CHAPTER 8 Chemical Equations and Reactions Joshua Jo








- Slides: 20

CHAPTER 8 Chemical Equations and Reactions Joshua Jo Bessy chen

Chemical equation represents, with symbols and formulas, the identities and relative amounts of the reactants and products in a chemical reaction. ex. (NH 4)2 Cr 2 O 7(g) N 2(9)+Cr 2 O 3(s)+4 H 2 O(g) Indications Characteristics Significance balancing

Indications Evolutions of heat and light Ex. Ammonium dichromate release energy to produce heat & light Production of a gas Ex. The reaction of vinegar & baking soda Formation of a precipitate Ex. combine of Water solutions of ammonium sulfide & cadmium nitrate (yellow precipitate)

characteristics Word: methane+oxygen carbon dioxide+water Formula: CH 4(g)+2 O 2(g) CO 2(g)+2 H 2 O(g)

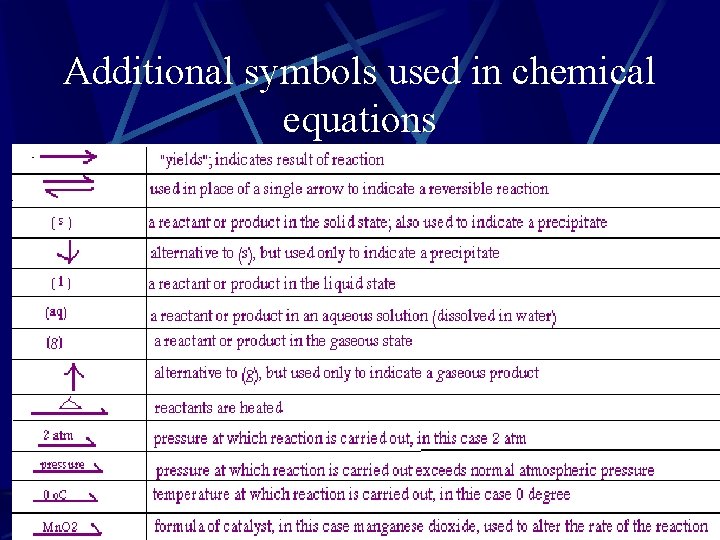
Additional symbols used in chemical equations

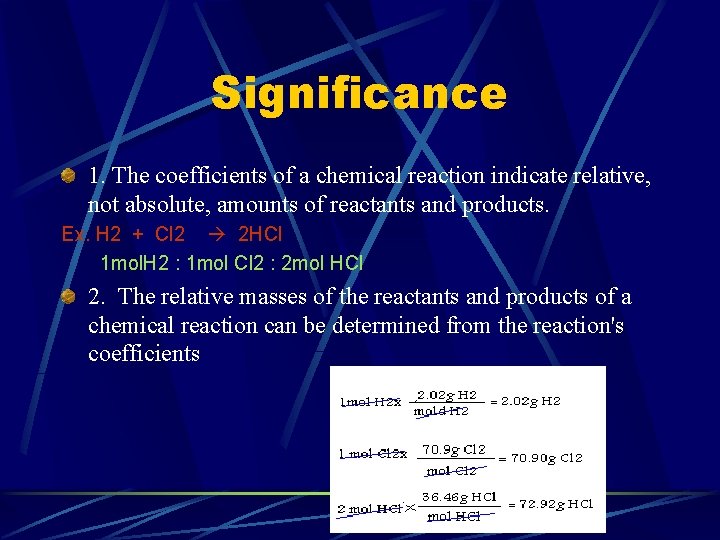
Significance 1. The coefficients of a chemical reaction indicate relative, not absolute, amounts of reactants and products. Ex. H 2 + Cl 2 2 HCl 1 mol. H 2 : 1 mol Cl 2 : 2 mol HCl 2. The relative masses of the reactants and products of a chemical reaction can be determined from the reaction's coefficients

Significance 3. The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction.

Balancing 1. Identify the names of the reactants and the products, and write a word equation. water hydrogen+oxygen 2. Write a formula equation by substituting correct formulas for the names of the reactants and the products H 2 O(l) H 2(g)+O 2(g)

Balancing 3. Count atoms to be sure that the equation is balanced 2 H 2 O(l) 2 H 2(g)+O 2(g) (4 H+2 O) = (4 H) + (2 O) 4. Balance the formula equation according to the law of conservation of mass 2 H 2 O(l) H 2(g)+O 2(g) Balance the different types of atoms one at a time. First balance the atoms of elements that are combined and that appear only once on each side of the equation. Balance polyatomic ions that appear on both sides of the equation as single units. Blance H and O after atoms of all other elements have been balanced.

Types of Chemical Reactions Synthesis Reaction - Two or more substances combine to form a new compound A + B → AB A+B+C → ABC

a. Reactions with Oxygen & Sulfur - Element + Oxygen → ex) 2 Mg(s) Oxide + O 2(g) → 2 Mg. O(s) - Element + Sulfur → Sulfide + S 8(s) → 8 Ba. S(s) b. Reactions with Halogens ex) 8 Ba(s) - Metal + Halogen → ionic compound ex) 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) Mg(s) + F 2(g) → Mg. F 2(s) c. Reactions with Oxides - Active metal oxide + water → Metal hydroxide ex) Ca. O(s) + H 2 O(l) → Ca(OH)2(s) - Nonmetal oxide + water → Oxyacid ex) SO 2(g) + H 2 O(l) → H 2 SO 3(aq)

Decomposition Reactions - A single compound breaks down to form two or more smaller compounds or elements - Most decomposition reactions take place only when energy in the form of electricity (electrolysis) or heat is added AB → A + B ABC → A+B+C

a. Decomposition of a Binary Compound into its element ex) 2 H 2 O(l) → 2 H 2(g) + O 2(g) b. Metal Carbonates - Metal Carbonate → Metal oxide + Carbon Dioxide gas ex) Ca. CO 3(s) → Ca. O(s) + CO 2(g) c. Metal Hydroxides (except those containing Group 1 metals) - Metal Hydroxide → Metal oxide + water ex) Ca(OH)2(s) → Ca. O(s) + H 2 O(g) d. Metal Chlorates - Metal chlorate → Metal chloride + Oxygen ex) 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g)

Single-Replacement Reactions - One element replaces a similar element in a compound - Many Reactions take place in aqueous solution. - More active metal replaces another. - Activity Series A + BX → AX + B Ex) 2 Al(s) + 3 Pb(NO 3)2(aq) → 3 Pb(s) + 2 Al(NO 3)3(aq)

Double-Replacement Reactions - The ions of two compounds exchange places in an aqueous solution to form two new compounds. - One of the compounds formed is usually a precipitate, an insoluble gas, or water. - The other compound remains dissolved in solution. AX + BY → AY + BX

a. Formation of a Precipitate - The cations of one reactant combine with the anions of another reactant to form an insoluble compound. ex) 2 KI(aq) + Pb(NO 3)2(aq) → Pb. I 2(s) + 2 KNO 3(aq) b. Formation of a Gas ex) Fe. S(s) + 2 HCl(aq) → H 2 S(g) + Fe. Cl 2(aq) c. Formation of Water ex) HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l)

Combustion Reactions - A substance combines with oxygen, releasing a large amount of energy in the form of light and heat. X + O 2(g) → XO 2 + heat Ex) C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g)

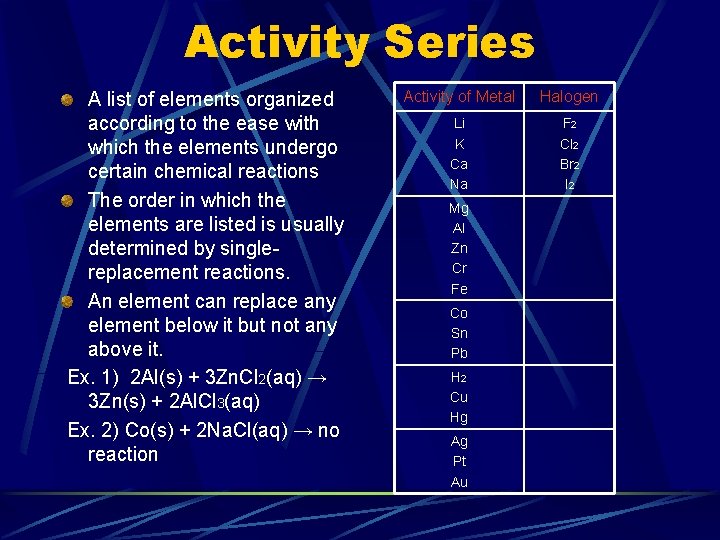
Activity Series A list of elements organized according to the ease with which the elements undergo certain chemical reactions The order in which the elements are listed is usually determined by singlereplacement reactions. An element can replace any element below it but not any above it. Ex. 1) 2 Al(s) + 3 Zn. Cl 2(aq) → 3 Zn(s) + 2 Al. Cl 3(aq) Ex. 2) Co(s) + 2 Na. Cl(aq) → no reaction Activity of Metal Halogen Li K Ca Na F 2 Cl 2 Br 2 I 2 Mg Al Zn Cr Fe Co Sn Pb H 2 Cu Hg Ag Pt Au

Animations http: //www. sci. brooklyn. cuny. edu/~marc iano/labs. htm

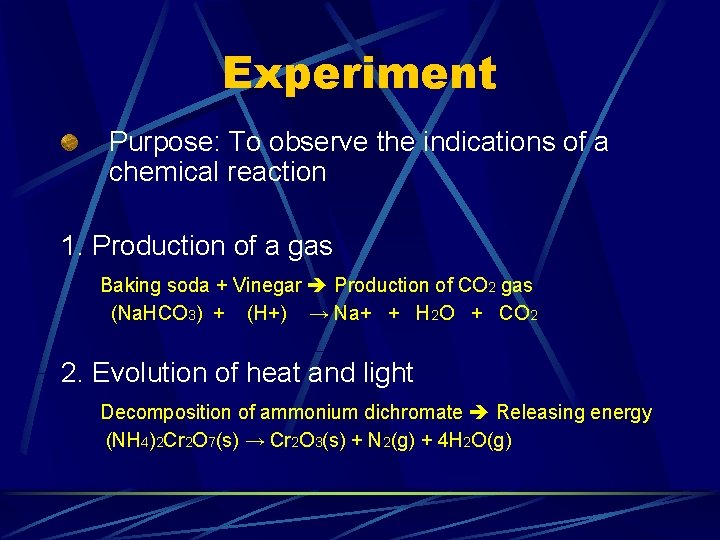
Experiment Purpose: To observe the indications of a chemical reaction 1. Production of a gas Baking soda + Vinegar Production of CO 2 gas (Na. HCO 3) + (H+) → Na+ + H 2 O + CO 2 2. Evolution of heat and light Decomposition of ammonium dichromate Releasing energy (NH 4)2 Cr 2 O 7(s) → Cr 2 O 3(s) + N 2(g) + 4 H 2 O(g)