. low melting points and high boiling points. b. low melting points and low boiling points. c. high melting points and high boiling points. d. high melting points and low boiling points. Slide 22 of 18 © Copyright Pearson Prentice Hall End Show

8. 1 Section Quiz 2. A molecular compound usually consists of a. two metal atoms and a nonmetal atom. b. two nonmetal atoms and a metal atom. c. two or more metal atoms. d. two or more nonmetal atoms. Slide 23 of 18 © Copyright Pearson Prentice Hall End Show

8. 1 Section Quiz 3. A molecular formula shows a. how many atoms of each element a molecule contains. b. a molecule's structure. c. which atoms are bonded together. d. how atoms are arranged in space. Slide 24 of 18 © Copyright Pearson Prentice Hall End Show
- Slides: 24