Chapter 8 Chemical Reactions 8 1 Nature of





















- Slides: 30

Chapter 8 Chemical Reactions

8 -1 Nature of Chem Rxn’s *form a new substance

-Characteristics of chem rxn’s *reactants: substance that enters into a chem rxn *products: substance being produced by the rxn

-Characteristics of chem rxn’s -reactants yields products -involve a change in nrg as well -nrg is either absorbed or released

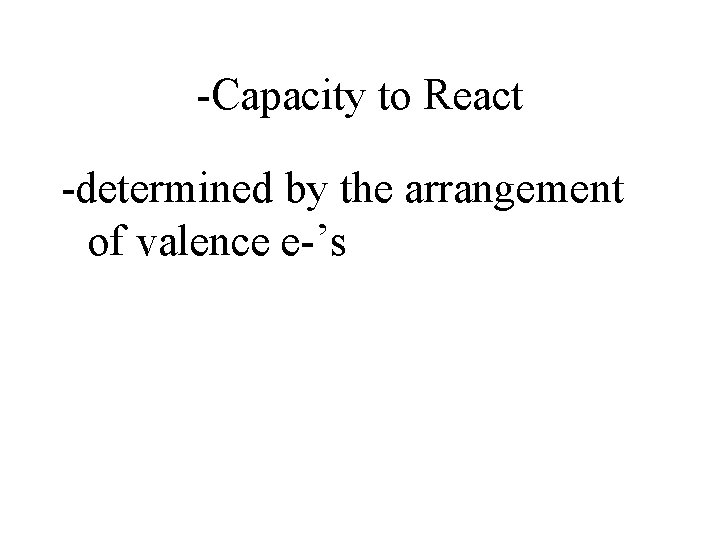
-Capacity to React -determined by the arrangement of valence e-’s

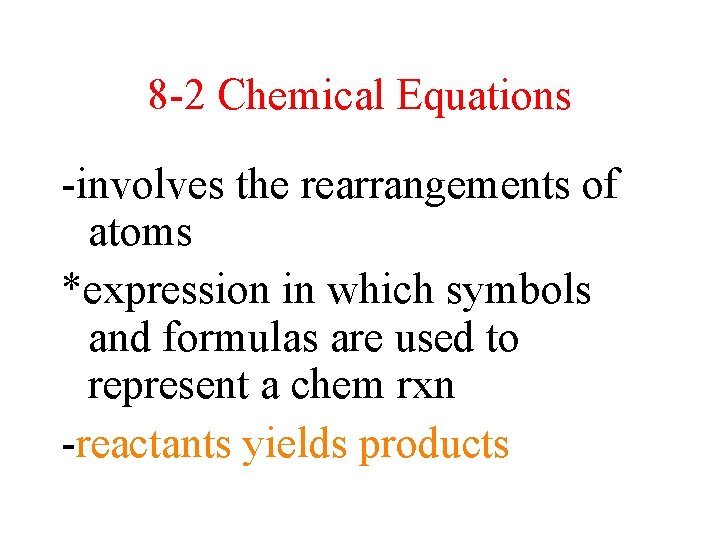
8 -2 Chemical Equations -involves the rearrangements of atoms *expression in which symbols and formulas are used to represent a chem rxn -reactants yields products

Conservation of mass -atoms can be neither created nor destroyed during a chem rxn

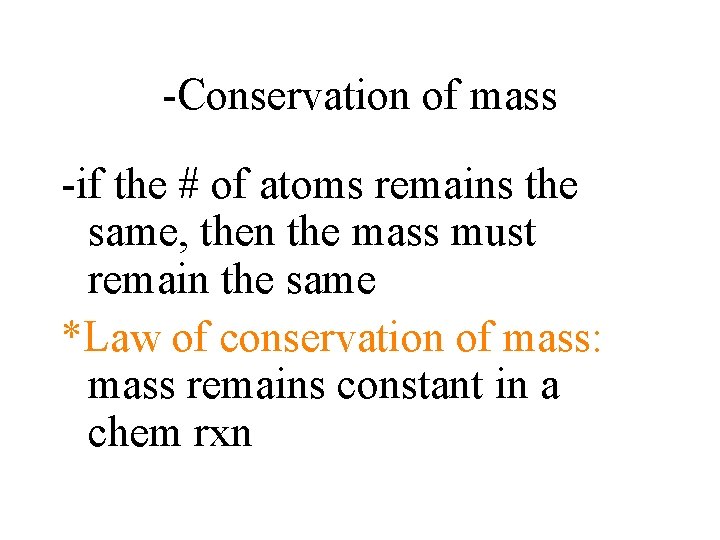
-Conservation of mass -if the # of atoms remains the same, then the mass must remain the same *Law of conservation of mass: mass remains constant in a chem rxn

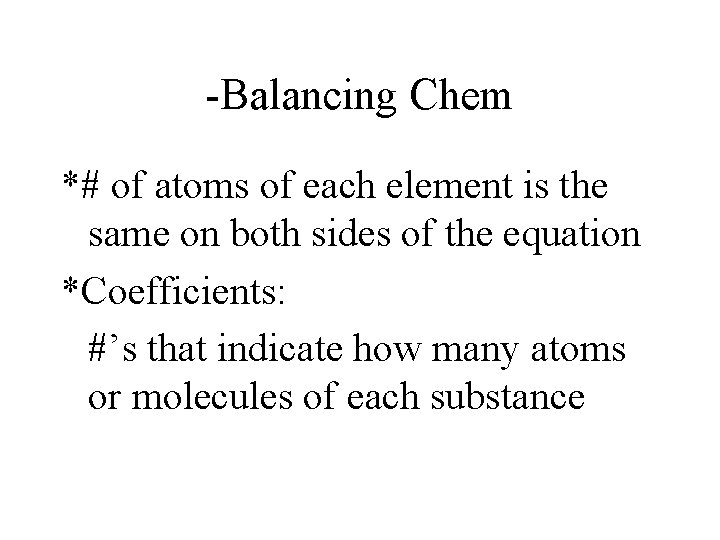
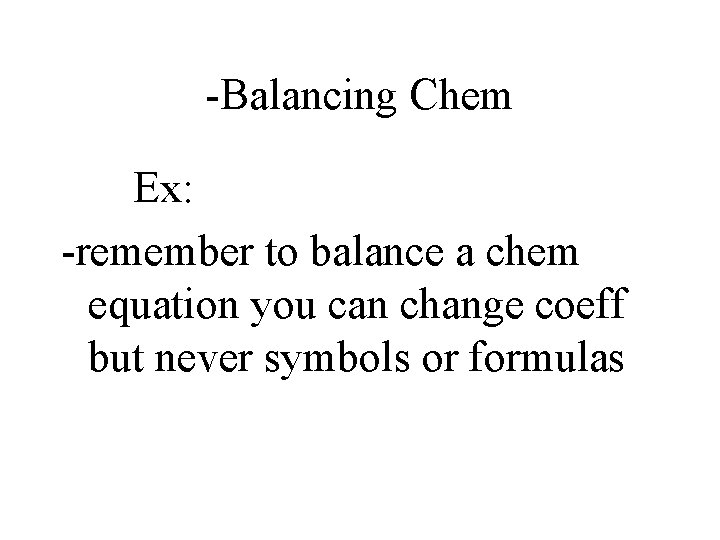
-Balancing Chem *# of atoms of each element is the same on both sides of the equation *Coefficients: #’s that indicate how many atoms or molecules of each substance

-Balancing Chem Ex: -remember to balance a chem equation you can change coeff but never symbols or formulas

-Balancing Chem -steps: 1. Write the chem equat(look for diatomics)

-Balancing Chem 2. Write down the oxidation #’s 3. Make oxidation #’s = 0 4. Balance left and right side

8 -3 Types of Chem Rxn’s -synthesis, decomposition, single replacement, and double replacement…. and Combustion

-Synthesis Rxn *two simple substances form a more complex substance EX: corrosion of metals

-Decomposition Rxn *complex substance breaks down into two or more simpler substances Ex

-Single-replacement rxn’s *an uncombined element replaces an element that is part of a cmpnd EX:

-Double-Replacement Rxn #14(reaction of lead nitrate) #15(yellow precip reaction) *different atoms in two different cmpnds replace eachother -2 cmpnds react to form 2 new cmpnds EX:

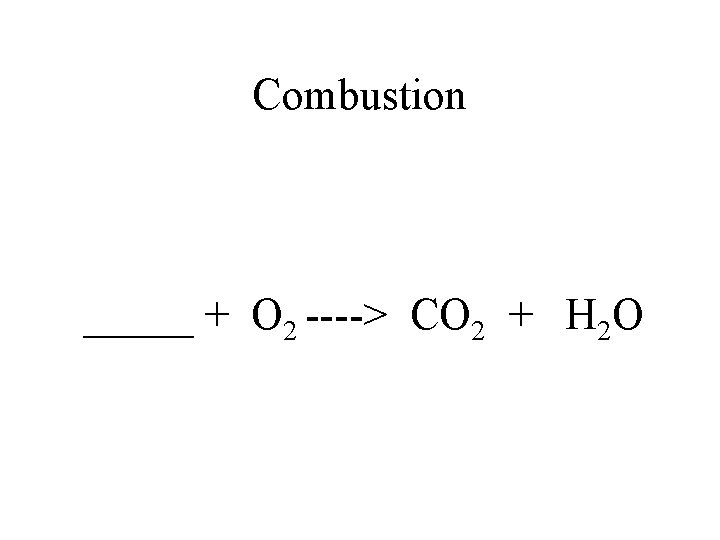
Combustion _____ + O 2 ----> CO 2 + H 2 O

8 -4 Energy of Chem Rxn’s -chem rxn = change in nrg -nrg is never created or destroyed in chem rxn’s -Two types of chem rxn’s

1. Exothermic Rxn *nrg is released -Ex: combustion -nrg of product is less than the nrg of the reactant - Equation: R -----> P + nrg

2. Endothermic Rxn’s *nrg is absorbed -nrg of the products is more than the nrg of the reactants -Equation: R + nrg ----> P

-Activation Energy *nrg needed to start a rxn -form short-lived, high nrg, extremely unstable molecule -all chem rxn’s require activation nrg -nrg diagrams: see ipad

8 -5 Rates of Chem Rxn *Kinetics: study of rxn rates *Rxn rates: how quickly reactants turn into products

-Collision Theory *relates molecular coll to rxn rate -reacting molecules must collide with sufficient nrg

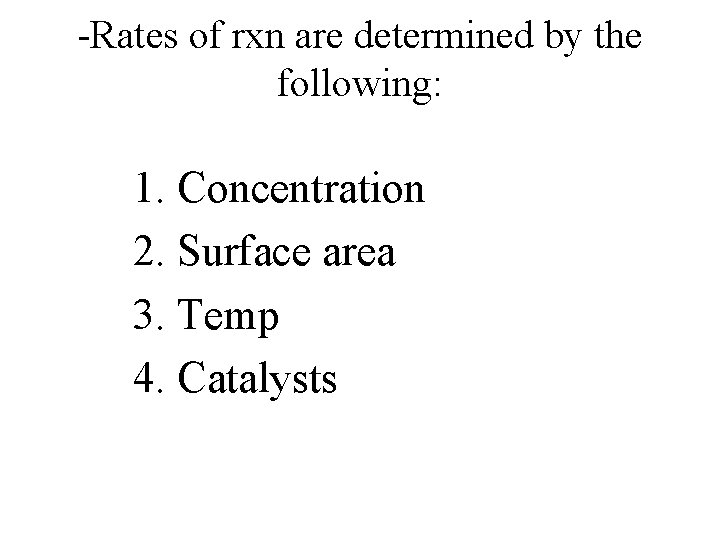
-Rates of rxn are determined by the following: 1. Concentration 2. Surface area 3. Temp 4. Catalysts

1. Concentration *measure of the amount of that subst in a given unit of vol. -high conc. = many particles per vol -usually if you increase the conc of reactants you increase the rate of the rxn

2. Surface Area -increase in surface area increases the collision between reacting molecules

3. Temperature -increase in temp generally increases the rate of rxn

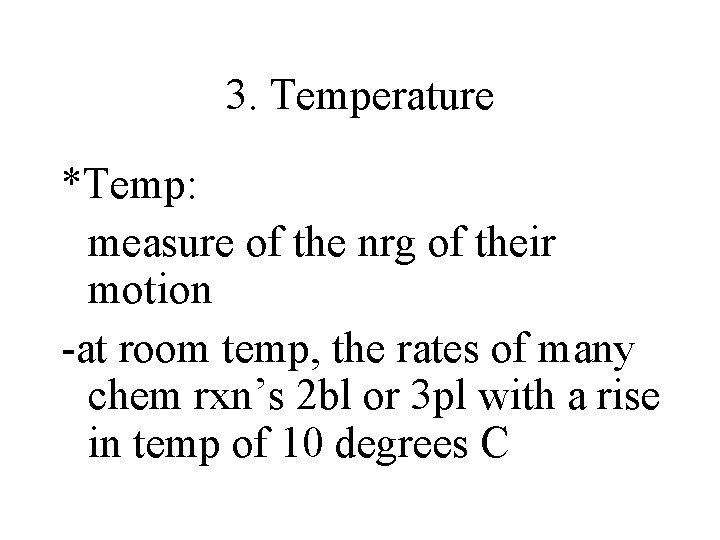
3. Temperature *Temp: measure of the nrg of their motion -at room temp, the rates of many chem rxn’s 2 bl or 3 pl with a rise in temp of 10 degrees C

4. Catalysts *substance that increases the rate of rxn but is not itself changed by the rxn -reduces the activation nrg required to start a chem rxn