Chapter 8 Chemical Equations Flames and sparks result









- Slides: 47

Chapter 8 Chemical Equations Flames and sparks result when aluminum foil is dropped Into liquid bromine. Introduction to General, Organic, and Biochemistry 10 e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena

Chapter Outline 8. 1 The Chemical Equation 8. 2 Writing and Balancing Chemical Equations 8. 3 Information in a Chemical Equation 8. 4 Types of Chemical Equations 8. 5 Heat in Chemical Reactions 8. 6 Global Warming: The Greenhouse Effect Copyright 2012 John Wiley & Sons, Inc

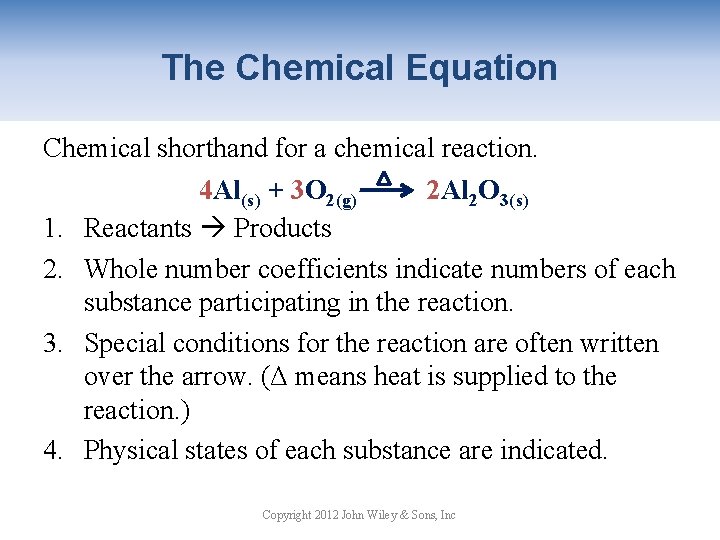
The Chemical Equation Chemical shorthand for a chemical reaction. 1. 2. 3. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) Reactants Products Whole number coefficients indicate numbers of each substance participating in the reaction. Special conditions for the reaction are often written over the arrow. (Δ means heat is supplied to the reaction. ) Physical states of each substance are indicated. Copyright 2012 John Wiley & Sons, Inc

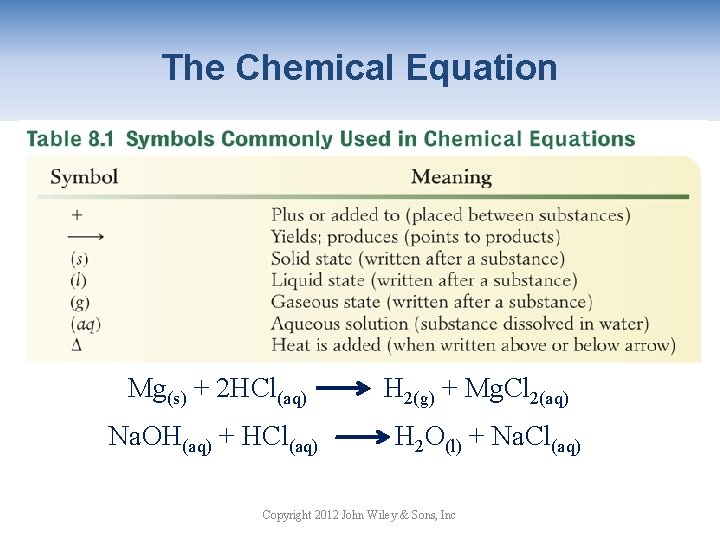
The Chemical Equation Mg(s) + 2 HCl(aq) Na. OH(aq) + HCl(aq) H 2(g) + Mg. Cl 2(aq) H 2 O(l) + Na. Cl(aq) Copyright 2012 John Wiley & Sons, Inc

Your Turn! In the reaction: Cu. SO 4 + Ba. Br 2 Cu. Br 2 + Ba. SO 4 a. Br 2 and Ba. SO 4 are reactants b. Ba. SO 4 and Cu. Br 2 are products c. Cu. SO 4 and Ba. SO 4 are reactants d. Cu. SO 4 and Ba. Br 2 are products Copyright 2012 John Wiley & Sons, Inc

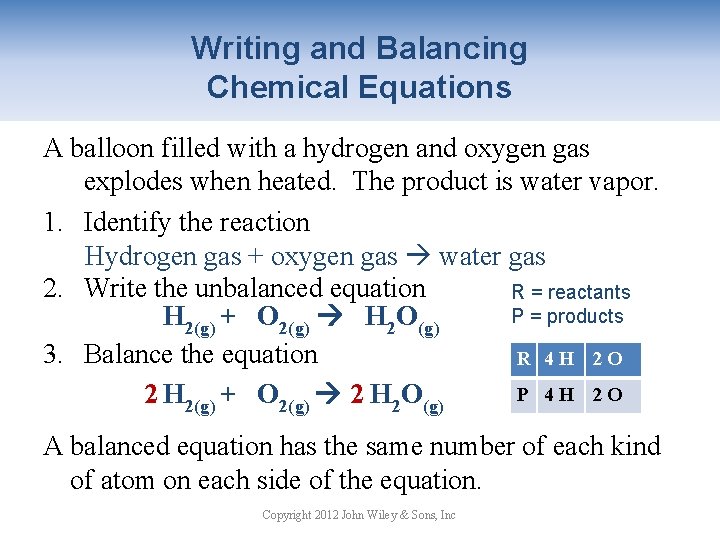
Writing and Balancing Chemical Equations A balloon filled with a hydrogen and oxygen gas explodes when heated. The product is water vapor. 1. Identify the reaction Hydrogen gas + oxygen gas water gas 2. Write the unbalanced equation R = reactants P = products H 2(g) + O 2(g) H 2 O(g) 3. Balance the equation R 42 H 2 O P 42 H 22 O 1 O 2 H 2(g) + O 2(g) 2 H 2 O(g) A balanced equation has the same number of each kind of atom on each side of the equation. Copyright 2012 John Wiley & Sons, Inc

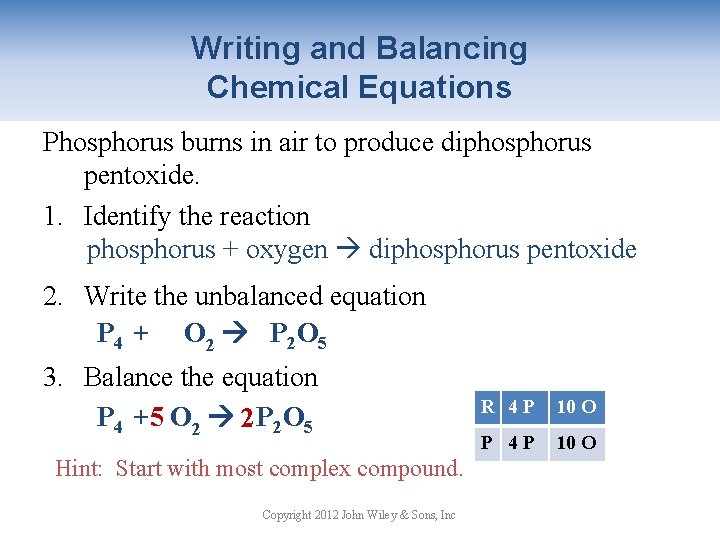
Writing and Balancing Chemical Equations Phosphorus burns in air to produce diphosphorus pentoxide. 1. Identify the reaction phosphorus + oxygen diphosphorus pentoxide 2. Write the unbalanced equation P 4 + O 2 P 2 O 5 3. Balance the equation P 4 + 5 O 2 2 P 2 O 5 Hint: Start with most complex compound. Copyright 2012 John Wiley & Sons, Inc R 4 P 2 OO 10 P 42 P 5 OO 10

Your Turn! Given the unbalanced equation: HCl + NH 3 NH 4 Cl When properly balanced, the sum of the balancing coefficients is a. 7 b. 5 HCl + NH 3 NH 4 Cl c. 3 d. 4 e. 6 Copyright 2012 John Wiley & Sons, Inc

Your Turn! Given the unbalanced equation: NH 3 H 2 + N 2 When properly balanced, the sum of the balancing coefficients is a. 3 b. 6 2 NH 3 3 H 2 + N 2 c. 9 d. 12 Copyright 2012 John Wiley & Sons, Inc

Writing and Balancing Chemical Equations Zinc metal reacts with silver nitrate to produce zinc nitrate and silver metal. 1. Identify the reaction zinc + silver nitrate zinc nitrate + silver 2. Write the unbalanced equation Zn + Ag. NO 3 Zn(NO 3)2 + Ag 3. Balance the equation Zn + 2 Ag. NO 3 Zn(NO 3)2 + 2 Ag Hint: Balance polyatomic ions as a unit. Copyright 2012 John Wiley & Sons, Inc R 1 Zn 21 Ag 21 NO 3 P 1 Zn 21 Ag 2 NO 3

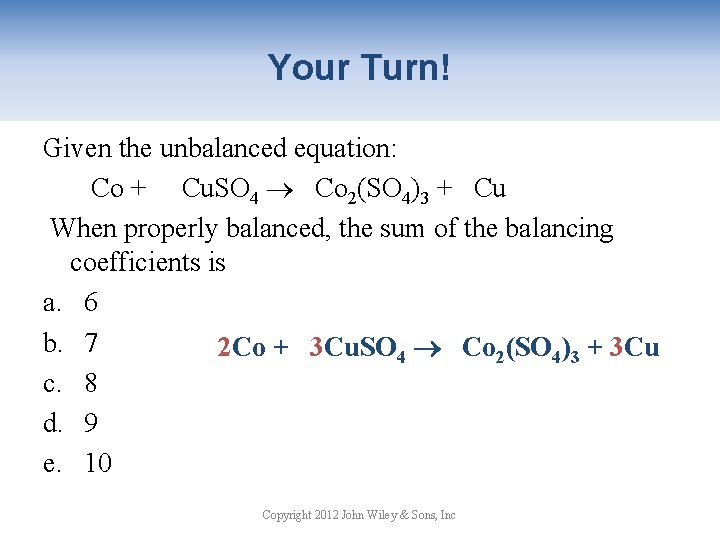
Your Turn! Given the unbalanced equation: Co + Cu. SO 4 Co 2(SO 4)3 + Cu When properly balanced, the sum of the balancing coefficients is a. 6 b. 7 2 Co + 3 Cu. SO 4 Co 2(SO 4)3 + 3 Cu c. 8 d. 9 e. 10 Copyright 2012 John Wiley & Sons, Inc

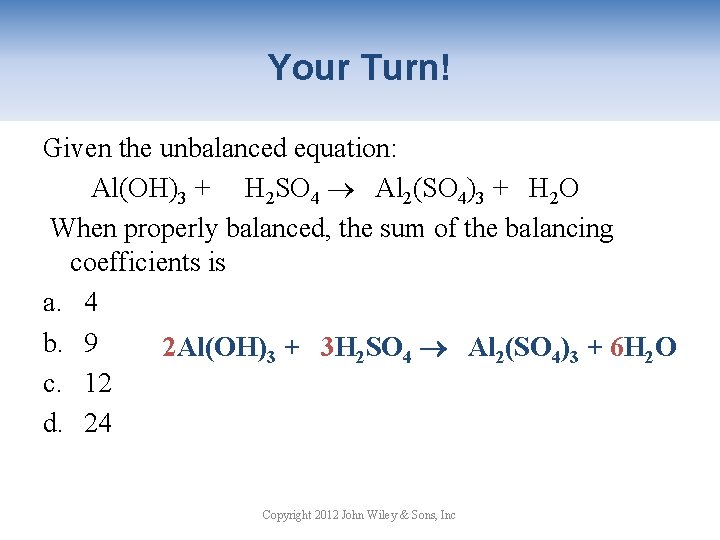
Your Turn! Given the unbalanced equation: Al(OH)3 + H 2 SO 4 Al 2(SO 4)3 + H 2 O When properly balanced, the sum of the balancing coefficients is a. 4 b. 9 2 Al(OH)3 + 3 H 2 SO 4 Al 2(SO 4)3 + 6 H 2 O c. 12 d. 24 Copyright 2012 John Wiley & Sons, Inc

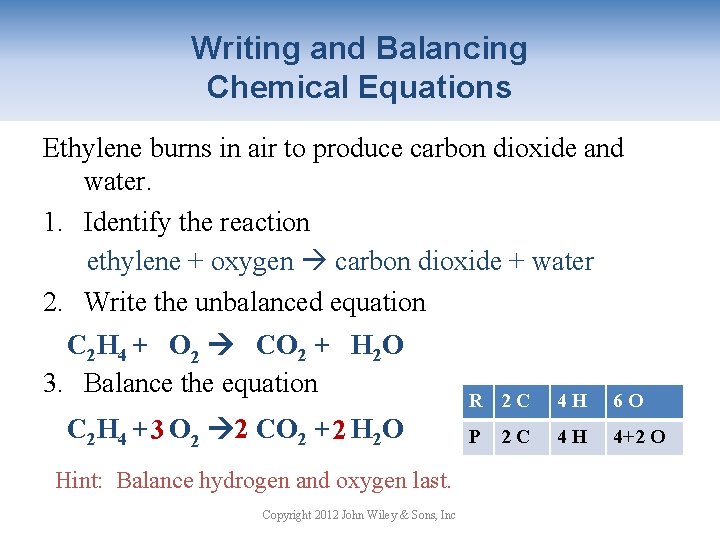
Writing and Balancing Chemical Equations Ethylene burns in air to produce carbon dioxide and water. 1. Identify the reaction ethylene + oxygen carbon dioxide + water 2. Write the unbalanced equation C 2 H 4 + O 2 CO 2 + H 2 O 3. Balance the equation C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O Hint: Balance hydrogen and oxygen last. Copyright 2012 John Wiley & Sons, Inc R 2 C 4 H 62 O P 21 C 42 H 2+1 O 4+1 4+2

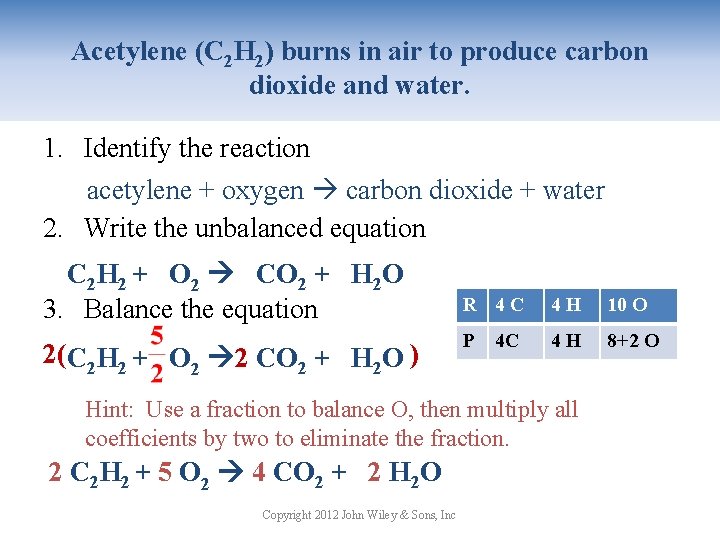
Acetylene (C 2 H 2) burns in air to produce carbon dioxide and water. 1. Identify the reaction acetylene + oxygen carbon dioxide + water 2. Write the unbalanced equation C 2 H 2 + O 2 CO 2 + H 2 O 3. Balance the equation 2(C 2 H 2 + O 2 2 CO 2 + H 2 O ) R 42 C 42 H 52 OO 10 P 4 C 21 C 42 H 2+1 O 4+1 8+2 Hint: Use a fraction to balance O, then multiply all coefficients by two to eliminate the fraction. 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O Copyright 2012 John Wiley & Sons, Inc

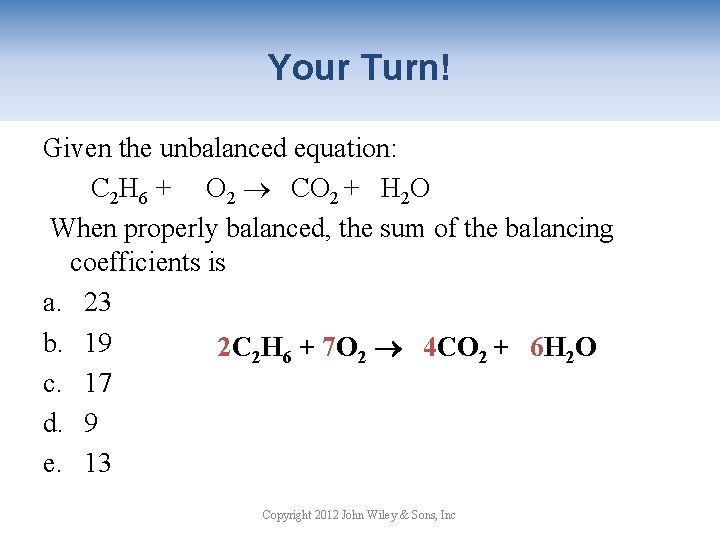
Your Turn! Given the unbalanced equation: C 2 H 6 + O 2 CO 2 + H 2 O When properly balanced, the sum of the balancing coefficients is a. 23 b. 19 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O c. 17 d. 9 e. 13 Copyright 2012 John Wiley & Sons, Inc

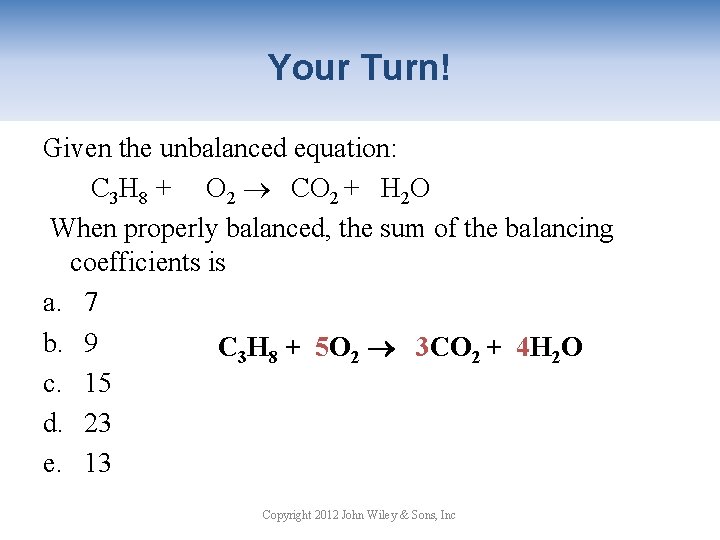
Your Turn! Given the unbalanced equation: C 3 H 8 + O 2 CO 2 + H 2 O When properly balanced, the sum of the balancing coefficients is a. 7 b. 9 C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O c. 15 d. 23 e. 13 Copyright 2012 John Wiley & Sons, Inc

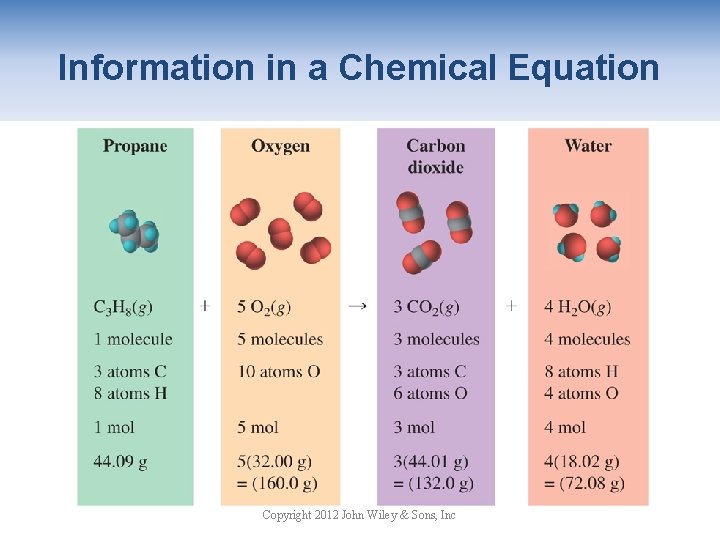
Information in a Chemical Equation Copyright 2012 John Wiley & Sons, Inc

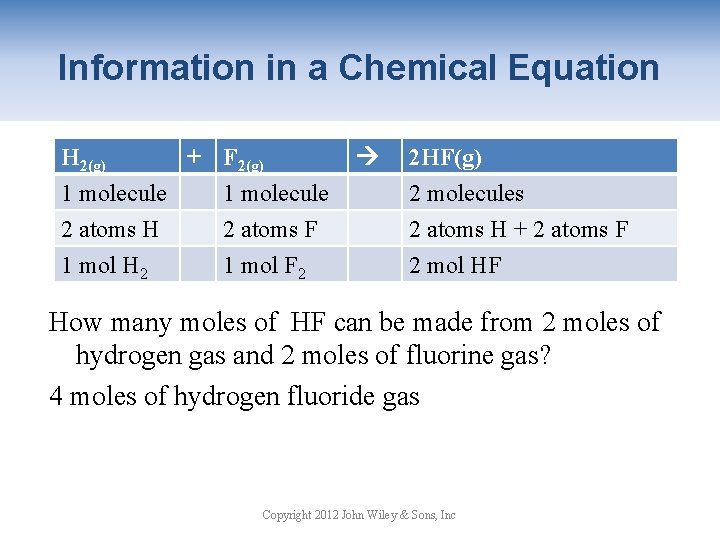
Information in a Chemical Equation H 2(g) + F 2(g) 1 molecule 2 atoms H 2 atoms F 1 mol H 2 1 mol F 2 2 HF(g) 2 molecules 2 atoms H + 2 atoms F 2 mol HF How many moles of HF can be made from 2 moles of hydrogen gas and 2 moles of fluorine gas? 4 moles of hydrogen fluoride gas Copyright 2012 John Wiley & Sons, Inc

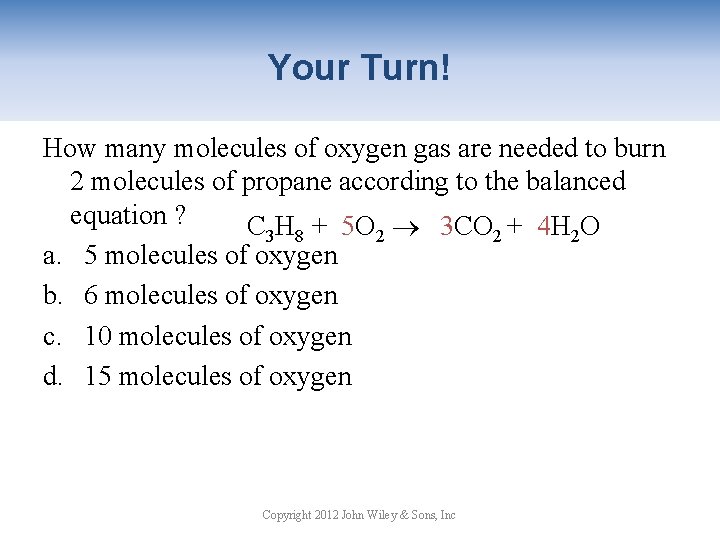
Your Turn! How many molecules of oxygen gas are needed to burn 2 molecules of propane according to the balanced equation ? C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O a. 5 molecules of oxygen b. 6 molecules of oxygen c. 10 molecules of oxygen d. 15 molecules of oxygen Copyright 2012 John Wiley & Sons, Inc

Types of Chemical Equations 1. Combination Reactions A + B AB 2. Decomposition Reactions AB A + B 3. Single-Displacement A + BC B + AC or A + BC C + BA 4. Double-Displacement A B+ CD AD + CB Copyright 2012 John Wiley & Sons, Inc

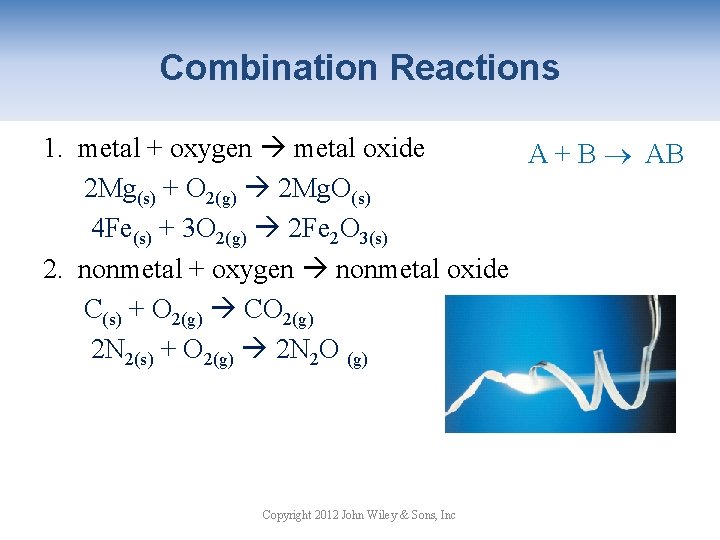
Combination Reactions 1. metal + oxygen metal oxide A + B AB 2 Mg(s) + O 2(g) 2 Mg. O(s) 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) 2. nonmetal + oxygen nonmetal oxide C(s) + O 2(g) CO 2(g) 2 N 2(s) + O 2(g) 2 N 2 O (g) Copyright 2012 John Wiley & Sons, Inc

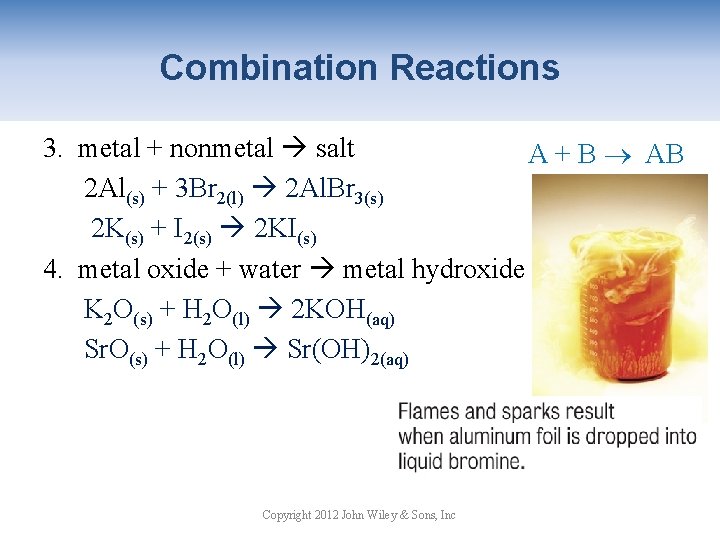
Combination Reactions 3. metal + nonmetal salt A + B AB 2 Al(s) + 3 Br 2(l) 2 Al. Br 3(s) 2 K(s) + I 2(s) 2 KI(s) 4. metal oxide + water metal hydroxide K 2 O(s) + H 2 O(l) 2 KOH(aq) Sr. O(s) + H 2 O(l) Sr(OH)2(aq) Copyright 2012 John Wiley & Sons, Inc

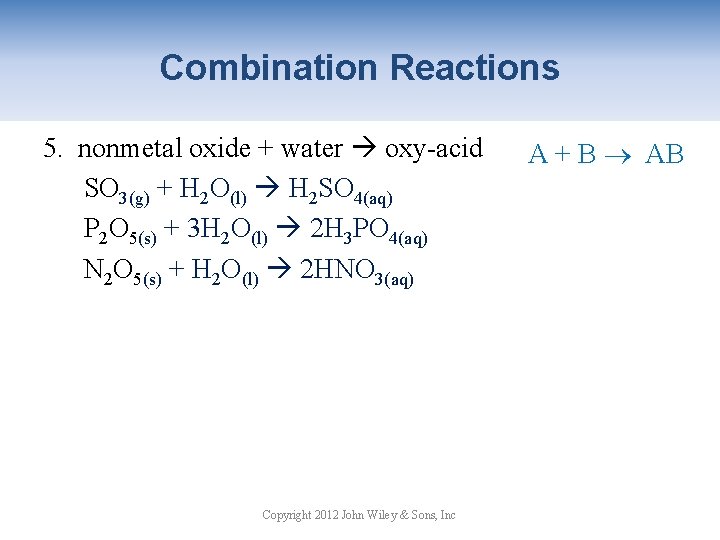
Combination Reactions 5. nonmetal oxide + water oxy-acid SO 3(g) + H 2 O(l) H 2 SO 4(aq) P 2 O 5(s) + 3 H 2 O(l) 2 H 3 PO 4(aq) N 2 O 5(s) + H 2 O(l) 2 HNO 3(aq) Copyright 2012 John Wiley & Sons, Inc A + B AB

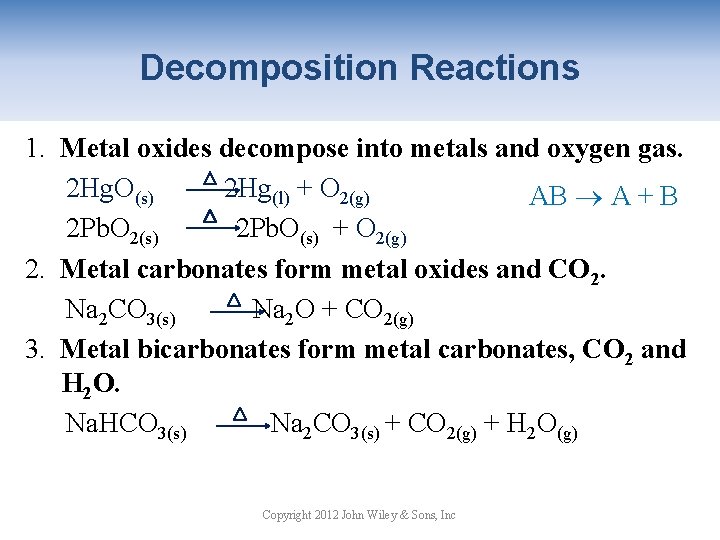
Decomposition Reactions 1. Metal oxides decompose into metals and oxygen gas. 2 Hg. O(s) 2 Hg(l) + O 2(g) AB A + B 2 Pb. O 2(s) 2 Pb. O(s) + O 2(g) 2. Metal carbonates form metal oxides and CO 2. Na 2 CO 3(s) Na 2 O + CO 2(g) 3. Metal bicarbonates form metal carbonates, CO 2 and H 2 O. Na. HCO 3(s) Na 2 CO 3(s) + CO 2(g) + H 2 O(g) Copyright 2012 John Wiley & Sons, Inc

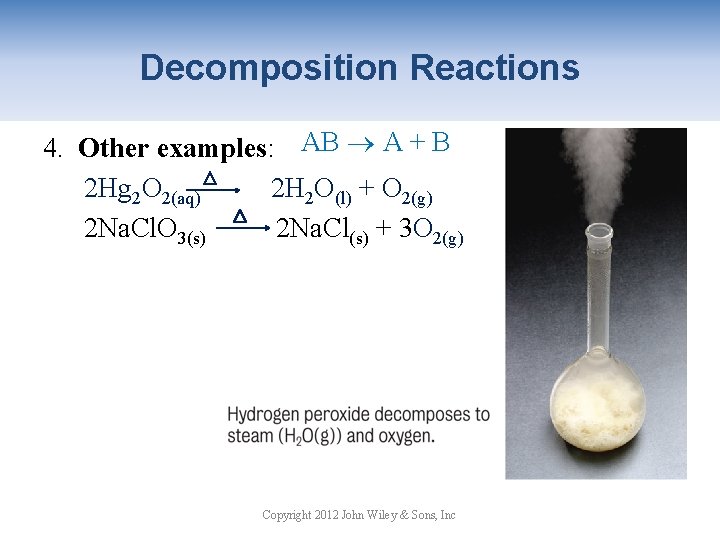
Decomposition Reactions 4. Other examples: AB A + B 2 Hg 2 O 2(aq) 2 H 2 O(l) + O 2(g) 2 Na. Cl. O 3(s) 2 Na. Cl(s) + 3 O 2(g) Copyright 2012 John Wiley & Sons, Inc

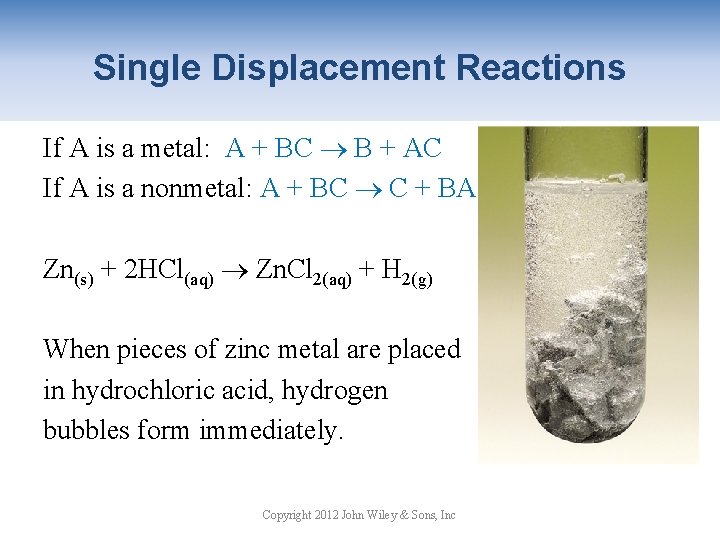
Single Displacement Reactions If A is a metal: A + BC B + AC If A is a nonmetal: A + BC C + BA Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) When pieces of zinc metal are placed in hydrochloric acid, hydrogen bubbles form immediately. Copyright 2012 John Wiley & Sons, Inc

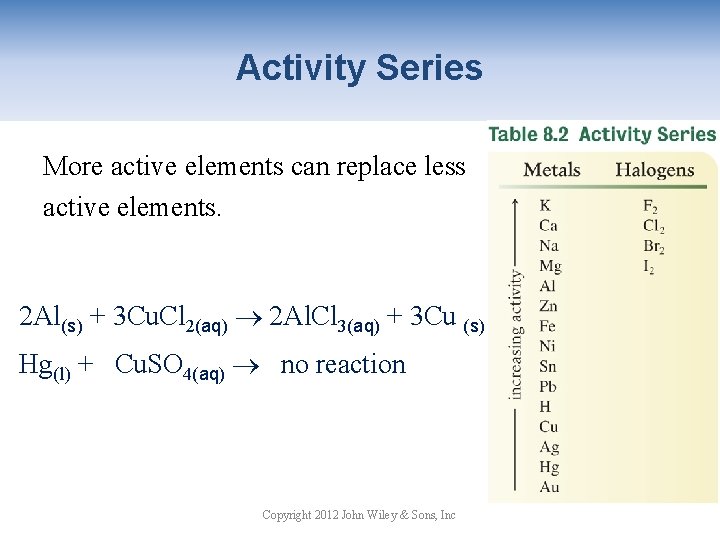
Activity Series More active elements can replace less active elements. 2 Al(s) + 3 Cu. Cl 2(aq) 2 Al. Cl 3(aq) + 3 Cu (s) Hg(l) + Cu. SO 4(aq) no reaction Copyright 2012 John Wiley & Sons, Inc

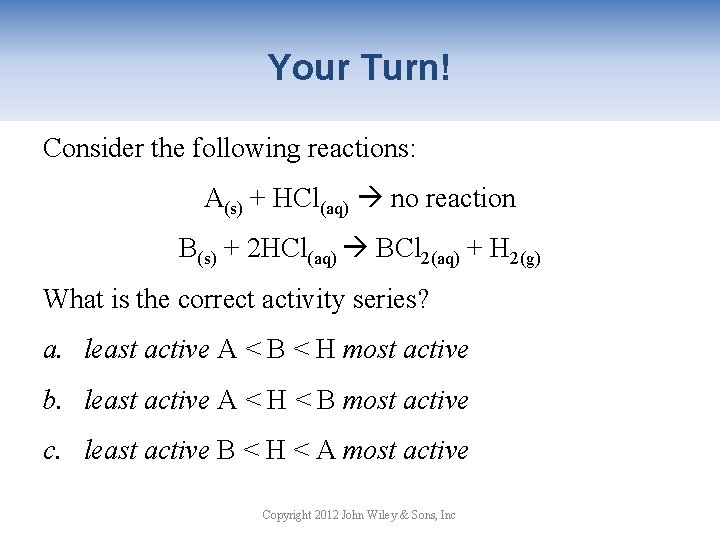
Your Turn! Consider the following reactions: A(s) + HCl(aq) no reaction B(s) + 2 HCl(aq) BCl 2(aq) + H 2(g) What is the correct activity series? a. least active A < B < H most active b. least active A < H < B most active c. least active B < H < A most active Copyright 2012 John Wiley & Sons, Inc

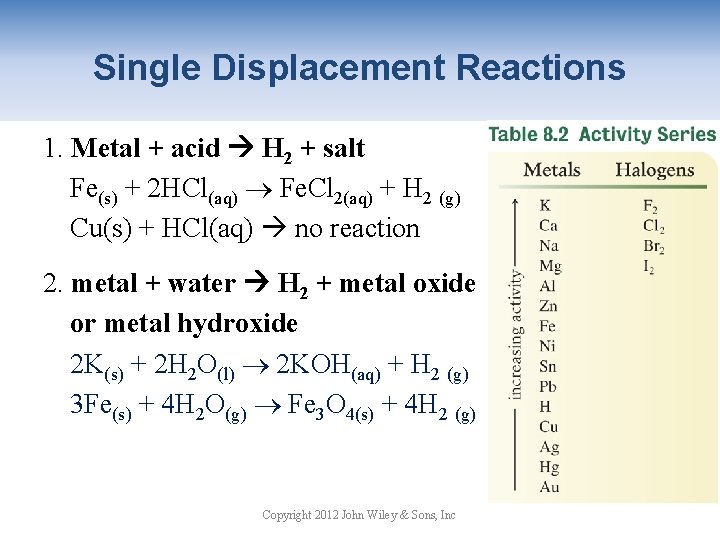
Single Displacement Reactions 1. Metal + acid H 2 + salt Fe(s) + 2 HCl(aq) Fe. Cl 2(aq) + H 2 (g) Cu(s) + HCl(aq) no reaction 2. metal + water H 2 + metal oxide or metal hydroxide 2 K(s) + 2 H 2 O(l) 2 KOH(aq) + H 2 (g) 3 Fe(s) + 4 H 2 O(g) Fe 3 O 4(s) + 4 H 2 (g) Copyright 2012 John Wiley & Sons, Inc

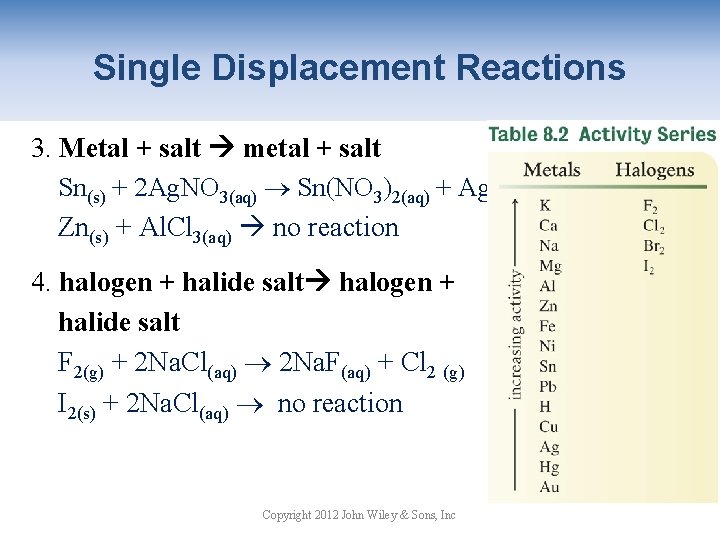
Single Displacement Reactions 3. Metal + salt metal + salt Sn(s) + 2 Ag. NO 3(aq) Sn(NO 3)2(aq) + Ag(s) Zn(s) + Al. Cl 3(aq) no reaction 4. halogen + halide salt F 2(g) + 2 Na. Cl(aq) 2 Na. F(aq) + Cl 2 (g) I 2(s) + 2 Na. Cl(aq) no reaction Copyright 2012 John Wiley & Sons, Inc

Your Turn! The reaction: Ba(s) + Pt. Cl 2(aq) Ba. Cl 2(aq) + Pt(s), will occur if a. Pt is more active than Ba b. Ba is more active than Pt c. Ba is more active than O d. O is more active than Pt Copyright 2012 John Wiley & Sons, Inc

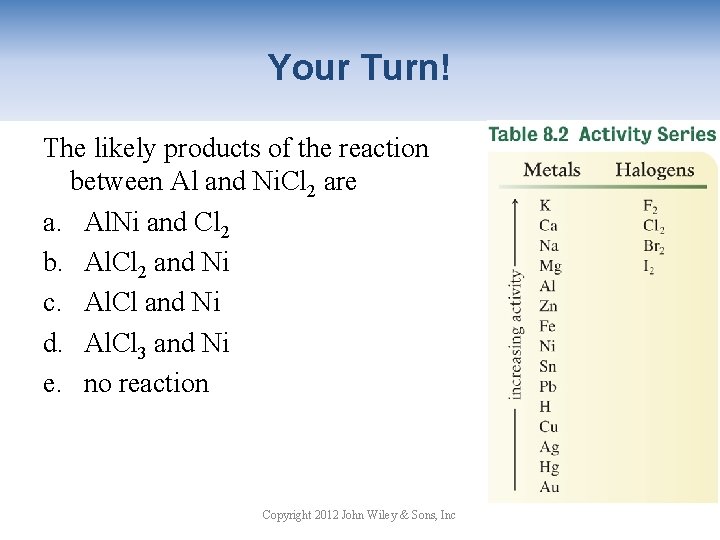
Your Turn! The likely products of the reaction between Al and Ni. Cl 2 are a. Al. Ni and Cl 2 b. Al. Cl 2 and Ni c. Al. Cl and Ni d. Al. Cl 3 and Ni e. no reaction Copyright 2012 John Wiley & Sons, Inc

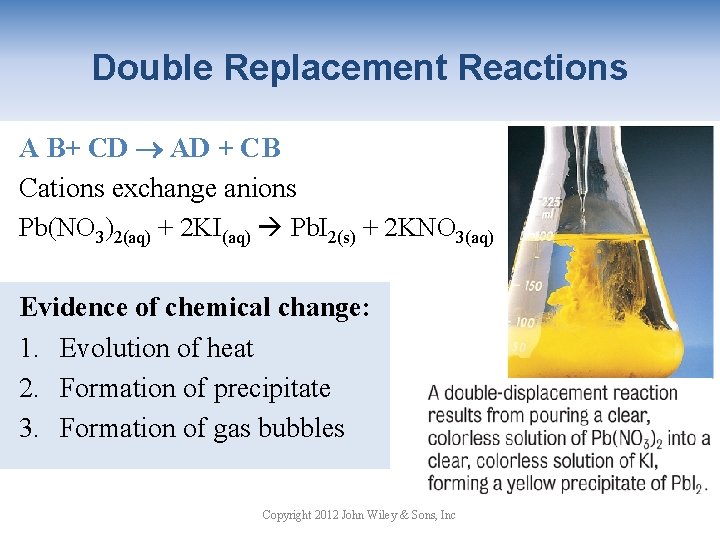
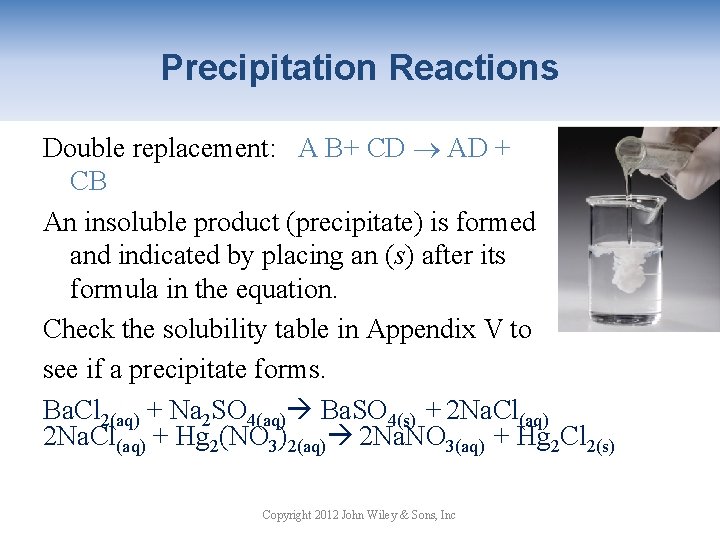
Double Replacement Reactions A B+ CD AD + CB Cations exchange anions Pb(NO 3)2(aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Evidence of chemical change: 1. Evolution of heat 2. Formation of precipitate 3. Formation of gas bubbles Copyright 2012 John Wiley & Sons, Inc

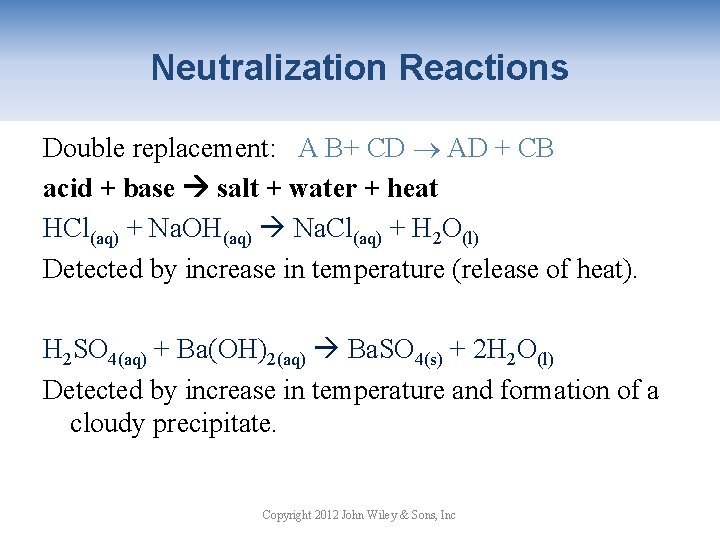
Neutralization Reactions Double replacement: A B+ CD AD + CB acid + base salt + water + heat HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Detected by increase in temperature (release of heat). H 2 SO 4(aq) + Ba(OH)2(aq) Ba. SO 4(s) + 2 H 2 O(l) Detected by increase in temperature and formation of a cloudy precipitate. Copyright 2012 John Wiley & Sons, Inc

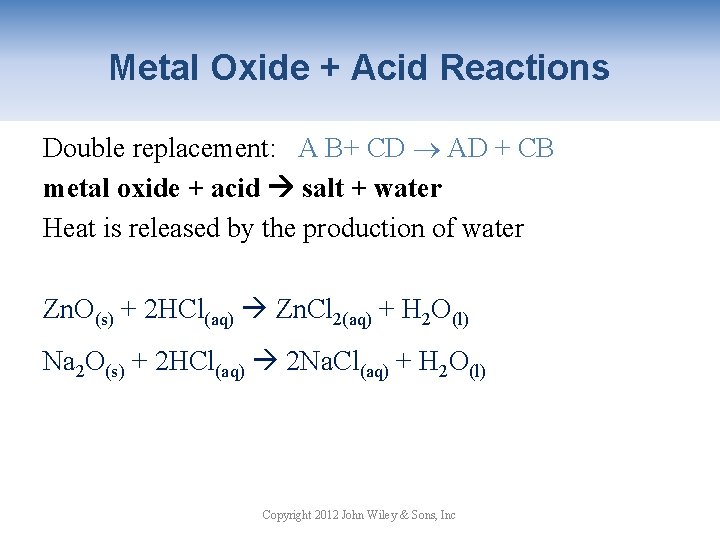
Metal Oxide + Acid Reactions Double replacement: A B+ CD AD + CB metal oxide + acid salt + water Heat is released by the production of water Zn. O(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 O(l) Na 2 O(s) + 2 HCl(aq) 2 Na. Cl(aq) + H 2 O(l) Copyright 2012 John Wiley & Sons, Inc

Precipitation Reactions Double replacement: A B+ CD AD + CB An insoluble product (precipitate) is formed and indicated by placing an (s) after its formula in the equation. Check the solubility table in Appendix V to see if a precipitate forms. Ba. Cl 2(aq) + Na 2 SO 4(aq) Ba. SO 4(s) + 2 Na. Cl(aq) + Hg 2(NO 3)2(aq) 2 Na. NO 3(aq) + Hg 2 Cl 2(s) Copyright 2012 John Wiley & Sons, Inc

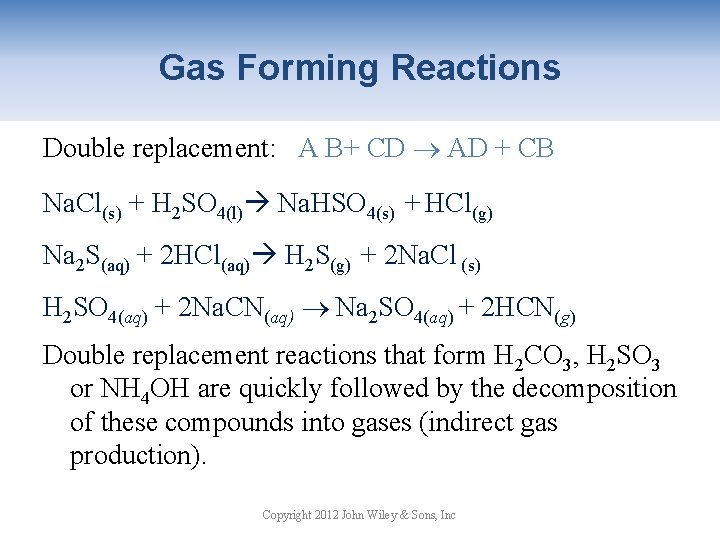
Gas Forming Reactions Double replacement: A B+ CD AD + CB Na. Cl(s) + H 2 SO 4(l) Na. HSO 4(s) + HCl(g) Na 2 S(aq) + 2 HCl(aq) H 2 S(g) + 2 Na. Cl (s) H 2 SO 4(aq) + 2 Na. CN(aq) Na 2 SO 4(aq) + 2 HCN(g) Double replacement reactions that form H 2 CO 3, H 2 SO 3 or NH 4 OH are quickly followed by the decomposition of these compounds into gases (indirect gas production). Copyright 2012 John Wiley & Sons, Inc

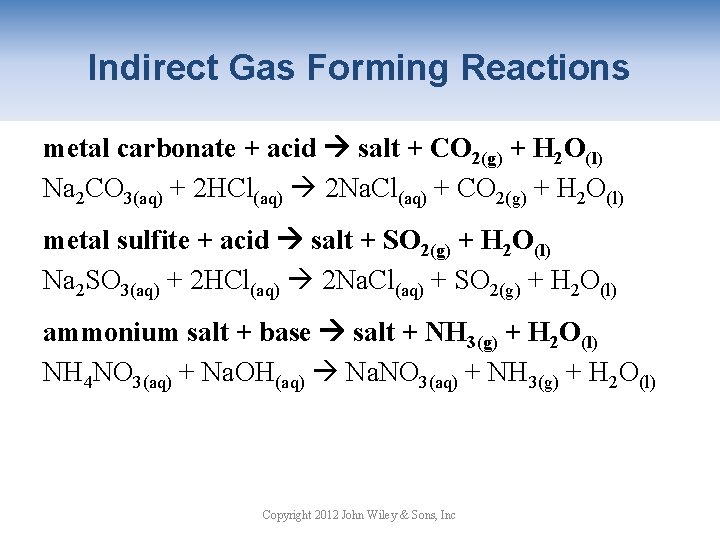
Indirect Gas Forming Reactions metal carbonate + acid salt + CO 2(g) + H 2 O(l) Na 2 CO 3(aq) + 2 HCl(aq) 2 Na. Cl(aq) + CO 2(g) + H 2 O(l) metal sulfite + acid salt + SO 2(g) + H 2 O(l) Na 2 SO 3(aq) + 2 HCl(aq) 2 Na. Cl(aq) + SO 2(g) + H 2 O(l) ammonium salt + base salt + NH 3(g) + H 2 O(l) NH 4 NO 3(aq) + Na. OH(aq) Na. NO 3(aq) + NH 3(g) + H 2 O(l) Copyright 2012 John Wiley & Sons, Inc

Your Turn! What are the likely products of the reaction of copper(II) oxide with nitric acid? a. Cu. NO 3 + H 2 O b. Cu(NO 3)2 + H 2 O c. Cu(NO 2)2 + H 2 O d. Cu. NO 2 + H 2 O Cu. O(s) + 2 HNO 3(aq) Cu(NO 3)2(aq) + H 2 O(l) Copyright 2012 John Wiley & Sons, Inc

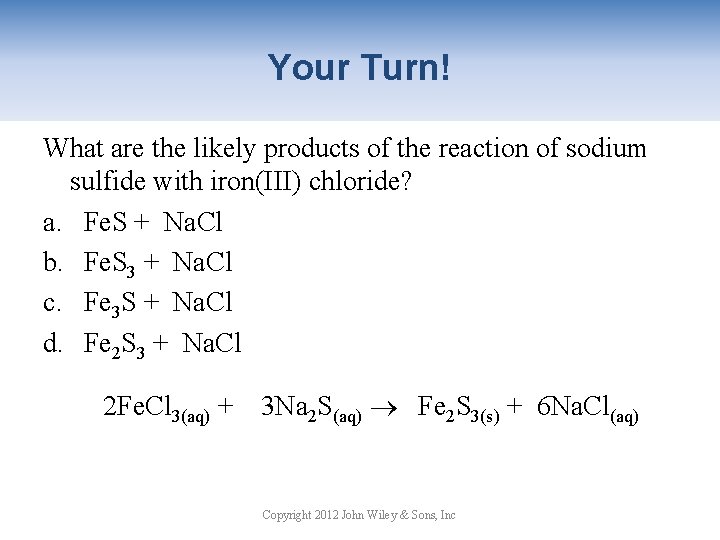
Your Turn! What are the likely products of the reaction of sodium sulfide with iron(III) chloride? a. Fe. S + Na. Cl b. Fe. S 3 + Na. Cl c. Fe 3 S + Na. Cl d. Fe 2 S 3 + Na. Cl 2 Fe. Cl 3(aq) + 3 Na 2 S(aq) Fe 2 S 3(s) + 6 Na. Cl(aq) Copyright 2012 John Wiley & Sons, Inc

Your Turn! What are the likely products of the reaction of sodium hydrogen carbonate with hydrochloric acid? a. Na. Cl + H 2 CO 3 b. Na. Cl + H 2 O + CO 2 c. Na. Cl + H 2 O + CO 3 d. Na. Cl + H 2 + CO 3 Na. HCO 3(aq) + HCl(aq) Na. Cl(aq) + H 2 O(l) + CO 2(g) Copyright 2012 John Wiley & Sons, Inc

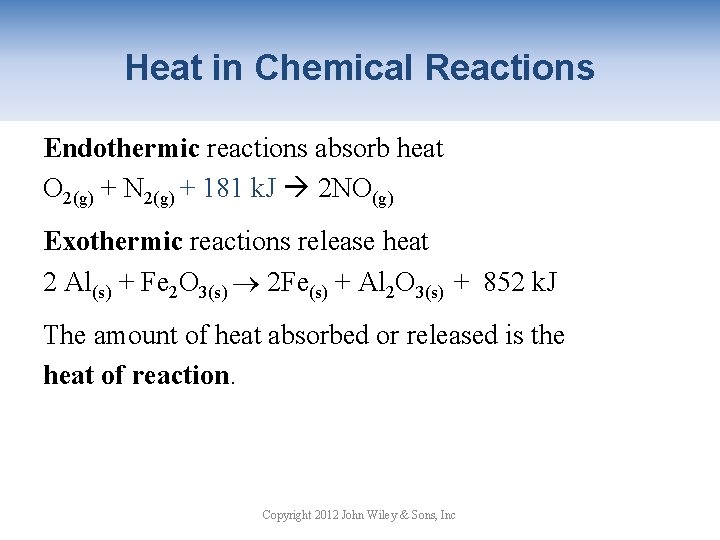
Heat in Chemical Reactions Endothermic reactions absorb heat O 2(g) + N 2(g) + 181 k. J 2 NO(g) Exothermic reactions release heat 2 Al(s) + Fe 2 O 3(s) 2 Fe(s) + Al 2 O 3(s) + 852 k. J The amount of heat absorbed or released is the heat of reaction. Copyright 2012 John Wiley & Sons, Inc

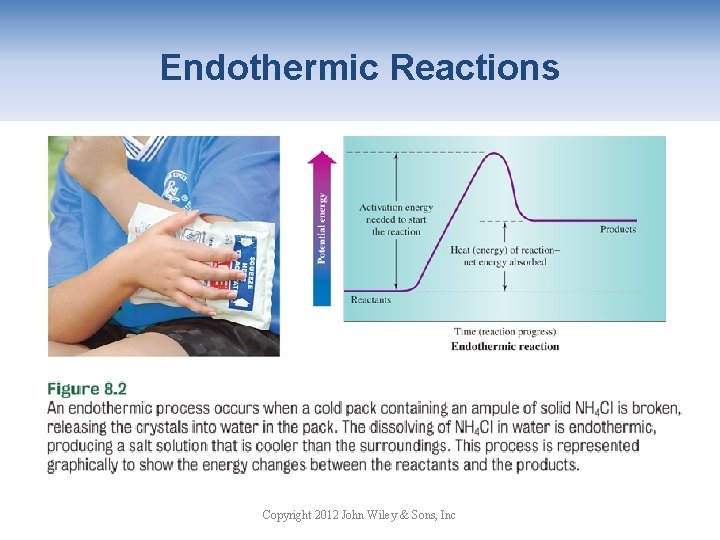
Endothermic Reactions Copyright 2012 John Wiley & Sons, Inc

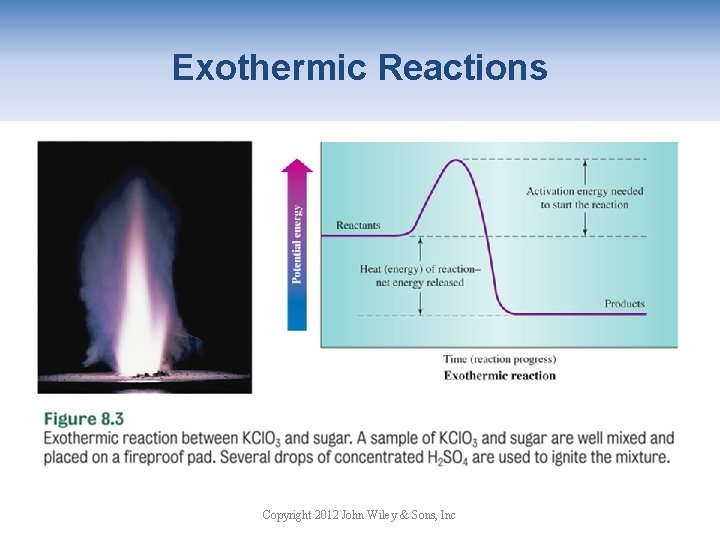
Exothermic Reactions Copyright 2012 John Wiley & Sons, Inc

Your Turn! Consider the reaction: H 2 + I 2 + 12. 6 k. J 2 HI. When one mole of HI is produced A. 12. 6 k. J of energy is absorbed B. 6. 3 k. J of energy is absorbed C. 12. 6 k. J of energy is released D. 6. 3 k. J of energy is released Copyright 2012 John Wiley & Sons, Inc

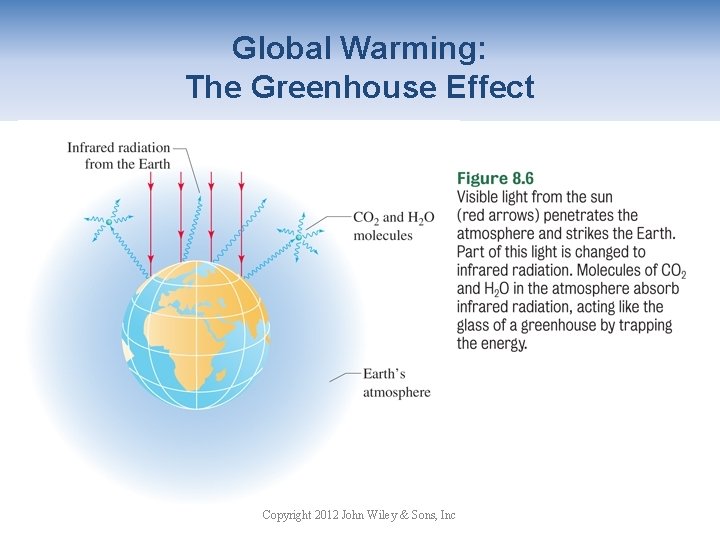
Global Warming: The Greenhouse Effect Copyright 2012 John Wiley & Sons, Inc

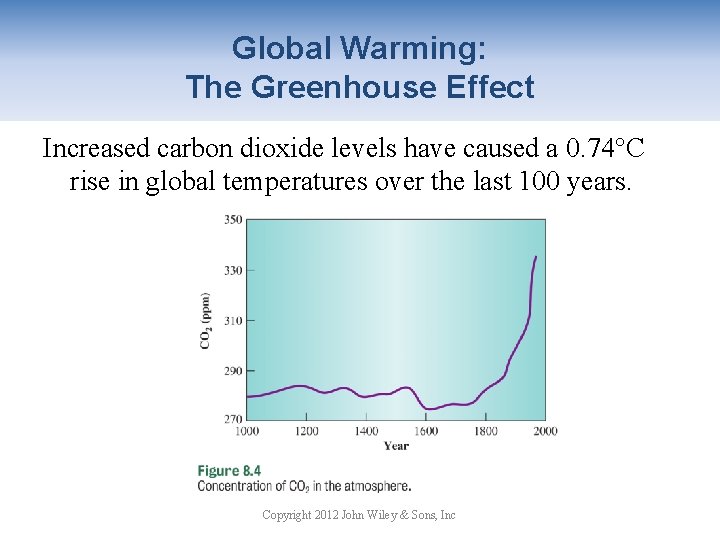
Global Warming: The Greenhouse Effect Increased carbon dioxide levels have caused a 0. 74°C rise in global temperatures over the last 100 years. Copyright 2012 John Wiley & Sons, Inc