Chapter 8 Acids and Bases 8 7 Buffers
Chapter 8 Acids and Bases 8. 7 Buffers 1

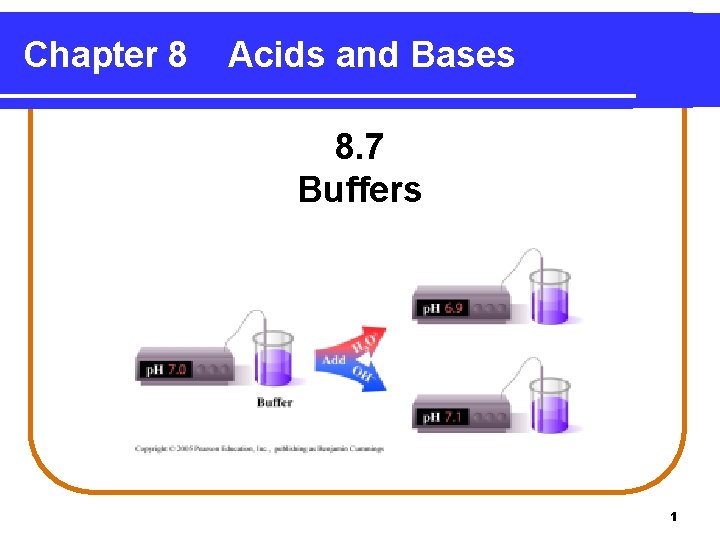
Buffers When an acid or base is added • to water, the p. H changes drastically. • to a buffer solution, the p. H is maintained; p. H does not change. 2

Buffers • resist changes in p. H from the addition of acid or base. • in the body, absorb H 3 O+ or OH- from foods and cellular processes to maintain p. H. • are important in the proper functioning of cells and blood. • in blood maintain a p. H close to 7. 4. A change in the p. H of the blood affects the uptake of oxygen and cellular processes. 3

Components of a Buffer A buffer solution • contains a combination of acid-base conjugate pairs. • may contain a weak acid and a salt of its conjugate base. • typically has equal concentrations of a weak acid and its salt. • may also contain a weak base and a salt of the conjugate acid. 4

Buffer Action In the acetic acid/acetate buffer with acetic acid (CH 3 COOH) and sodium acetate (CH 3 COONa) • The salt produces acetate ions and sodium ions. CH 3 COONa(aq) CH 3 COO-(aq) + Na+ (aq) • The salt is added to provide a higher concentration of the conjugate base CH 3 COO- than the weak acid alone. CH 3 COOH(aq) + H 2 O(l) CH 3 COO-(aq) + H 3 O+(aq) Large amount 5

Function of the Weak Acid in a Buffer The function of the weak acid in a buffer is to neutralize a base. The acetate ion produced adds to the available acetate. CH 3 COOH + OH− acetic acid base CH 3 COO− + H 2 O acetate ion water Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 6

Function of the Conjugate Base The function of the acetate ion CH 3 COO− is to neutralize H 3 O+ from acids. The acetic acid produced contributes to the available weak acid. CH 3 COO− + H 3 O+ acetate ion acid CH 3 COOH + H 2 O acetic acid water Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

Summary of Buffer Action Buffer action occurs as • the weak acid in a buffer neutralizes base. • the conjugate base in the buffer neutralizes acid. • the p. H of the solution is maintained. 8

Learning Check Which combination(s) make a buffer solution? A. B. C. D. HCl and KCl H 2 CO 3 and Na. HCO 3 H 3 PO 4 and Na. Cl CH 3 COOH and CH 3 COOK 9

Solution B. H 2 CO 3 + Na. HCO 3 A weak acid and its salt D. CH 3 COOH + CH 3 COOK A weak acid and its salt. 10
- Slides: 10