Chapter 8 Bulk Electrolysis Electrogravimetry and Coulometry F

Chapter 8 Bulk Electrolysis: Electrogravimetry and Coulometry
F 8 A Electrolytical Analysis F 8 B Electrogravimetric Methods F 8 C Coulometry F 8 D Other Coulometric Methods

What are electrolytical analysis and coulometry? André Marie Ampère (1775 -1863)
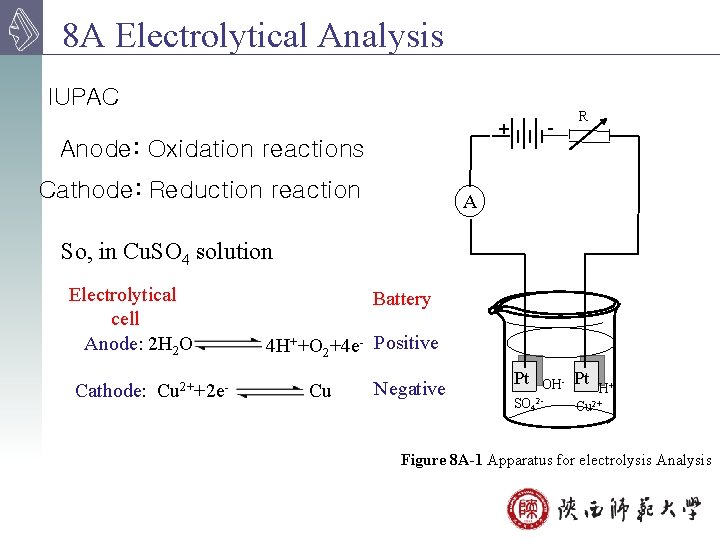
8 A Electrolytical Analysis IUPAC - + Anode: Oxidation reactions Cathode: Reduction reaction R A So, in Cu. SO 4 solution Electrolytical cell Anode: 2 H 2 O Cathode: Cu 2++2 e- Battery 4 H++O 2+4 e- Positive Cu Negative Pt SO 4 OH 2 - Pt H+ Cu 2+ Figure 8 A-1 Apparatus for electrolysis Analysis
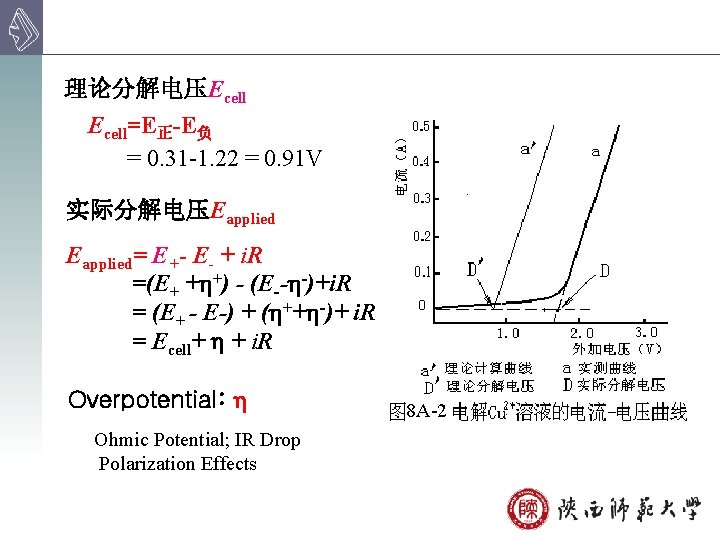
理论分解电压Ecell=E正-E负 = 0. 31 -1. 22 = 0. 91 V 实际分解电压Eapplied= E+- E- + i. R =(E+ + +) - (E-- -)+i. R = (E+ - E-) + ( ++ -)+ i. R = Ecell+ + i. R Overpotential: Ohmic Potential; IR Drop Polarization Effects 8 A-2
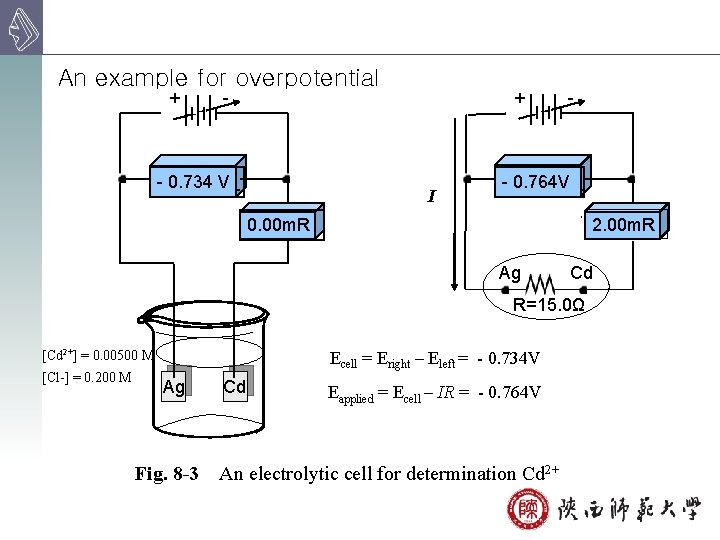
An example for overpotential + + - - 0. 734 V - I - - 0. 764 V 2. 00 m. R 0. 00 m. R Ag Cd R=15. 0Ω [Cd 2+] = 0. 00500 M [Cl-] = 0. 200 M Ecell = Eright – Eleft = - 0. 734 V Ag Fig. 8 -3 Cd Eapplied = Ecell – IR = - 0. 764 V An electrolytic cell for determination Cd 2+
The metal is deposited on a weighed platinum or other metal cathode, and the increase in mass is determined. Controlled-current electrolytical analysis Controlled-potential electrolytical analysis
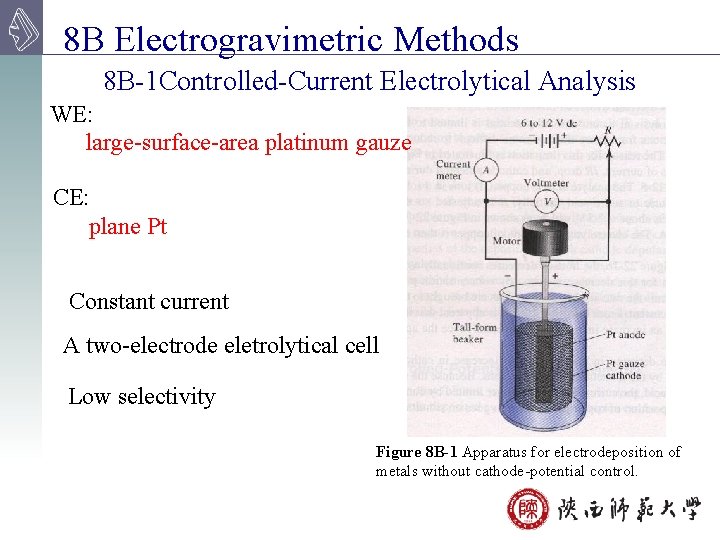
8 B Electrogravimetric Methods 8 B-1 Controlled-Current Electrolytical Analysis WE: large-surface-area platinum gauze CE: plane Pt Constant current A two-electrode eletrolytical cell Low selectivity Figure 8 B-1 Apparatus for electrodeposition of metals without cathode-potential control.
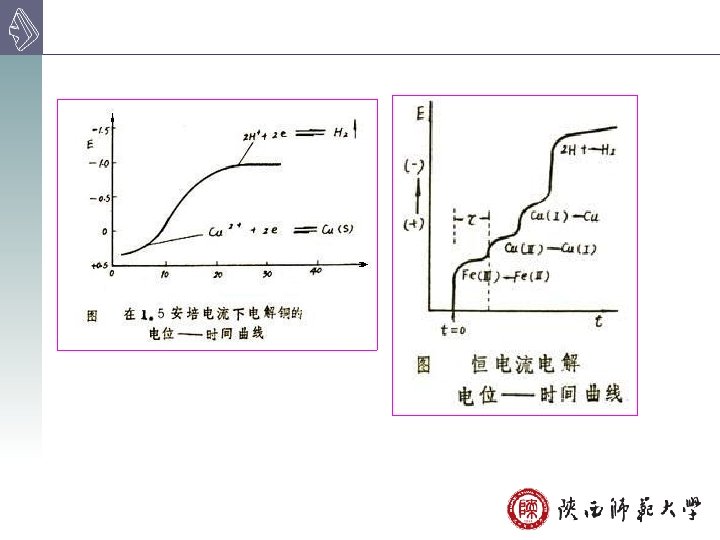
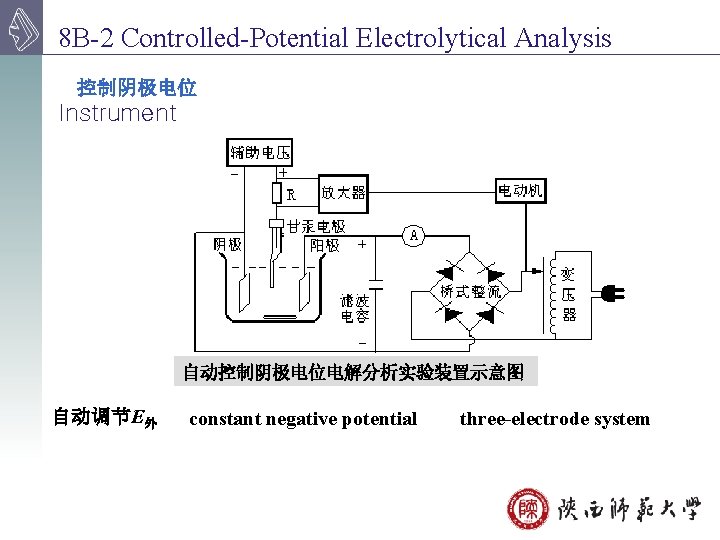
8 B-2 Controlled-Potential Electrolytical Analysis 控制阴极电位 Instrument 自动控制阴极电位电解分析实验装置示意图 自动调节E外 constant negative potential three-electrode system
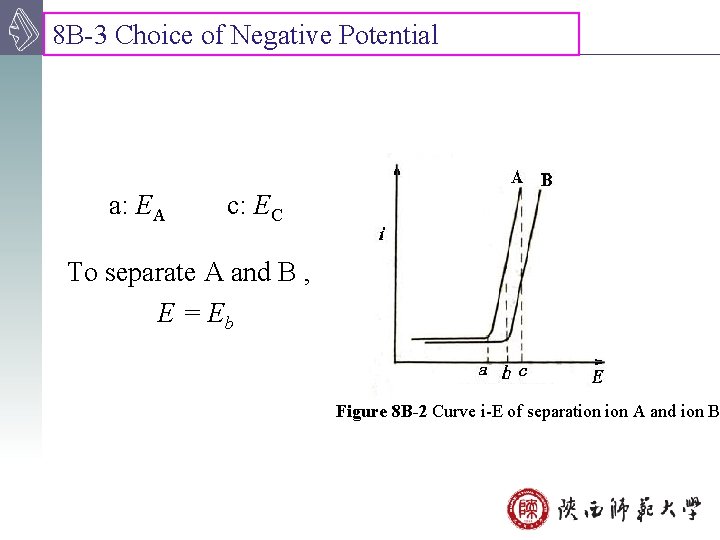
8 B-3 Choice of Negative Potential a: EA c: EC To separate A and B , E = Eb Figure 8 B-2 Curve i-E of separation A and ion B
Character and application Good selectivity Low speed For example: Seperation of Cu and Bi, Sb, Pb, Sn, Ni, Cd, Zn Seperation of Pb and Cd, Zn, Ni, Zn, Mn, Al, Fe
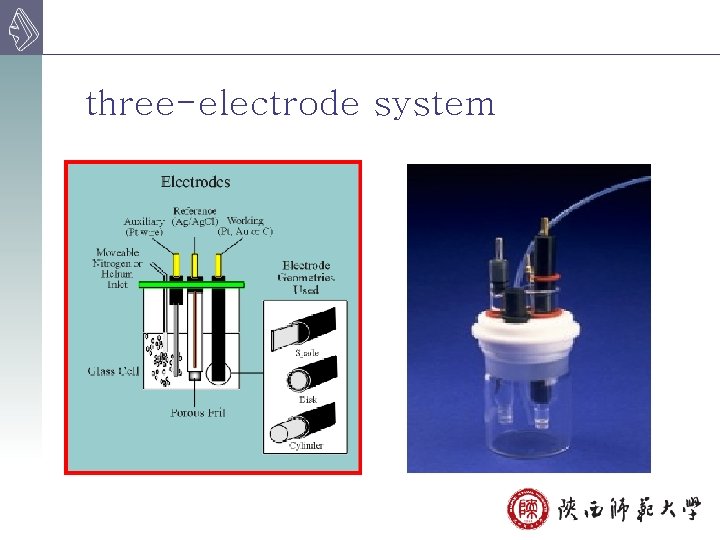
three-electrode system
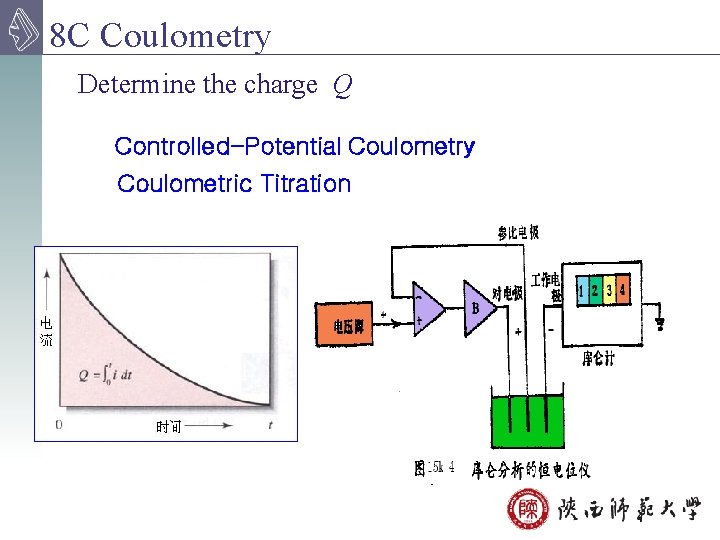
8 C Coulometry Determine the charge Q Controlled-Potential Coulometry Coulometric Titration
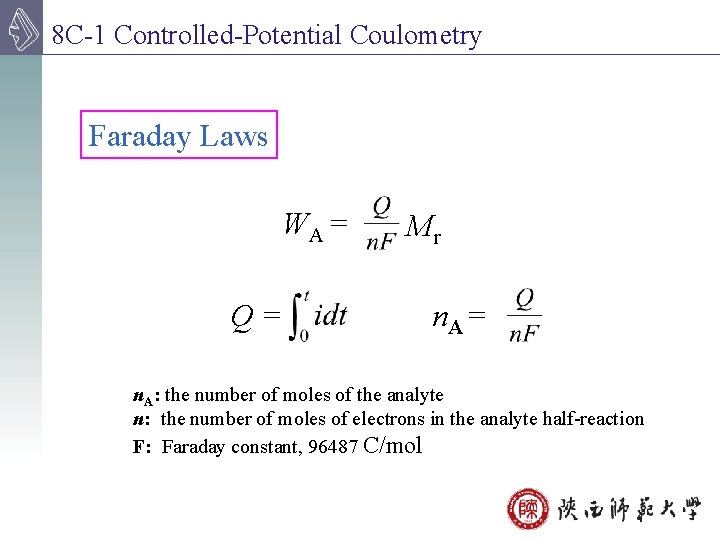
8 C-1 Controlled-Potential Coulometry Faraday Laws WA = Q= Mr n. A = n. A: the number of moles of the analyte n: the number of moles of electrons in the analyte half-reaction F: Faraday constant, 96487 C/mol
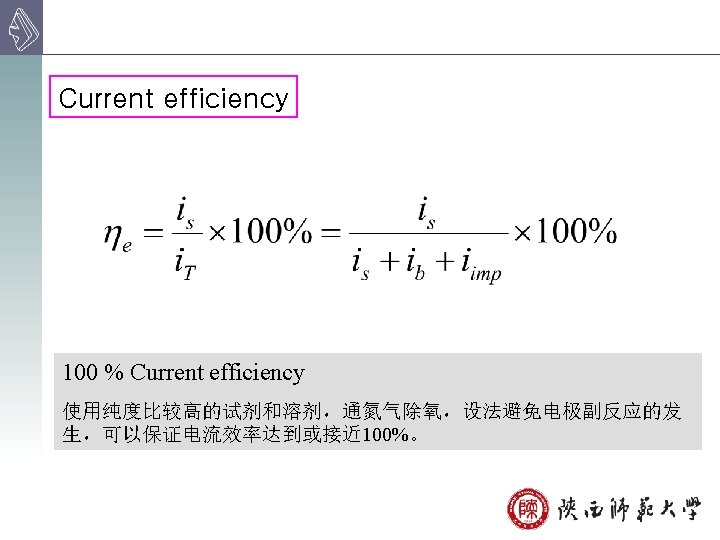
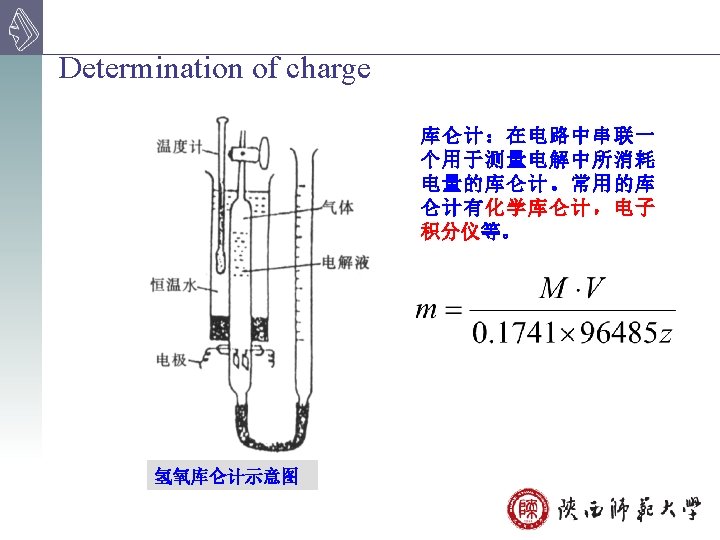

Advantage: accurate, sensitivity, good selectivity Disadvantage: difficult to ensure 100% current efficiency need long time Application: determine mixtures study the electrode process, and the mechanism of various reactions
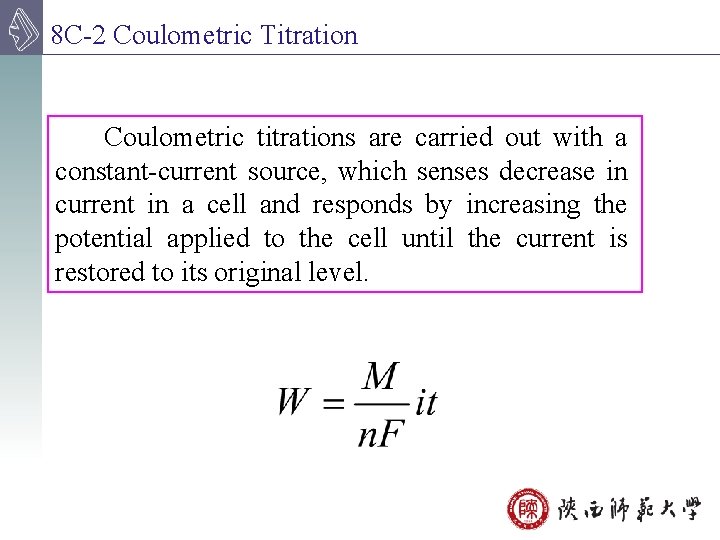
8 C-2 Coulometric Titration Coulometric titrations are carried out with a constant-current source, which senses decrease in current in a cell and responds by increasing the potential applied to the cell until the current is restored to its original level.
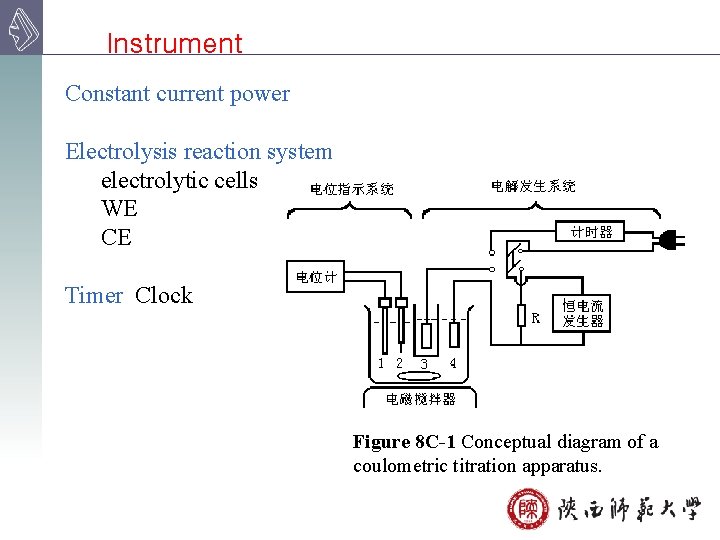
Instrument Constant current power Electrolysis reaction system electrolytic cells WE CE Timer Clock Figure 8 C-1 Conceptual diagram of a coulometric titration apparatus.
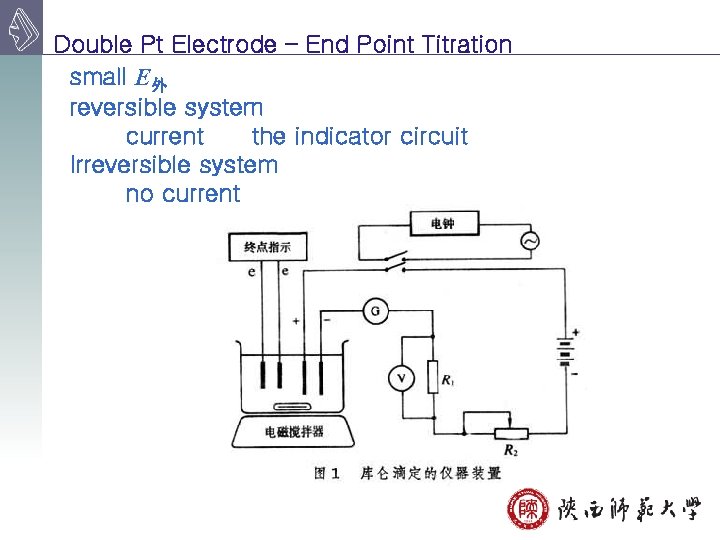
Double Pt Electrode – End Point Titration small E外 reversible system current the indicator circuit Irreversible system no current
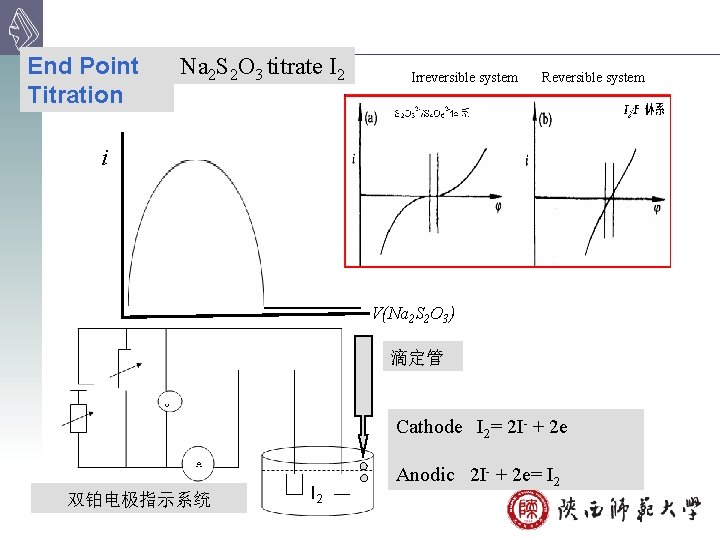
End Point Titration Na 2 S 2 O 3 titrate I 2 Irreversible system Reversible system i V(Na 2 S 2 O 3) 滴定管 Cathode I 2= 2 I- + 2 e 双铂电极指示系统 I 2 Anodic 2 I- + 2 e= I 2
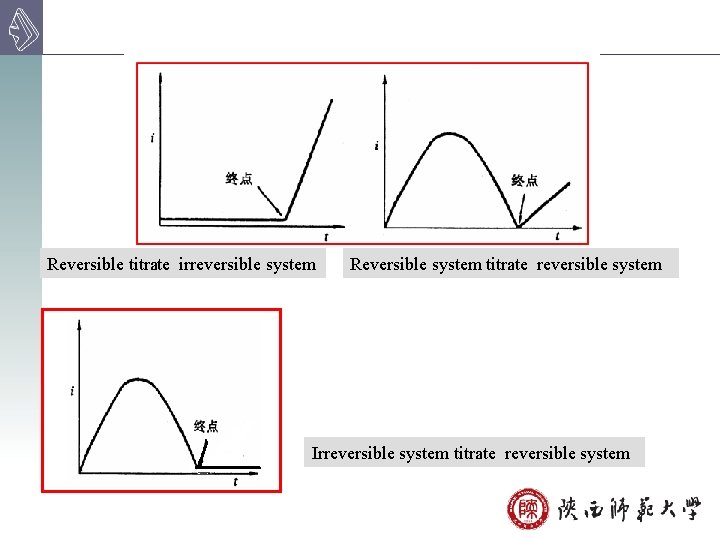
Reversible titrate irreversible system Reversible system titrate reversible system Irreversible system titrate reversible system

Character and Application High accuracy High sensitivity 10 -5~10 -9 g/m. L In situ produce unstable regents No standard solutions
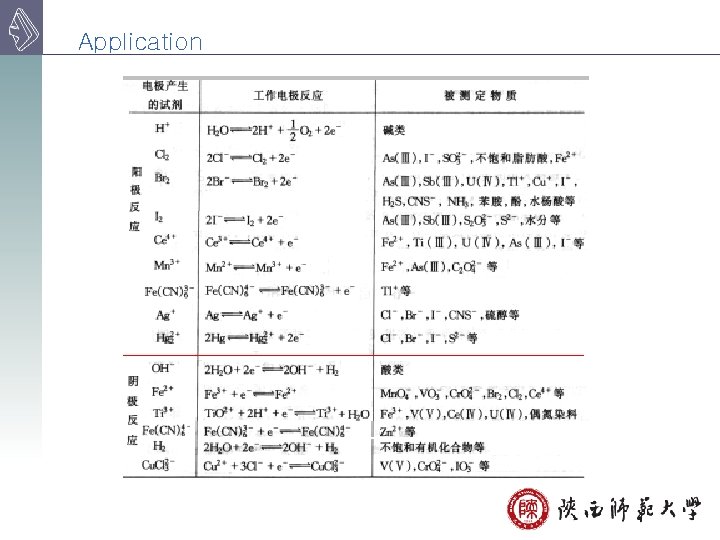
Application
8 D Other Coulometric Methods Automated coulometric titration Determination of COD Microcoulometric analysis Sensitive Fast speed Convenient
- Slides: 26