Chapter 8 Basic Concepts of Chemical Bonding Copyright

Chapter 8 Basic Concepts of Chemical Bonding Copyright © 2018, 2015, 2012 Pearson Education, Inc. All Rights Reserved
Chemical Bonds • A chemical bond is a strong attractive force between atoms or ions. • There are three basic types of bonds ØIonic: Electrostatic attraction between ions ØCovalent: Sharing of electrons between atoms ØMetallic: Free electrons hold metal atoms together 2
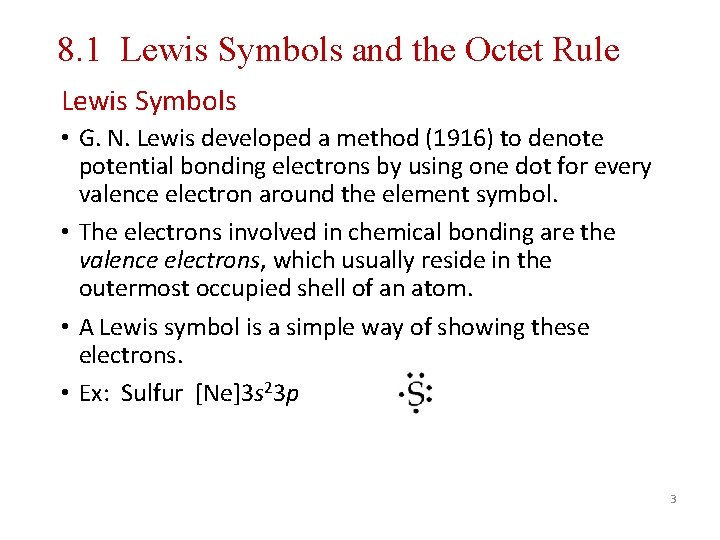
8. 1 Lewis Symbols and the Octet Rule Lewis Symbols • G. N. Lewis developed a method (1916) to denote potential bonding electrons by using one dot for every valence electron around the element symbol. • The electrons involved in chemical bonding are the valence electrons, which usually reside in the outermost occupied shell of an atom. • A Lewis symbol is a simple way of showing these electrons. • Ex: Sulfur [Ne]3 s 23 p 3
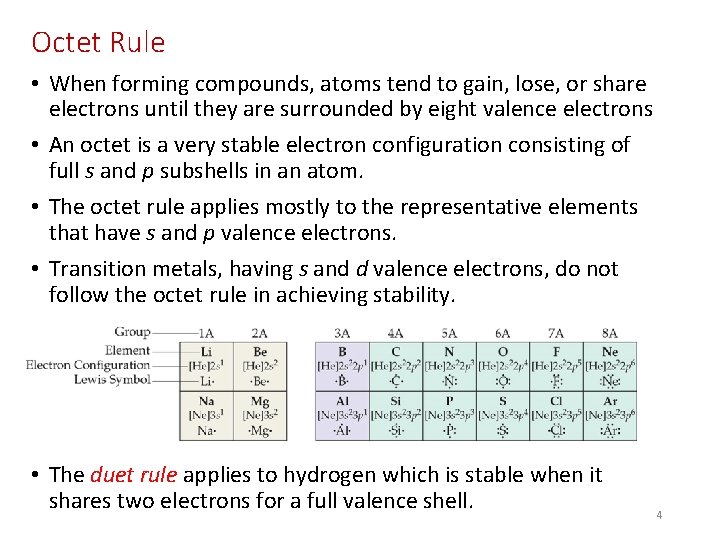
Octet Rule • When forming compounds, atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons • An octet is a very stable electron configuration consisting of full s and p subshells in an atom. • The octet rule applies mostly to the representative elements that have s and p valence electrons. • Transition metals, having s and d valence electrons, do not follow the octet rule in achieving stability. • The duet rule applies to hydrogen which is stable when it shares two electrons for a full valence shell. 4
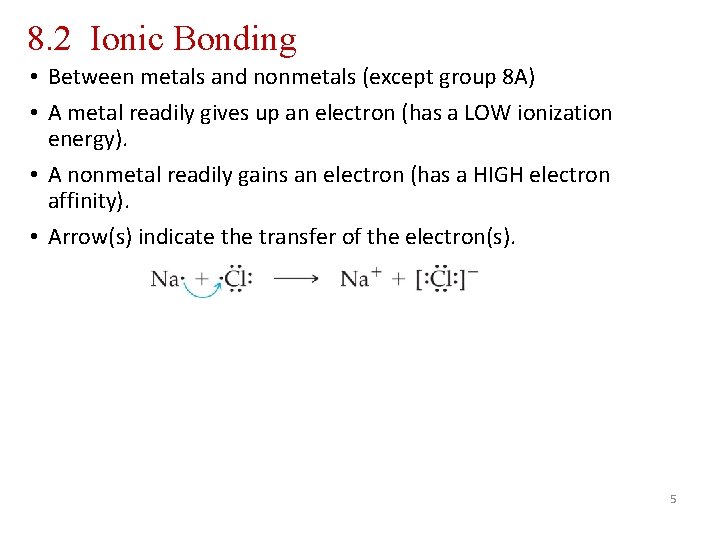
8. 2 Ionic Bonding • Between metals and nonmetals (except group 8 A) • A metal readily gives up an electron (has a LOW ionization energy). • A nonmetal readily gains an electron (has a HIGH electron affinity). • Arrow(s) indicate the transfer of the electron(s). 5
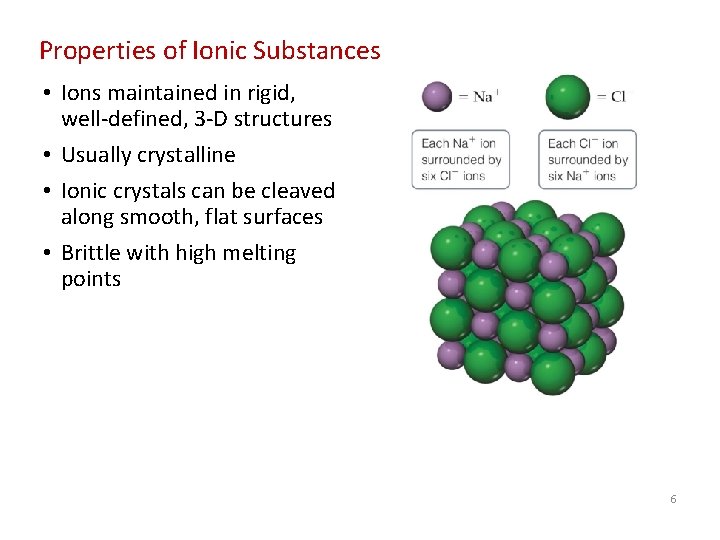
Properties of Ionic Substances • Ions maintained in rigid, well-defined, 3 -D structures • Usually crystalline • Ionic crystals can be cleaved along smooth, flat surfaces • Brittle with high melting points 6
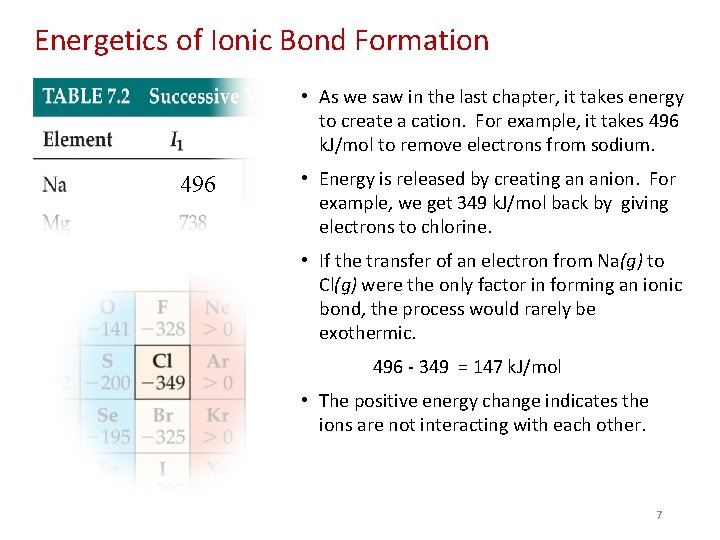
Energetics of Ionic Bond Formation • As we saw in the last chapter, it takes energy to create a cation. For example, it takes 496 k. J/mol to remove electrons from sodium. 496 • Energy is released by creating an anion. For example, we get 349 k. J/mol back by giving electrons to chlorine. • If the transfer of an electron from Na(g) to Cl(g) were the only factor in forming an ionic bond, the process would rarely be exothermic. 496 - 349 = 147 k. J/mol • The positive energy change indicates the ions are not interacting with each other. 7
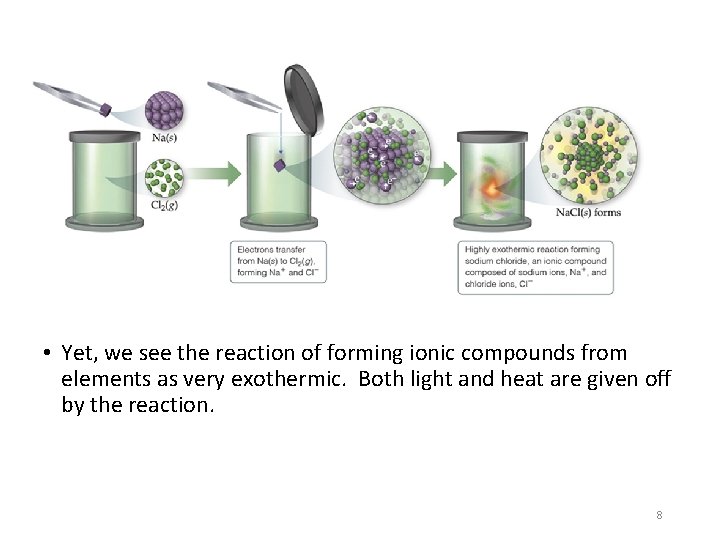
• Yet, we see the reaction of forming ionic compounds from elements as very exothermic. Both light and heat are given off by the reaction. 8
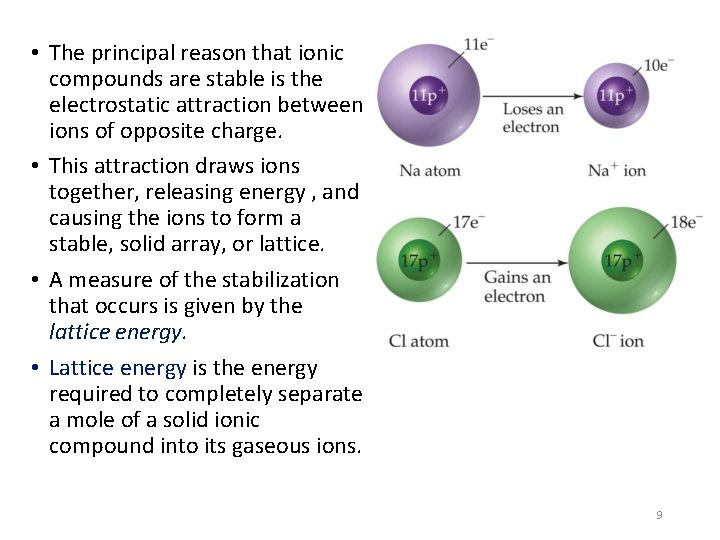
• The principal reason that ionic compounds are stable is the electrostatic attraction between ions of opposite charge. • This attraction draws ions together, releasing energy , and causing the ions to form a stable, solid array, or lattice. • A measure of the stabilization that occurs is given by the lattice energy. • Lattice energy is the energy required to completely separate a mole of a solid ionic compound into its gaseous ions. 9

• For Na. Cl, ΔHlattice = +788 k. J/mol • This process is highly endothermic. The reverse process – the formation of Na. Cl – is highly exothermic: ΔH = -788 k. J/mol • The energy released by the attraction of ions of unlike charge more than makes up for the endothermic nature of ionization energies, making the formation of ionic compounds from elements an exothermic process. 10
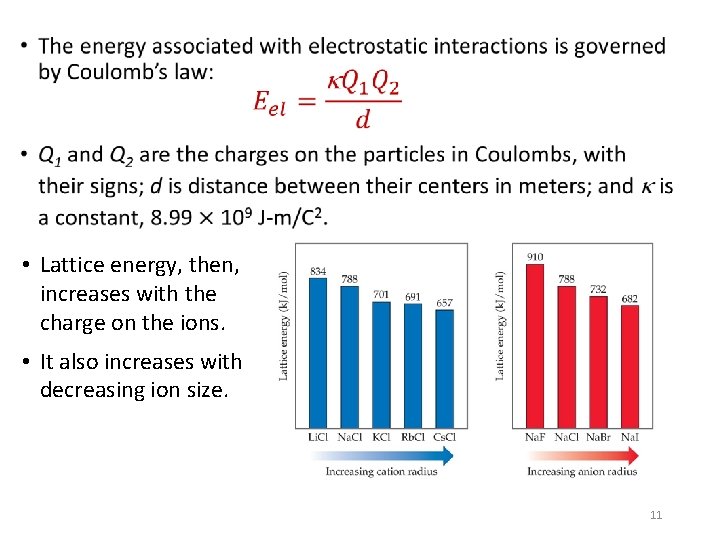
• • Lattice energy, then, increases with the charge on the ions. • It also increases with decreasing ion size. 11
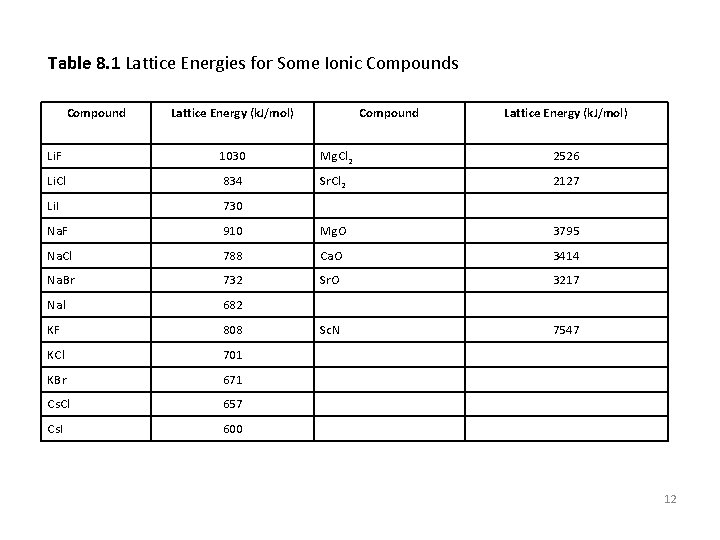
Table 8. 1 Lattice Energies for Some Ionic Compounds Compound Lattice Energy (k. J/mol) Li. F 1030 M g C l 2 2526 Li. Cl 834 S r C l 2 2127 Li. I 730 Blank blank Na. F 910 Mg. O 3795 Na. Cl 788 Ca O 3414 Na. B r 732 Sr. O 3217 N al 682 Blank KF 808 Sc. N KCl 701 blank KBr 671 blank Cs Cl 657 blank Cs I 600 blank blank 7547 12
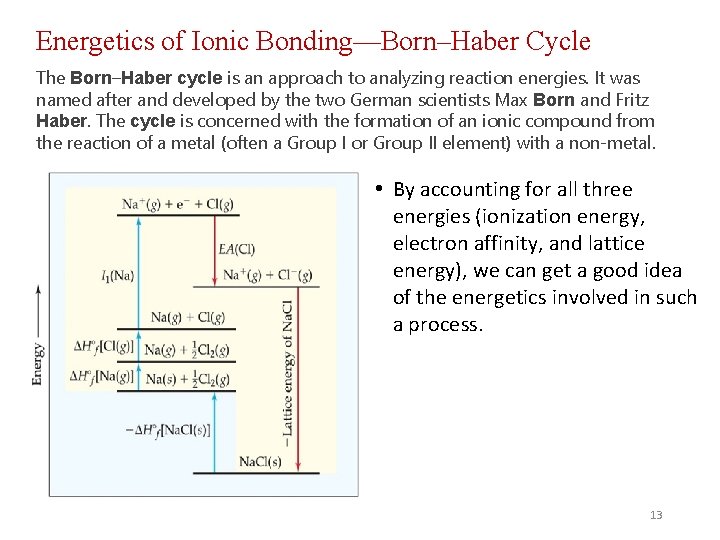
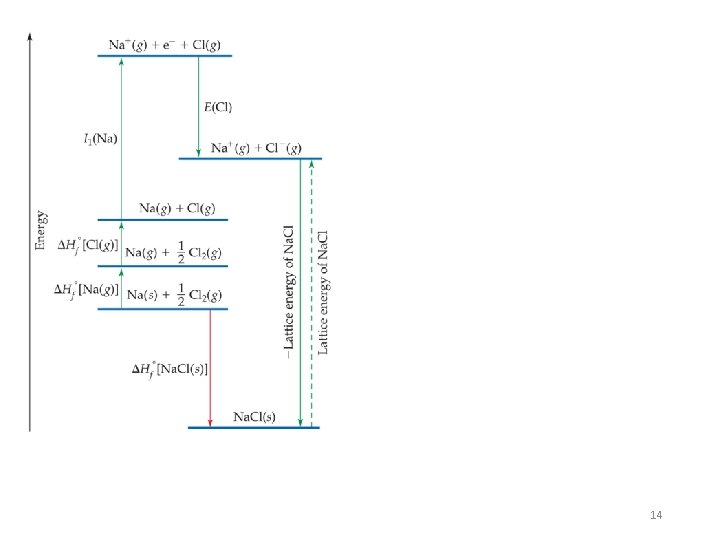
Energetics of Ionic Bonding—Born–Haber Cycle The Born–Haber cycle is an approach to analyzing reaction energies. It was named after and developed by the two German scientists Max Born and Fritz Haber. The cycle is concerned with the formation of an ionic compound from the reaction of a metal (often a Group I or Group II element) with a non-metal. • By accounting for all three energies (ionization energy, electron affinity, and lattice energy), we can get a good idea of the energetics involved in such a process. 13
14

Electron Configuration of Ions of the s- and p-Block Elements. • Main group metals lose electrons, resulting in the electron configuration of the previous noble gas. • Nonmetals gain electrons, resulting in the electron configuration of the nearest noble gas. Transition Metal Ions • Transition metals do Not follow the Octet rule. • Transition metals lose the Valence electrons First, Then lose the d- electrons necessary for the given ion charge. 15
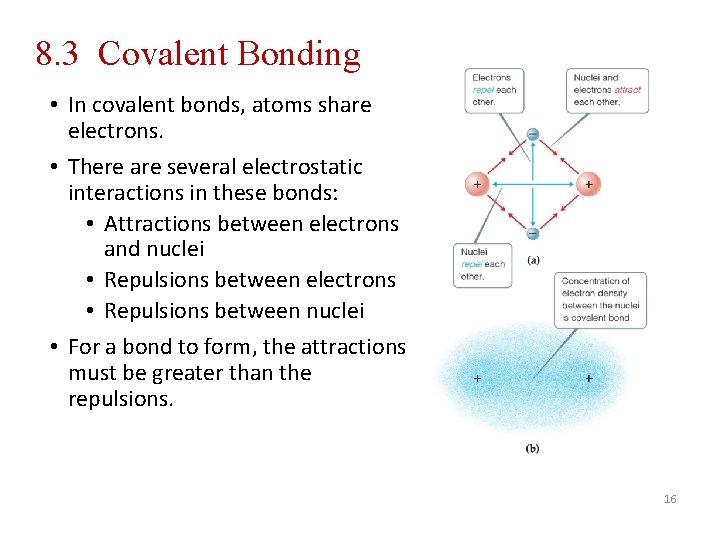
8. 3 Covalent Bonding • In covalent bonds, atoms share electrons. • There are several electrostatic interactions in these bonds: • Attractions between electrons and nuclei • Repulsions between electrons • Repulsions between nuclei • For a bond to form, the attractions must be greater than the repulsions. 16
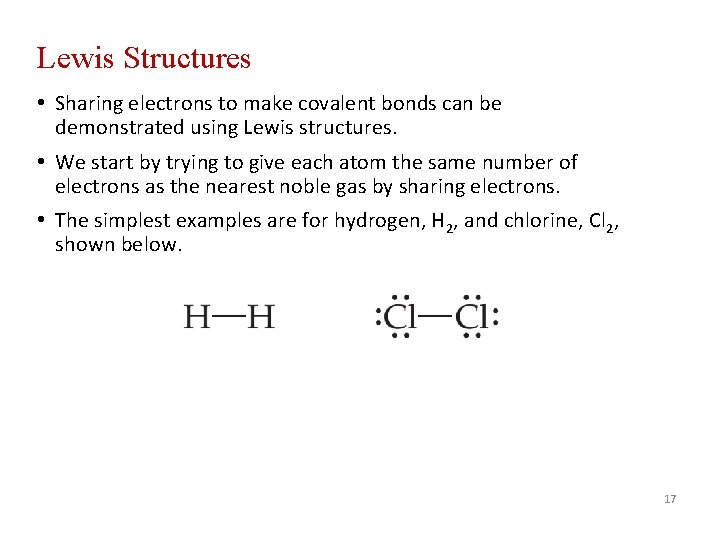
Lewis Structures • Sharing electrons to make covalent bonds can be demonstrated using Lewis structures. • We start by trying to give each atom the same number of electrons as the nearest noble gas by sharing electrons. • The simplest examples are for hydrogen, H 2, and chlorine, Cl 2, shown below. 17
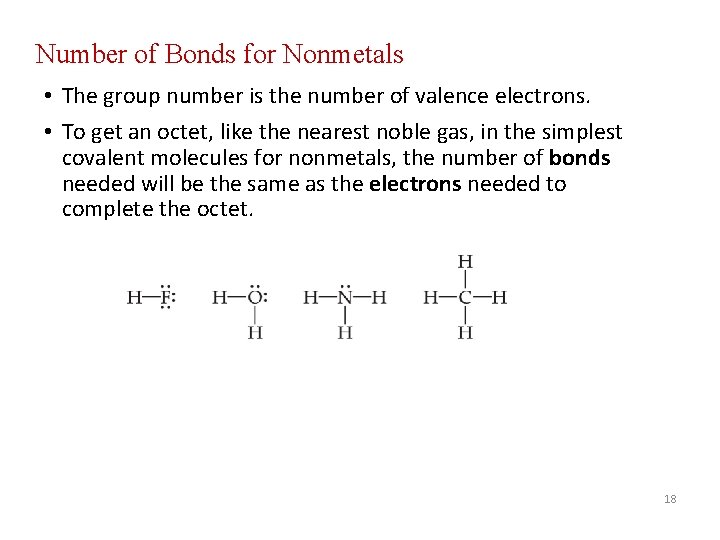
Number of Bonds for Nonmetals • The group number is the number of valence electrons. • To get an octet, like the nearest noble gas, in the simplest covalent molecules for nonmetals, the number of bonds needed will be the same as the electrons needed to complete the octet. 18
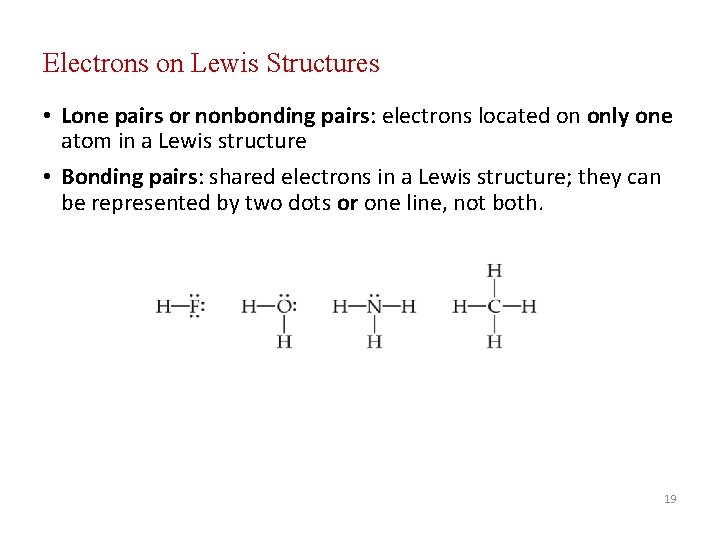
Electrons on Lewis Structures • Lone pairs or nonbonding pairs: electrons located on only one atom in a Lewis structure • Bonding pairs: shared electrons in a Lewis structure; they can be represented by two dots or one line, not both. 19
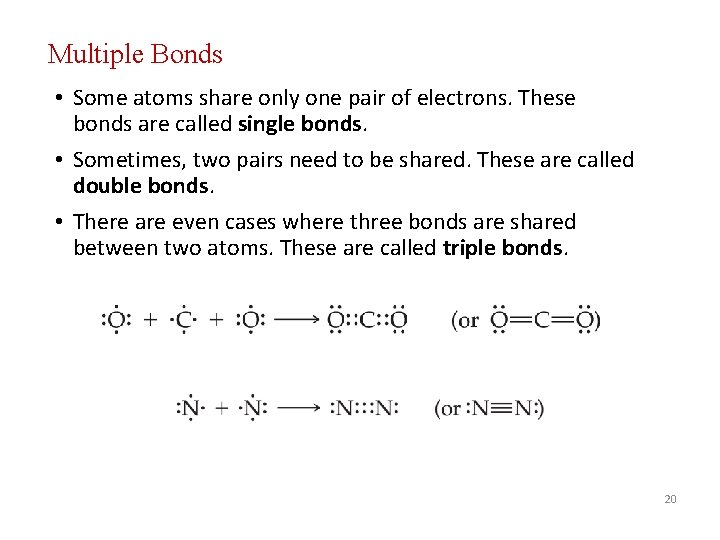
Multiple Bonds • Some atoms share only one pair of electrons. These bonds are called single bonds. • Sometimes, two pairs need to be shared. These are called double bonds. • There are even cases where three bonds are shared between two atoms. These are called triple bonds. 20
8. 4 Bond Polarity and Electronegativity • The electrons in a covalent bond are not always shared equally. • Bond polarity is a measure of how equally or unequally the electrons in a covalent bond are shared. • In a nonpolar covalent bond, the electrons are shared equally. • In a polar covalent bond, one of the atoms attracts electrons to itself with a greater force. 21
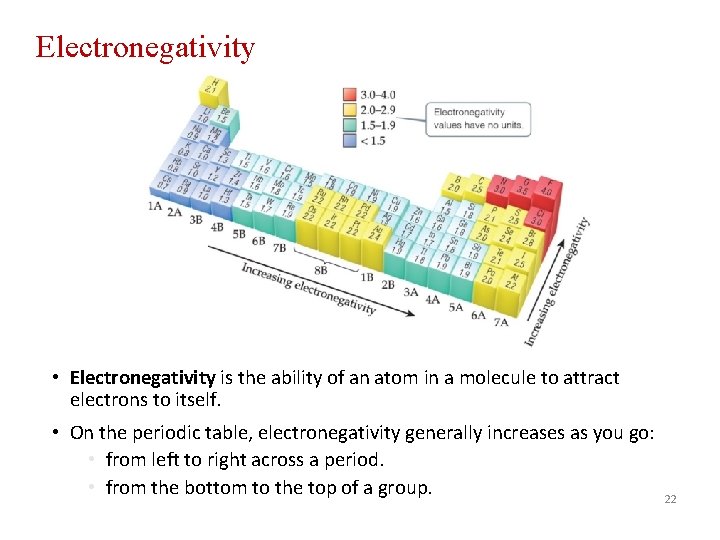
Electronegativity • Electronegativity is the ability of an atom in a molecule to attract electrons to itself. • On the periodic table, electronegativity generally increases as you go: • from left to right across a period. • from the bottom to the top of a group. 22
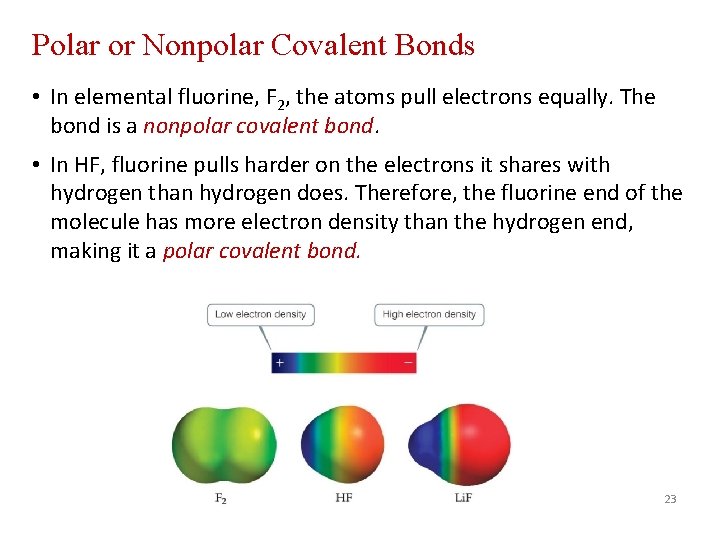
Polar or Nonpolar Covalent Bonds • In elemental fluorine, F 2, the atoms pull electrons equally. The bond is a nonpolar covalent bond. • In HF, fluorine pulls harder on the electrons it shares with hydrogen than hydrogen does. Therefore, the fluorine end of the molecule has more electron density than the hydrogen end, making it a polar covalent bond. 23
Electronegativity and Polar Covalent Bonds • When two atoms share electrons unequally, a polar covalent bond results. • Electrons tend to spend more time around the more electronegative atom. The result is a partial negative charge (not a complete transfer of charge). It is represented by • The other atom is “more positive, ” or 24
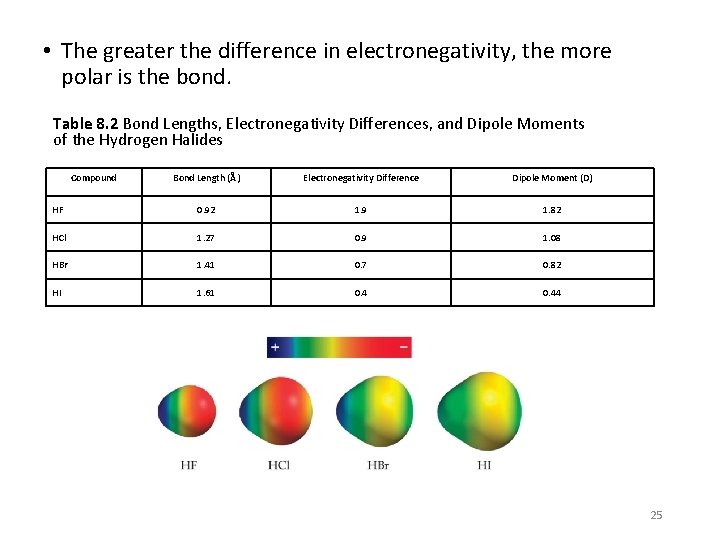
• The greater the difference in electronegativity, the more polar is the bond. Table 8. 2 Bond Lengths, Electronegativity Differences, and Dipole Moments of the Hydrogen Halides Compound Bond Length (Å) Electronegativity Difference Dipole Moment (D) HF 0. 92 1. 9 1. 82 HCl 1. 27 0. 9 1. 08 HBr 1. 41 0. 7 0. 82 HI 1. 61 0. 44 25
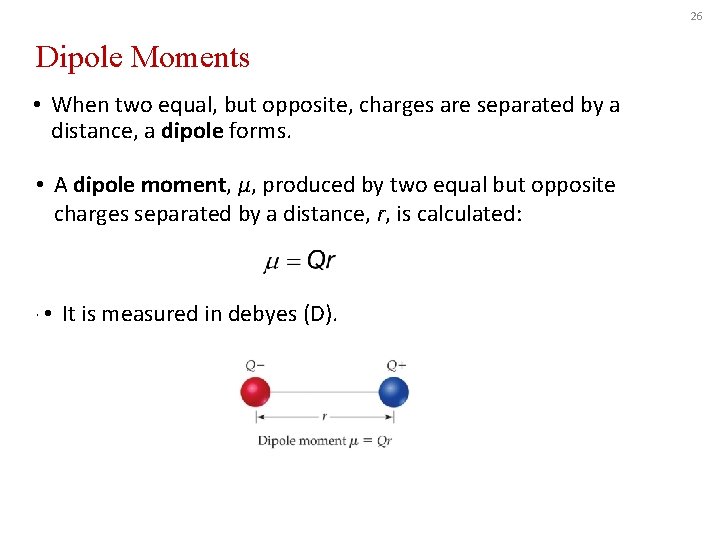
26 Dipole Moments • When two equal, but opposite, charges are separated by a distance, a dipole forms. • A dipole moment, μ, produced by two equal but opposite charges separated by a distance, r, is calculated: • • It is measured in debyes (D).
Comparing Ionic and Covalent Bonding • Simplest approach: Metal + nonmetal is ionic; nonmetal + nonmetal is covalent. • There are many exceptions: It doesn’t take into account oxidation number of a metal (higher oxidation numbers can give covalent bonding). • Electronegativity difference can be used; the table still doesn’t take into account oxidation number. • Properties of compounds are often best: Lower melting points mean covalent bonding, for example. 27
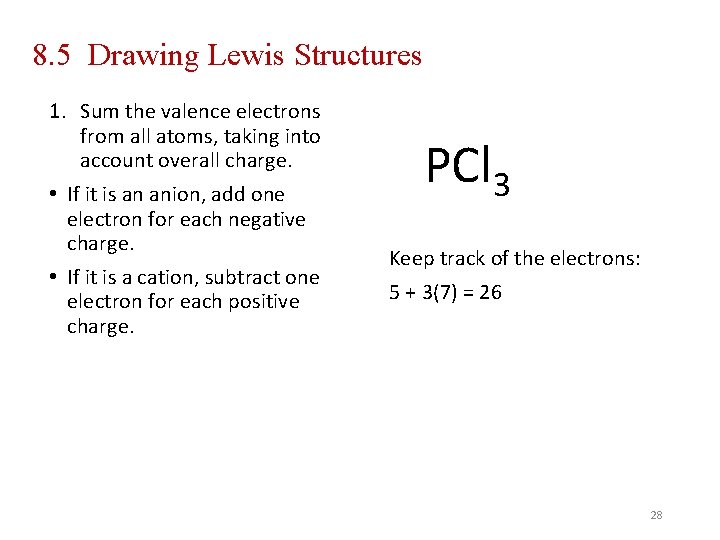
8. 5 Drawing Lewis Structures 1. Sum the valence electrons from all atoms, taking into account overall charge. • If it is an anion, add one electron for each negative charge. • If it is a cation, subtract one electron for each positive charge. PCl 3 Keep track of the electrons: 5 + 3(7) = 26 28
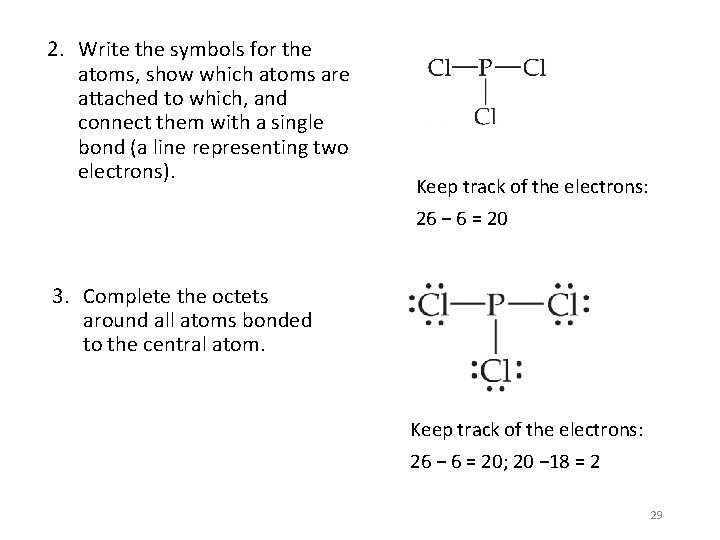
2. Write the symbols for the atoms, show which atoms are attached to which, and connect them with a single bond (a line representing two electrons). Keep track of the electrons: 26 − 6 = 20 3. Complete the octets around all atoms bonded to the central atom. Keep track of the electrons: 26 − 6 = 20; 20 − 18 = 2 29
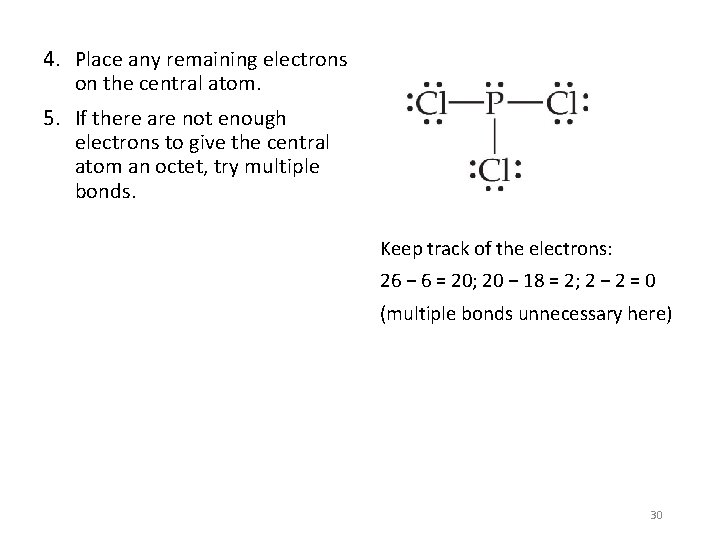
4. Place any remaining electrons on the central atom. 5. If there are not enough electrons to give the central atom an octet, try multiple bonds. Keep track of the electrons: 26 − 6 = 20; 20 − 18 = 2; 2 − 2 = 0 (multiple bonds unnecessary here) 30
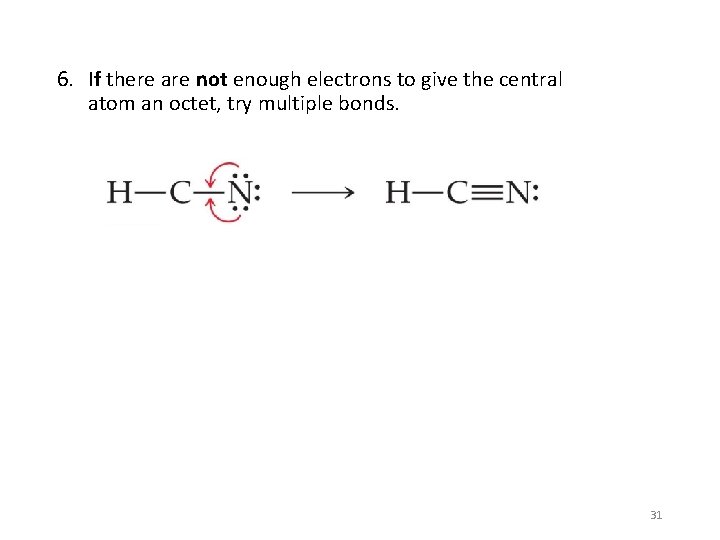
6. If there are not enough electrons to give the central atom an octet, try multiple bonds. 31
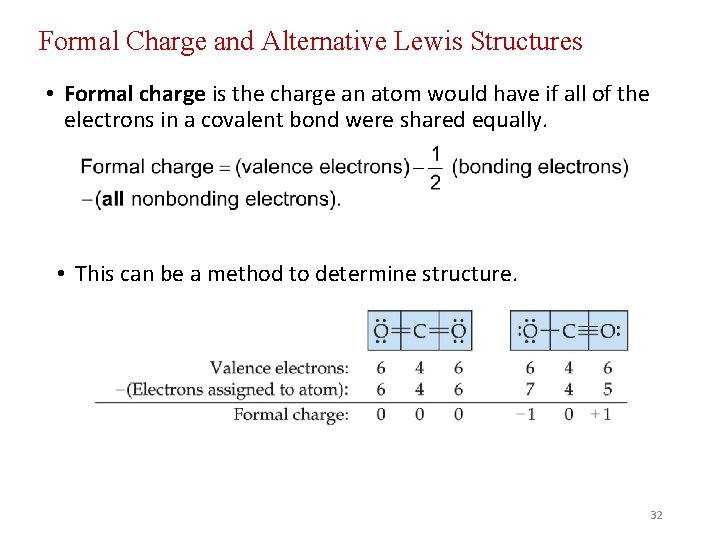
Formal Charge and Alternative Lewis Structures • Formal charge is the charge an atom would have if all of the electrons in a covalent bond were shared equally. • This can be a method to determine structure. 32
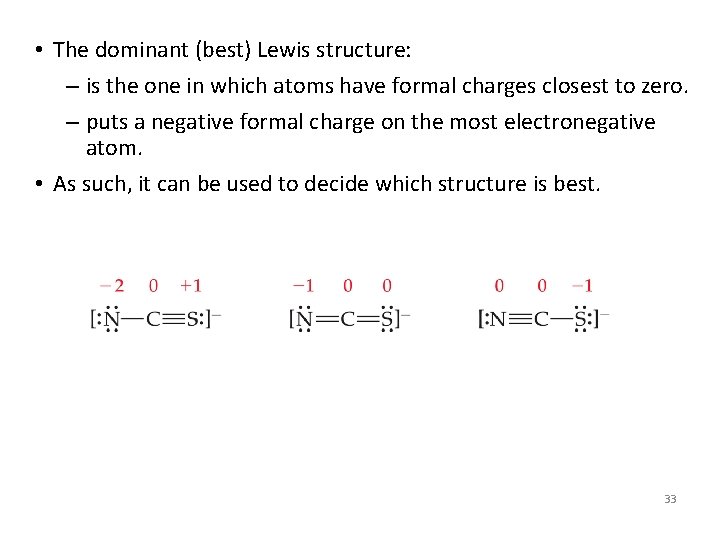
• The dominant (best) Lewis structure: – is the one in which atoms have formal charges closest to zero. – puts a negative formal charge on the most electronegative atom. • As such, it can be used to decide which structure is best. 33
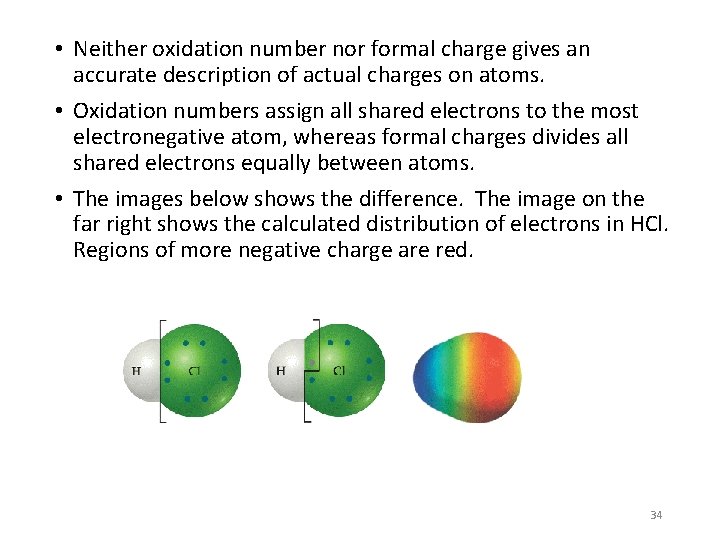
• Neither oxidation number nor formal charge gives an accurate description of actual charges on atoms. • Oxidation numbers assign all shared electrons to the most electronegative atom, whereas formal charges divides all shared electrons equally between atoms. • The images below shows the difference. The image on the far right shows the calculated distribution of electrons in HCl. Regions of more negative charge are red. 34
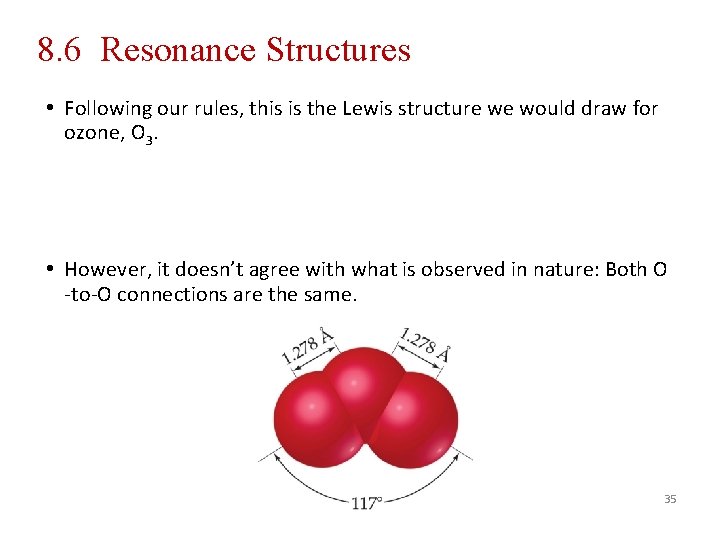
8. 6 Resonance Structures • Following our rules, this is the Lewis structure we would draw for ozone, O 3. • However, it doesn’t agree with what is observed in nature: Both O -to-O connections are the same. 35
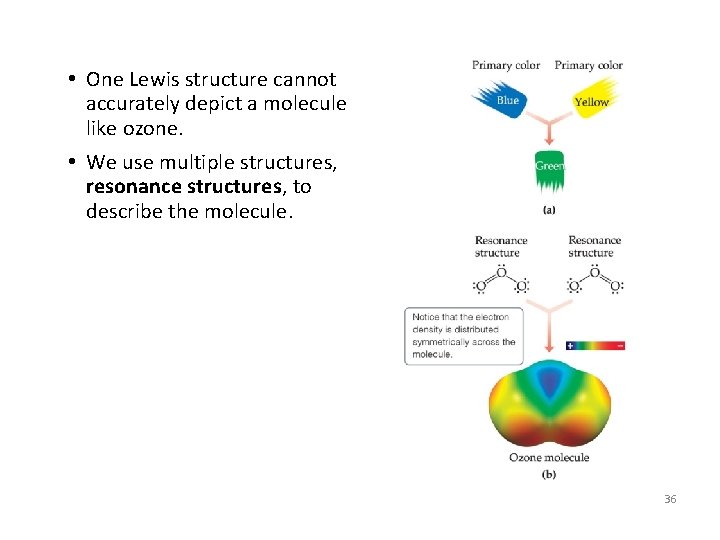
• One Lewis structure cannot accurately depict a molecule like ozone. • We use multiple structures, resonance structures, to describe the molecule. 36
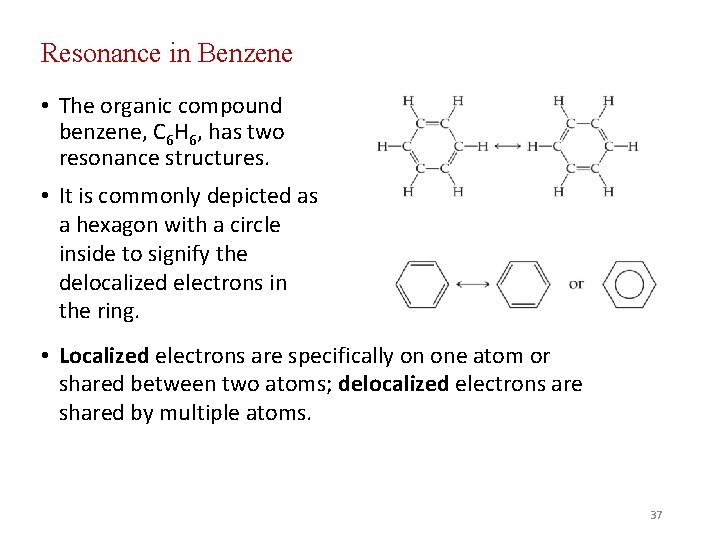
Resonance in Benzene • The organic compound benzene, C 6 H 6, has two resonance structures. • It is commonly depicted as a hexagon with a circle inside to signify the delocalized electrons in the ring. • Localized electrons are specifically on one atom or shared between two atoms; delocalized electrons are shared by multiple atoms. 37
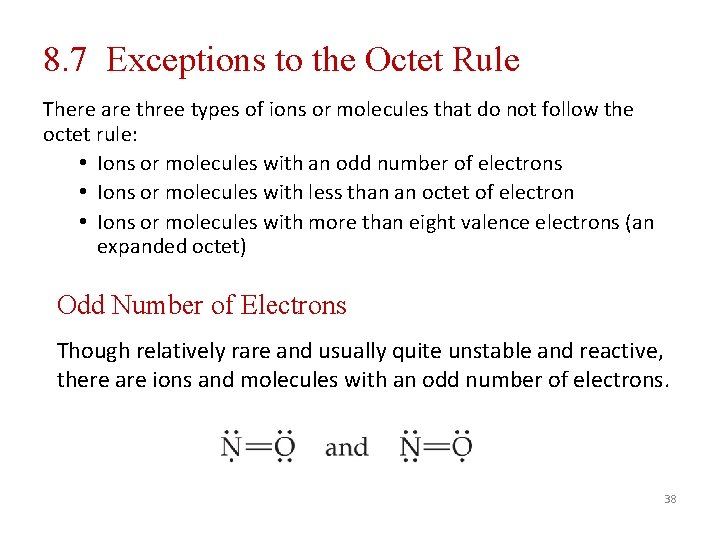
8. 7 Exceptions to the Octet Rule There are three types of ions or molecules that do not follow the octet rule: • Ions or molecules with an odd number of electrons • Ions or molecules with less than an octet of electron • Ions or molecules with more than eight valence electrons (an expanded octet) Odd Number of Electrons Though relatively rare and usually quite unstable and reactive, there are ions and molecules with an odd number of electrons. 38
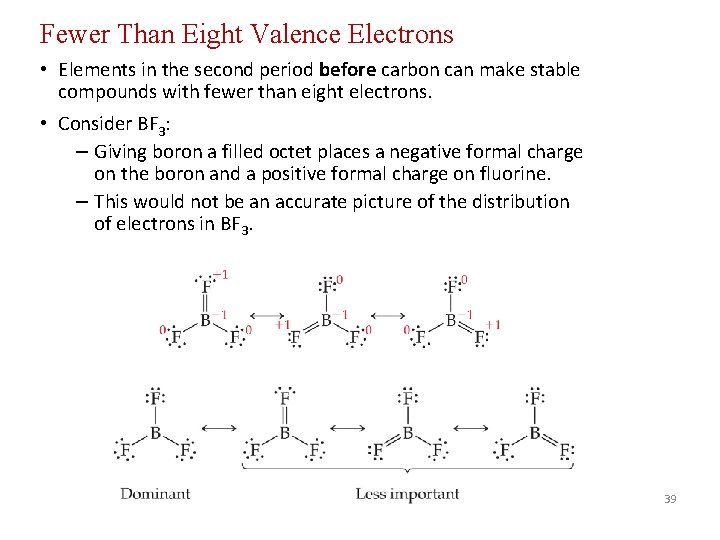
Fewer Than Eight Valence Electrons • Elements in the second period before carbon can make stable compounds with fewer than eight electrons. • Consider BF 3: – Giving boron a filled octet places a negative formal charge on the boron and a positive formal charge on fluorine. – This would not be an accurate picture of the distribution of electrons in BF 3. 39
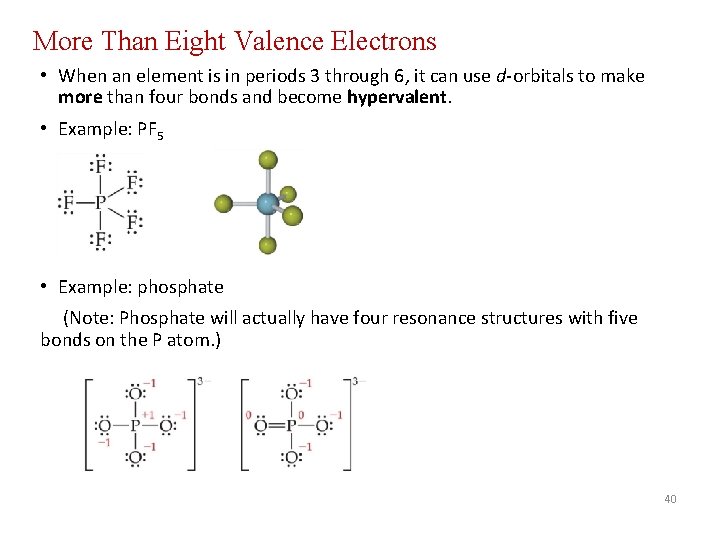
More Than Eight Valence Electrons • When an element is in periods 3 through 6, it can use d-orbitals to make more than four bonds and become hypervalent. • Example: PF 5 • Example: phosphate (Note: Phosphate will actually have four resonance structures with five bonds on the P atom. ) 40
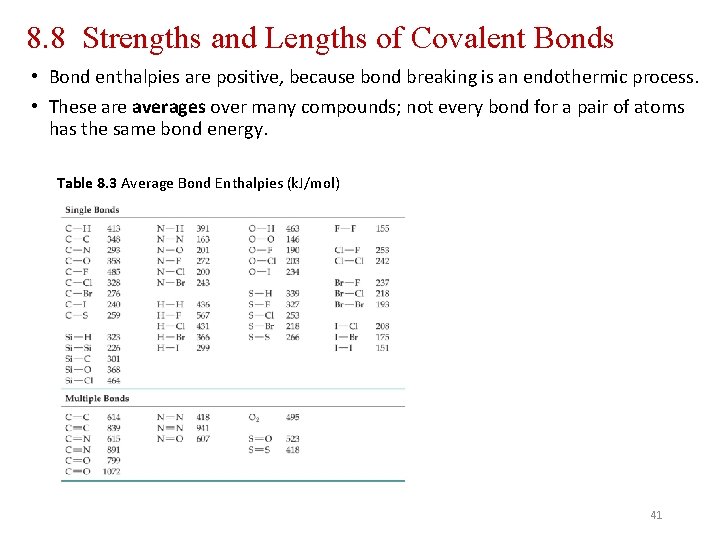
8. 8 Strengths and Lengths of Covalent Bonds • Bond enthalpies are positive, because bond breaking is an endothermic process. • These are averages over many compounds; not every bond for a pair of atoms has the same bond energy. Table 8. 3 Average Bond Enthalpies (k. J/mol) 41
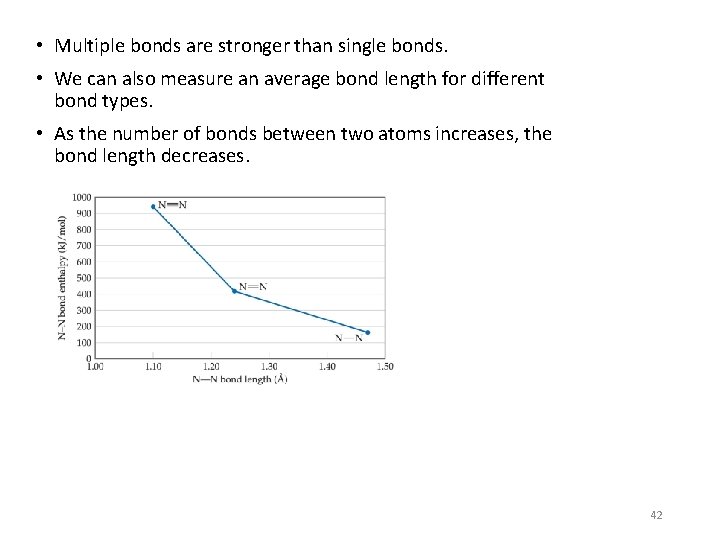
• Multiple bonds are stronger than single bonds. • We can also measure an average bond length for different bond types. • As the number of bonds between two atoms increases, the bond length decreases. 42
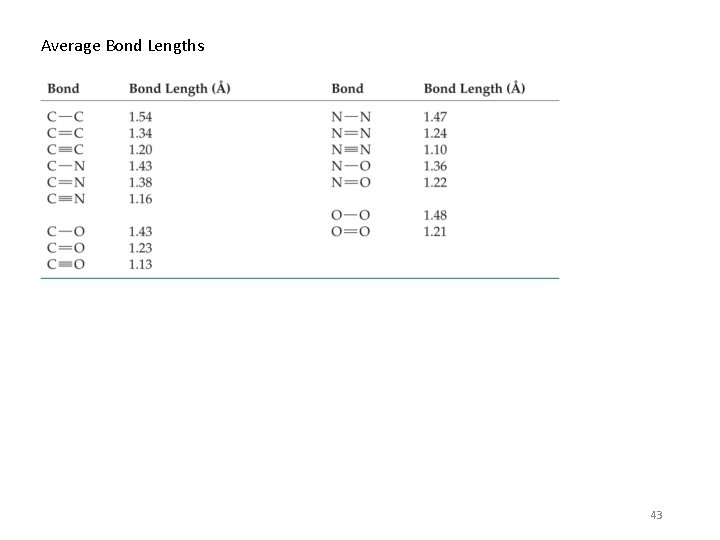
Average Bond Lengths 43

44 Copyright
- Slides: 44