Chapter 8 CHEMICAL BONDING PART 2 Resonance Only

Chapter 8 CHEMICAL BONDING PART 2

Resonance Only occurs with double and triple bonds What is it? A sharing of a double bond between atoms
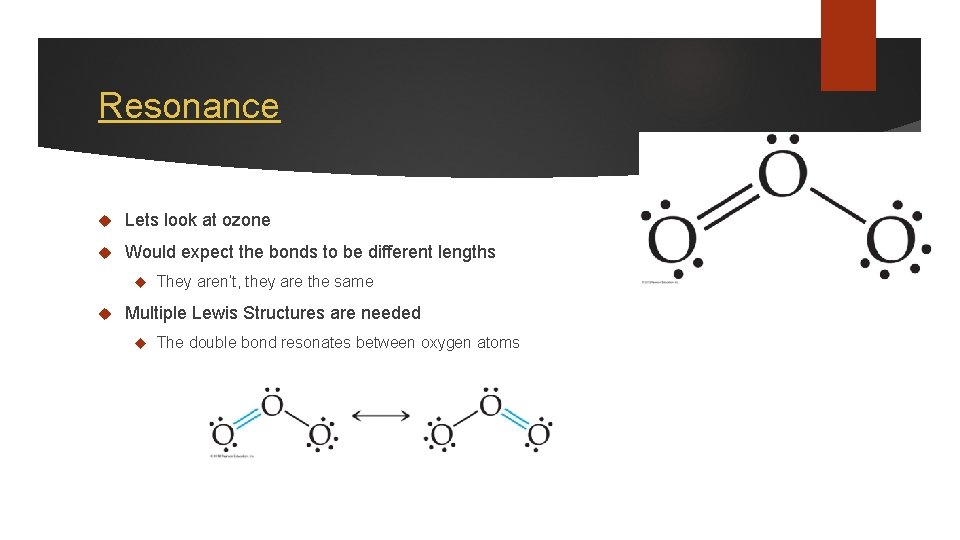
Resonance Lets look at ozone Would expect the bonds to be different lengths They aren’t, they are the same Multiple Lewis Structures are needed The double bond resonates between oxygen atoms
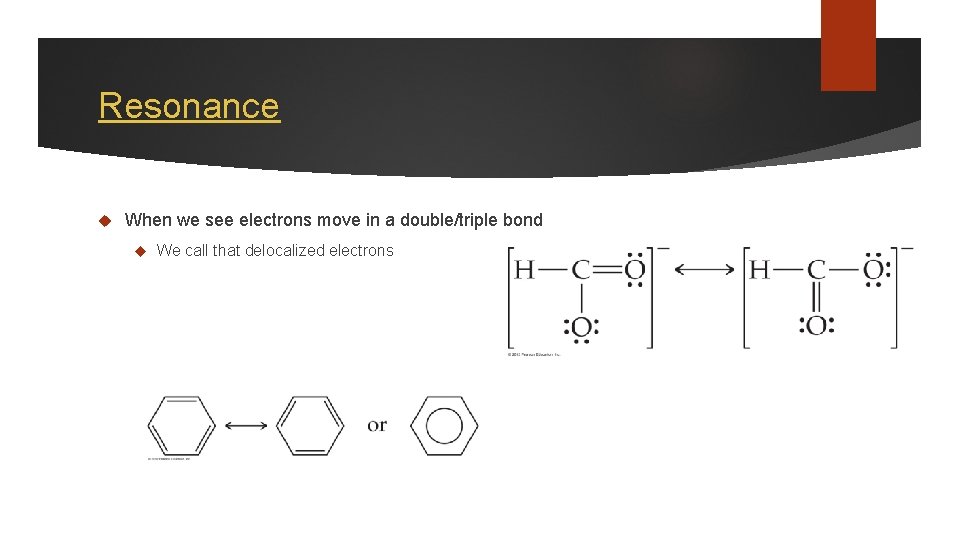
Resonance When we see electrons move in a double/triple bond We call that delocalized electrons

You Try Draw all of the Lewis Structures for the formate ion, HCO 2 -1

Exceptions to the Octet Rule Three types of ions/molecules that do not follow the octet Odd number of electrons Less than an octet More than eight valence electrons (an expanded octet)

Odd Number of Electrons Very rare and very unstable
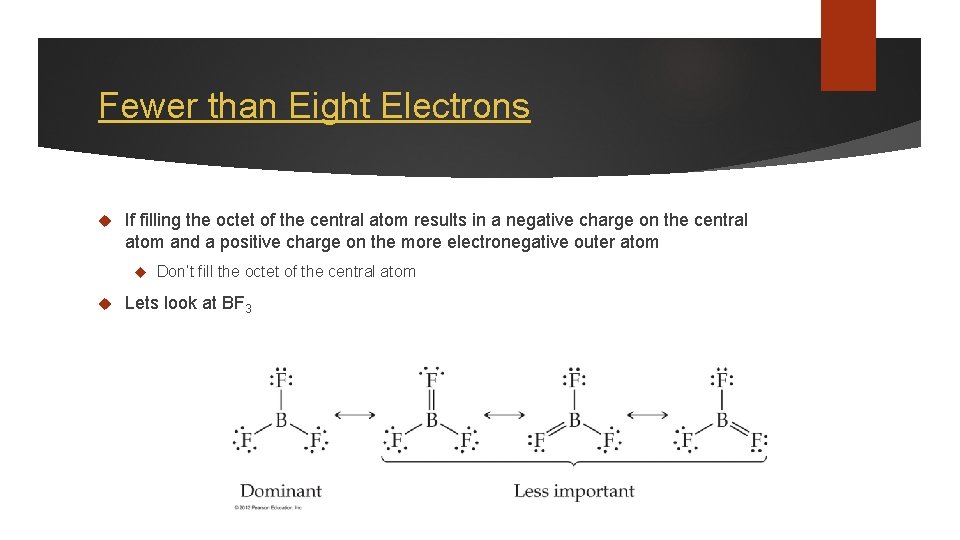
Fewer than Eight Electrons If filling the octet of the central atom results in a negative charge on the central atom and a positive charge on the more electronegative outer atom Don’t fill the octet of the central atom Lets look at BF 3
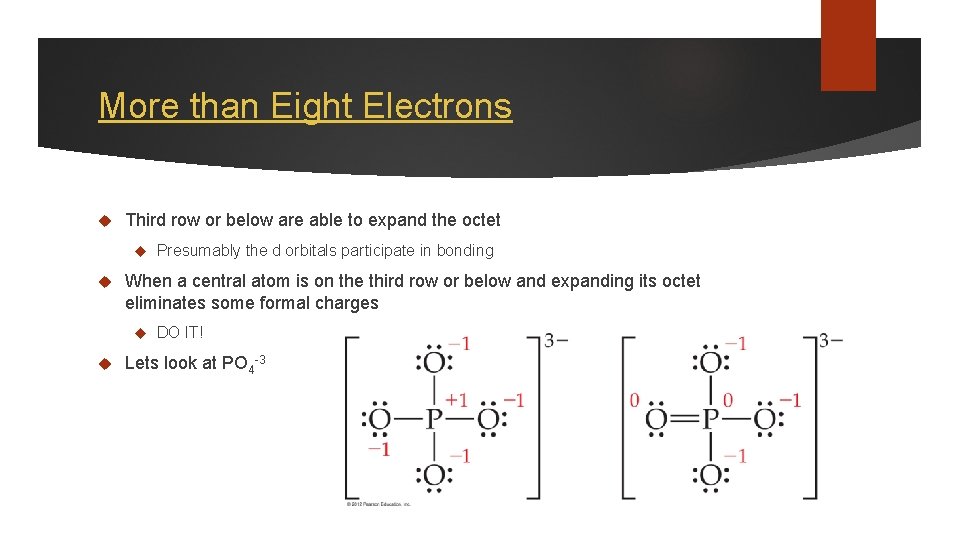
More than Eight Electrons Third row or below are able to expand the octet When a central atom is on the third row or below and expanding its octet eliminates some formal charges Presumably the d orbitals participate in bonding DO IT! Lets look at PO 4 -3

Covalent Bond Strength is measured by determining how much energy is needed to break the bond Called bond enthalpy Bond enthalpies are always positive…. Why? Bond enthalpies are reported as averages…. Why?
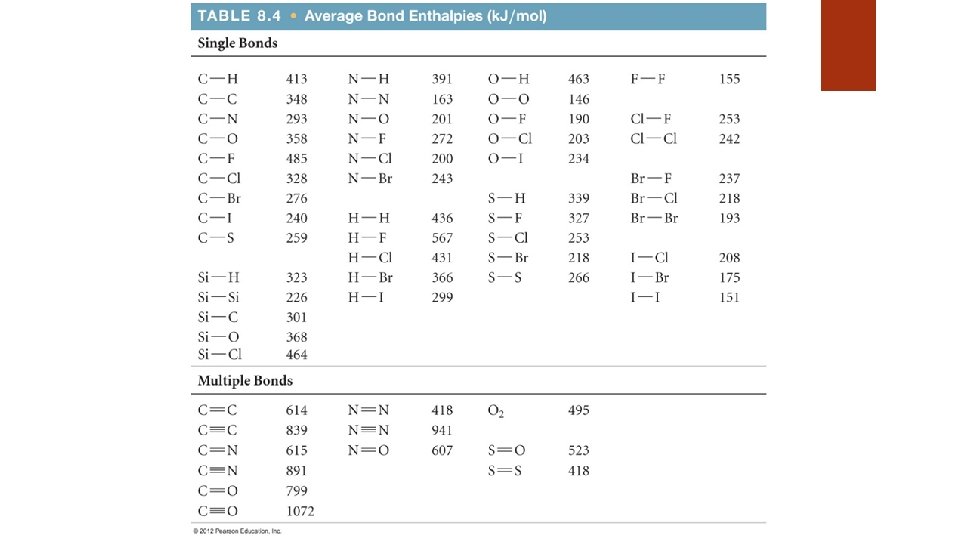
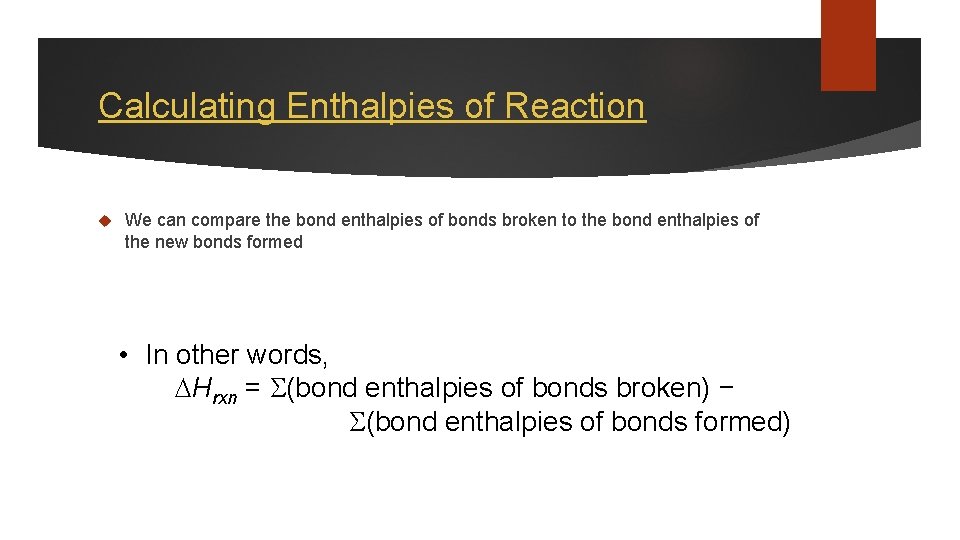
Calculating Enthalpies of Reaction We can compare the bond enthalpies of bonds broken to the bond enthalpies of the new bonds formed • In other words, Hrxn = (bond enthalpies of bonds broken) − (bond enthalpies of bonds formed)

Example Calculate ΔH for the following reaction CH 4(g) + Cl 2(g) CH 3 Cl (g) + HCl(g)

You Try Calculate ΔH for the burning of ethane. -2831 k. J
- Slides: 14