Chapter 8 COVALENT BONDING Molecular Compounds Are compounds
Chapter 8 COVALENT BONDING
Molecular Compounds… Are compounds composed of molecules Are also known as Covalent Compounds Are held together by covalent bonds Covalent Bond : atoms held together by the SHARING of electrons
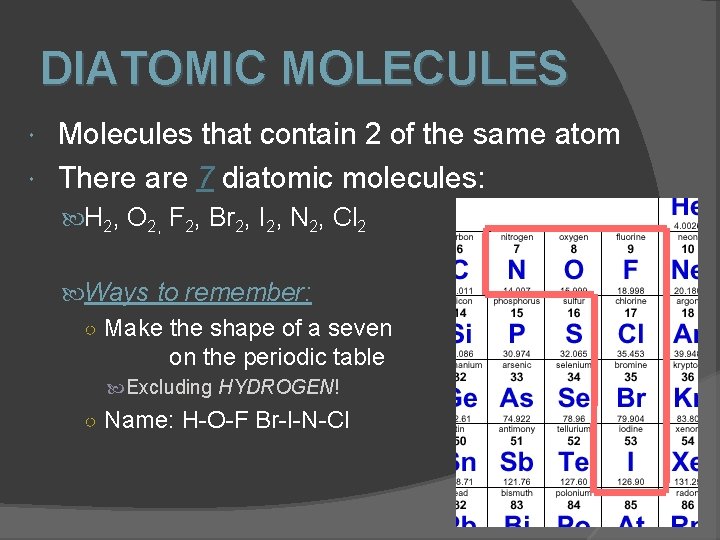
DIATOMIC MOLECULES Molecules that contain 2 of the same atom There are 7 diatomic molecules: H 2, O 2, F 2, Br 2, I 2, N 2, Cl 2 Ways to remember: ○ Make the shape of a seven on the periodic table Excluding HYDROGEN! ○ Name: H-O-F Br-I-N-Cl
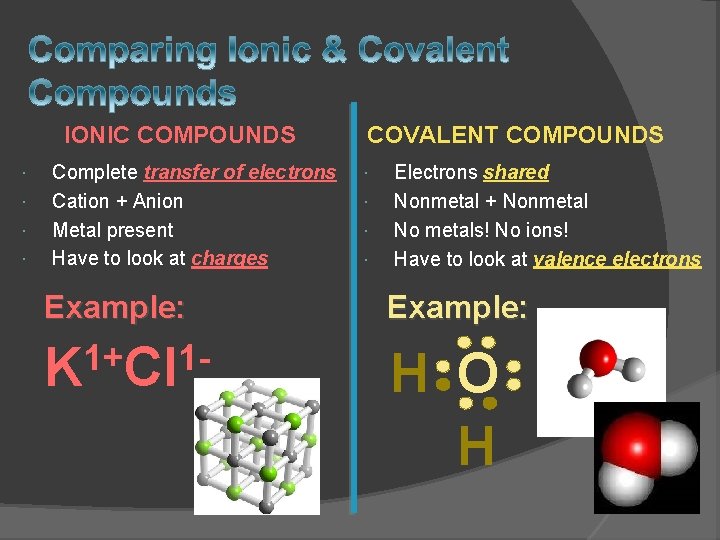
IONIC COMPOUNDS Complete transfer of electrons Cation + Anion Metal present Have to look at charges COVALENT COMPOUNDS Electrons shared Nonmetal + Nonmetal No metals! No ions! Have to look at valence electrons Example: 1+ 1 K Cl H O H

Properties of Covalent Bonds Electrons are shared so that the atoms can attain the electron configuration of noble gases. Weak inter-particle forces in contrast to ionic compounds. Many are liquids or gases at room temperature, but some are solids (i. e. sugar). Have low melting and boiling points compared to ionic compounds. Do not conduct electricity. Less soluble in water than ionic compounds, in general.
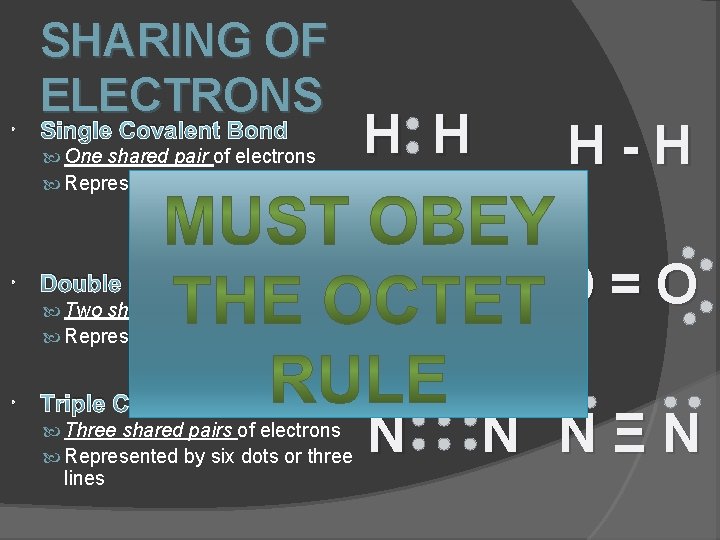
SHARING OF ELECTRONS Single Covalent Bond H H H-H O O O=O One shared pair of electrons Represented by two dots or one line Double Covalent Bond Two shared pairs of electrons Represented by four dots or two lines Triple Covalent Bond Three shared pairs of electrons Represented by six dots or three lines N N NΞN
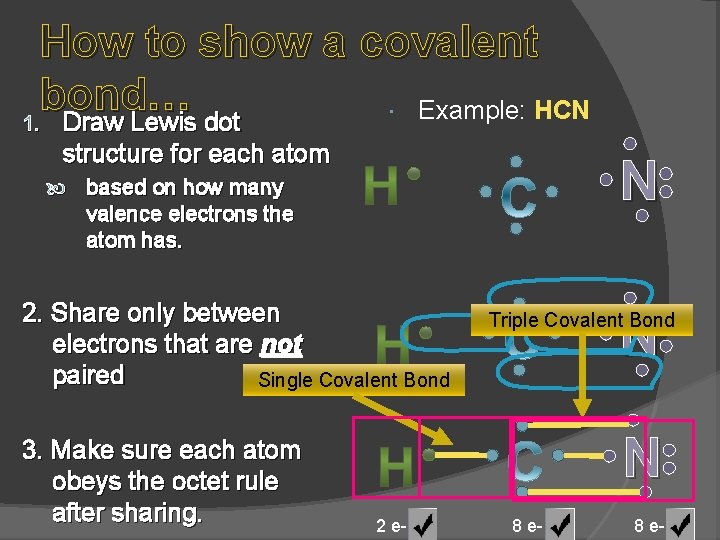
How to show a covalent bond… Example: HCN 1. Draw Lewis dot structure for each atom N based on how many valence electrons the atom has. 2. Share only between electrons that are not paired Single Covalent Bond 3. Make sure each atom obeys the octet rule after sharing. N Triple Covalent Bond N 2 e- 8 e-
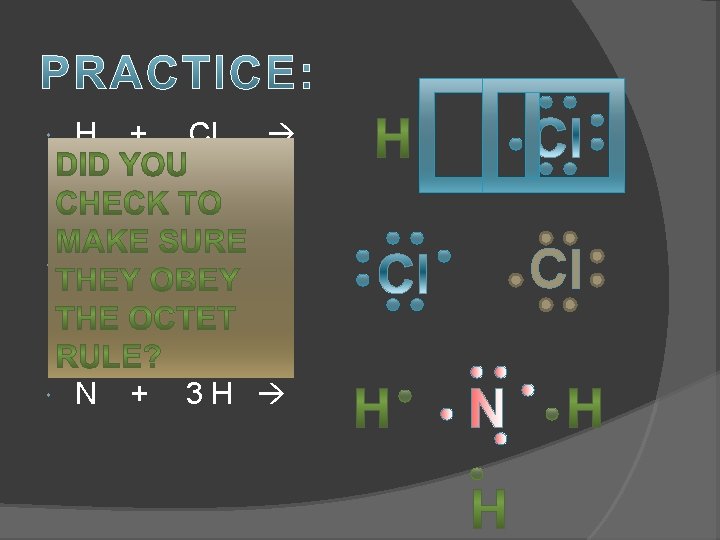
H + Cl Cl + Cl N 3 H + Cl
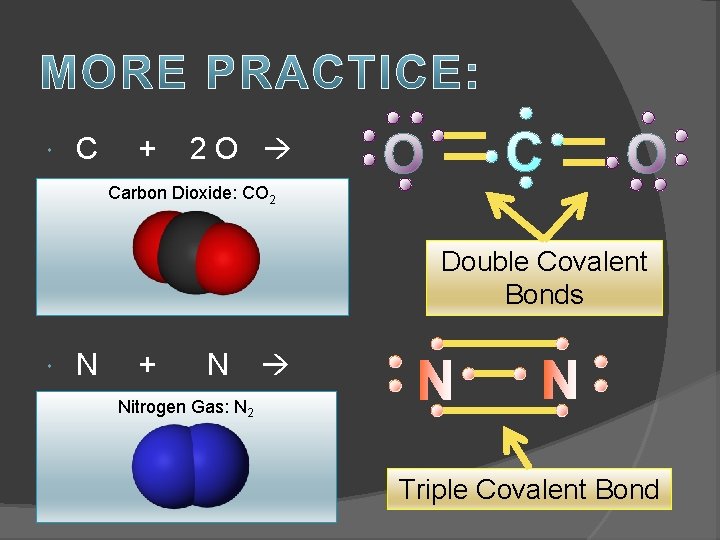
C + 2 O Carbon Dioxide: CO 2 Double Covalent Bonds N + N Nitrogen Gas: N 2 Triple Covalent Bond

The octet rule cannot be satisfied in molecules whose total number of valence electrons is an odd number. What does this mean? ! Let’s take a closer The octet rule cannot be satisfied in molecules in which an atom has fewer, look… or more than a complete octet of valence electrons.
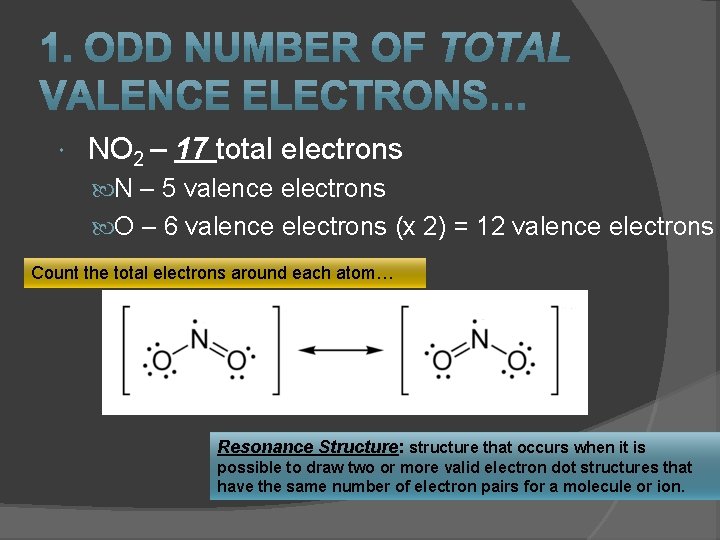
NO 2 – 17 total electrons N – 5 valence electrons O – 6 valence electrons (x 2) = 12 valence electrons Count the total electrons around each atom… Resonance Structure: structure that occurs when it is possible to draw two or more valid electron dot structures that have the same number of electron pairs for a molecule or ion.
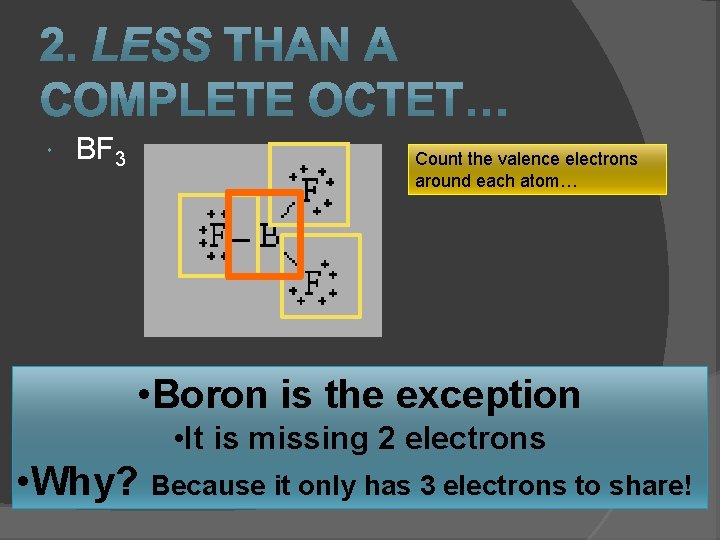
BF 3 Count the valence electrons around each atom… • Boron is the exception • It is missing 2 electrons • Why? Because it only has 3 electrons to share!
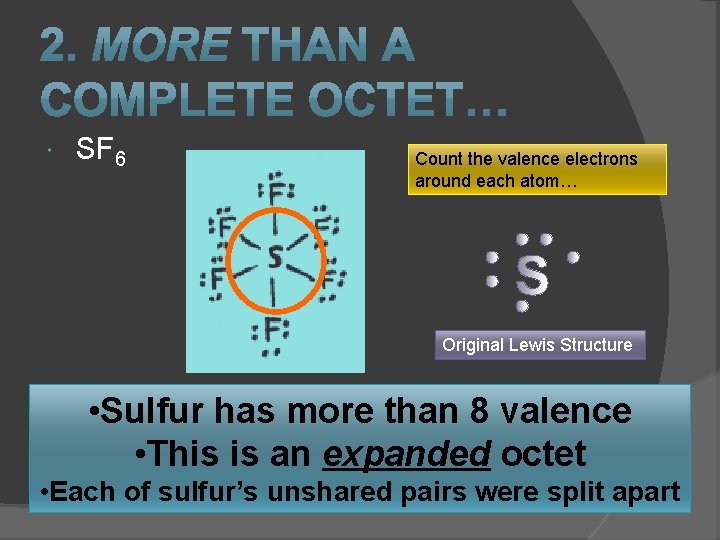
SF 6 Count the valence electrons around each atom… Original Lewis Structure • Sulfur has more than 8 valence • This is an expanded octet • Each of sulfur’s unshared pairs were split apart

Quick review of Ionic, Metallic, & Covalent Bonds

Ionic, Metallic, or Covalent? 1. Two non metals 1. Covalent 2. Transfer of electrons 2. Ionic 3. Metal and nonmetal 3. Ionic 4. Two metals 4. Metallic 5. Crystal Structure 5. Ionic 6. Sharing of electrons 6. Covalent 7. Conducts electricity 7. Metallic 8. High melting point 1. Ionic 9. Solid at room 2. Ionic & Metallic without being melted temperature 10. Sea of electrons QUESTION 3. Metallic ANSWER

Ionic, Metallic, or Covalent? 1. Brittle 1. Ionic 2. Conducts electricity 2. Ionic 3. Strong inter-particle 1. Ionic after being melted or dissolved in water forces 2. Covalent 4. Forms molecules 3. Covalent 5. Low melting point 4. Covalent 6. Does not conduct electricity 7. Liquid or gas at room 1. Covalent temperature QUESTION ANSWER

Review: Naming Ionic Compounds Name of cation followed by name of anion Anion - change ending of nonmetal to -ide Use a roman numeral to represent the charge for a cation ONLY if it is a transition metal Transition metal with a specific charge: Ag 1+, Zn 2+, Cd 2+ ○ Do NOT need roman numeral in the name!

Naming Covalent Compounds In order to name molecular compounds, a different naming system is required. WHY? ○ How else would we differentiate between CO & CO 2? Still follow the same naming rules, but add PREFIXES to distinguish how many atoms of each element

Prefixes – need to memorize 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca

Naming Covalent Compounds Write the name of the first element. Add a prefix if there is more than one of that first atom. Write the name of the second element and change the ending to –ide Add a prefix to the second atom even if there is only one! If “ao” or “oo” appears in the name, remove the first “a” or “o”
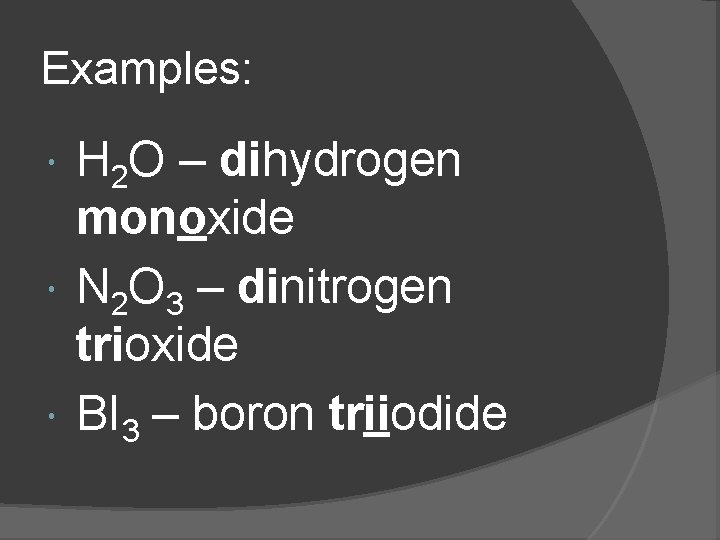
Examples: H 2 O – dihydrogen monoxide N 2 O 3 – dinitrogen trioxide BI 3 – boron triiodide
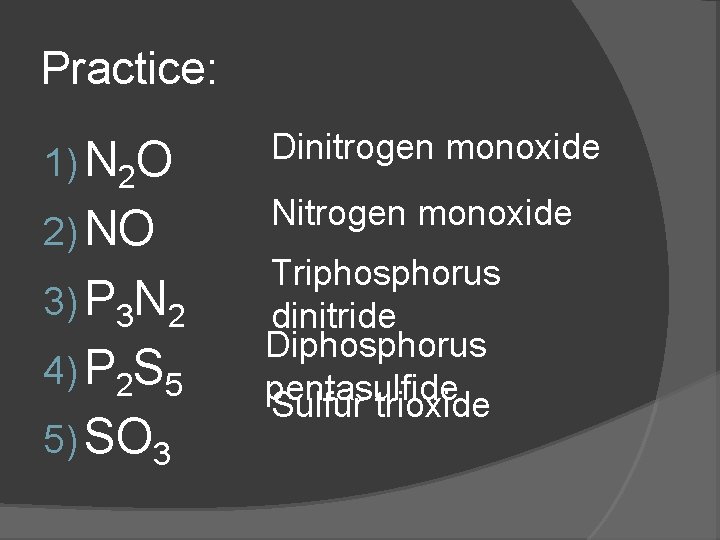
Practice: 1) N 2 O 2) NO 3) P 3 N 2 4) P 2 S 5 5) SO 3 Dinitrogen monoxide Nitrogen monoxide Triphosphorus dinitride Diphosphorus pentasulfide Sulfur trioxide

Naming Diatomic Molecules 7 diatomic elements: H 2, O 2, F 2, Br 2, I 2, N 2, Cl 2 You will name the molecule the same as the element! H 2 = hydrogen or hydrogen gas
- Slides: 23