Chapter 8 A Solutions 1 CHAPTER OUTLINE Type







- Slides: 44

Chapter 8 A Solutions 1

CHAPTER OUTLINE § § § § Type of Solutions Electrolytes & Non-electrolytes Equivalents of Electrolytes Solubility & Saturation Soluble & Insoluble Salts Formation of a Solid Precipitation Reactions 2

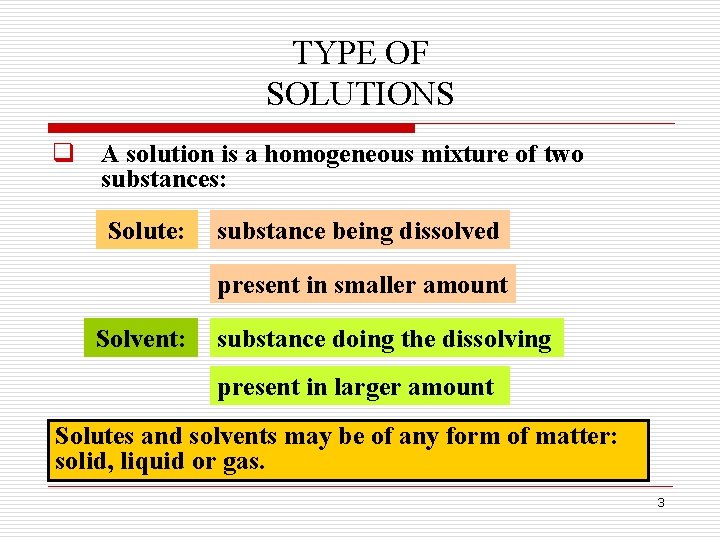
TYPE OF SOLUTIONS q A solution is a homogeneous mixture of two substances: Solute: substance being dissolved present in smaller amount Solvent: substance doing the dissolving present in larger amount Solutes and solvents may be of any form of matter: solid, liquid or gas. 3

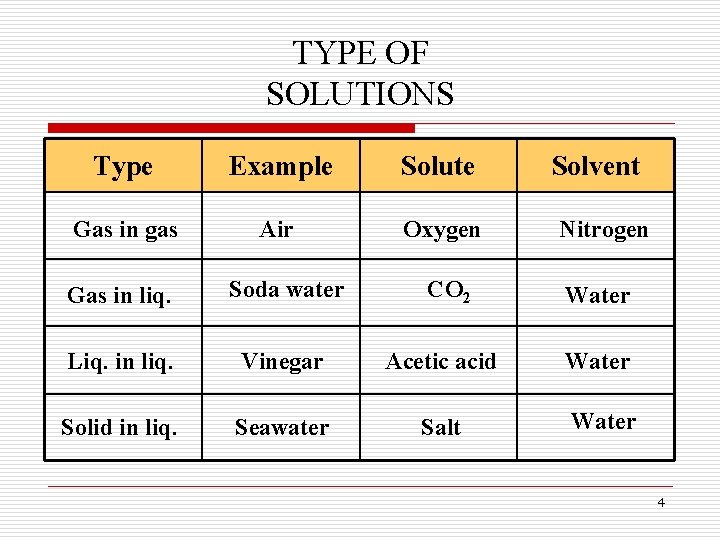
TYPE OF SOLUTIONS Type Example Solute Solvent Gas in gas Air Oxygen Nitrogen CO 2 Water Gas in liq. Soda water Liq. in liq. Vinegar Acetic acid Water Solid in liq. Seawater Salt Water 4

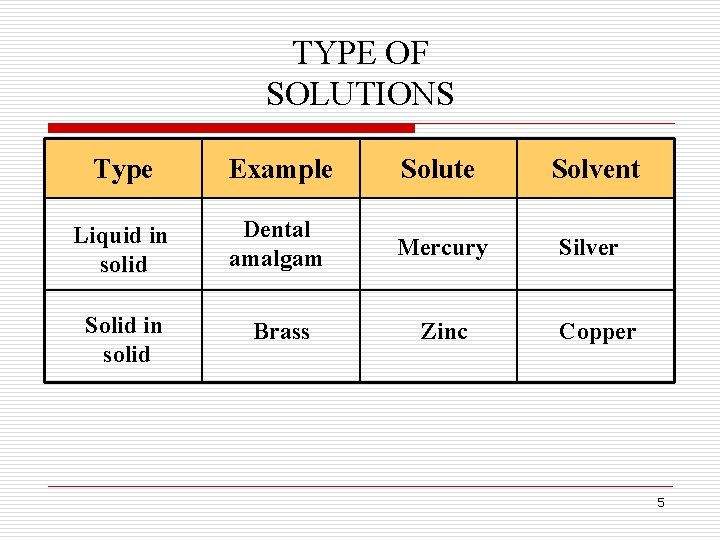
TYPE OF SOLUTIONS Type Example Solute Solvent Liquid in solid Dental amalgam Mercury Silver Solid in solid Brass Zinc Copper 5

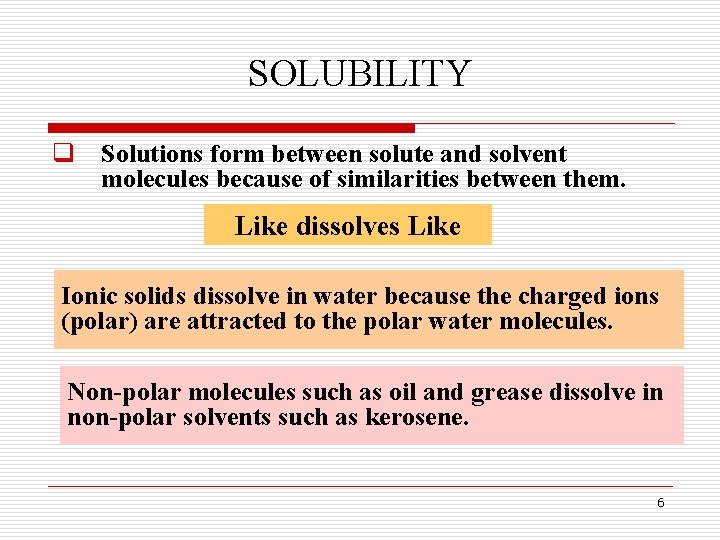
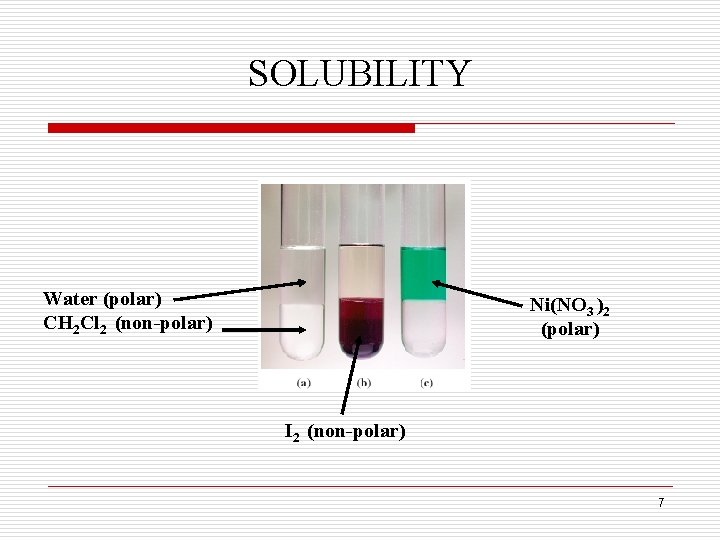
SOLUBILITY q Solutions form between solute and solvent molecules because of similarities between them. Like dissolves Like Ionic solids dissolve in water because the charged ions (polar) are attracted to the polar water molecules. Non-polar molecules such as oil and grease dissolve in non-polar solvents such as kerosene. 6

SOLUBILITY Water (polar) CH 2 Cl 2 (non-polar) Ni(NO 3 )2 (polar) I 2 (non-polar) 7

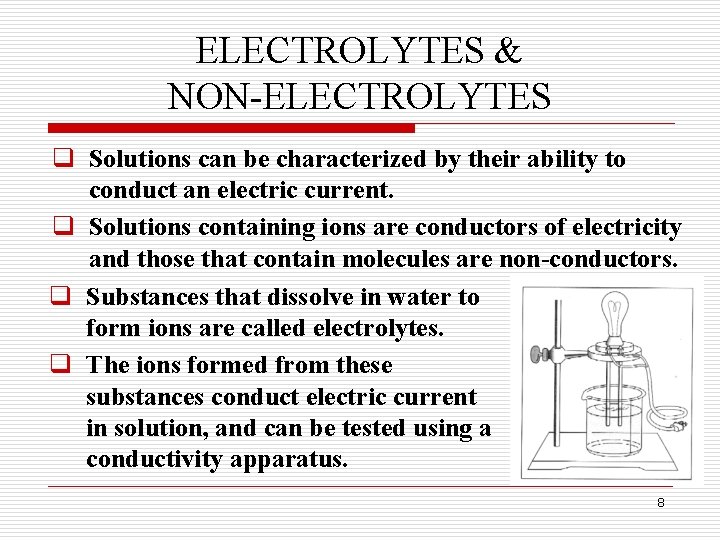
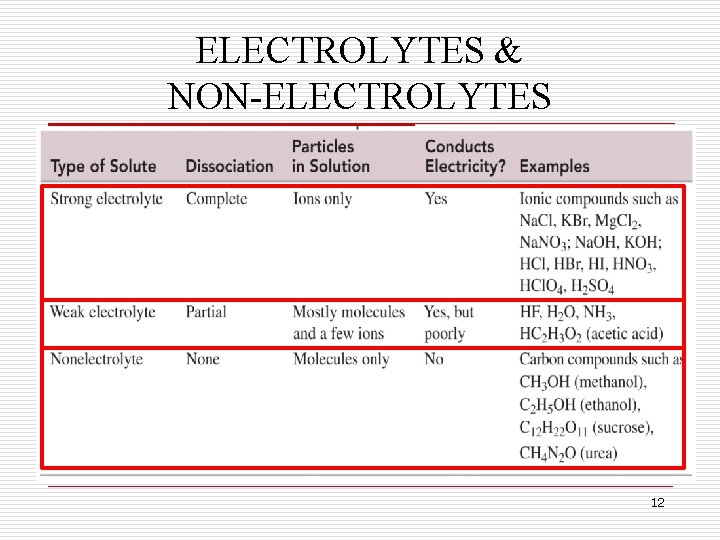
ELECTROLYTES & NON-ELECTROLYTES q Solutions can be characterized by their ability to conduct an electric current. q Solutions containing ions are conductors of electricity and those that contain molecules are non-conductors. q Substances that dissolve in water to form ions are called electrolytes. q The ions formed from these substances conduct electric current in solution, and can be tested using a conductivity apparatus. 8

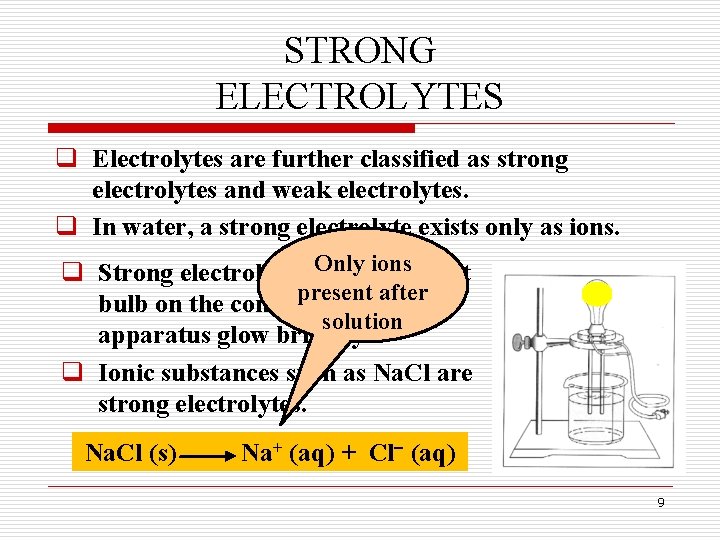
STRONG ELECTROLYTES q Electrolytes are further classified as strong electrolytes and weak electrolytes. q In water, a strong electrolyte exists only as ions. Only ions q Strong electrolytes make the light present after bulb on the conductivity solution apparatus glow brightly. q Ionic substances such as Na. Cl are strong electrolytes. Na. Cl (s) Na+ (aq) + Cl (aq) 9

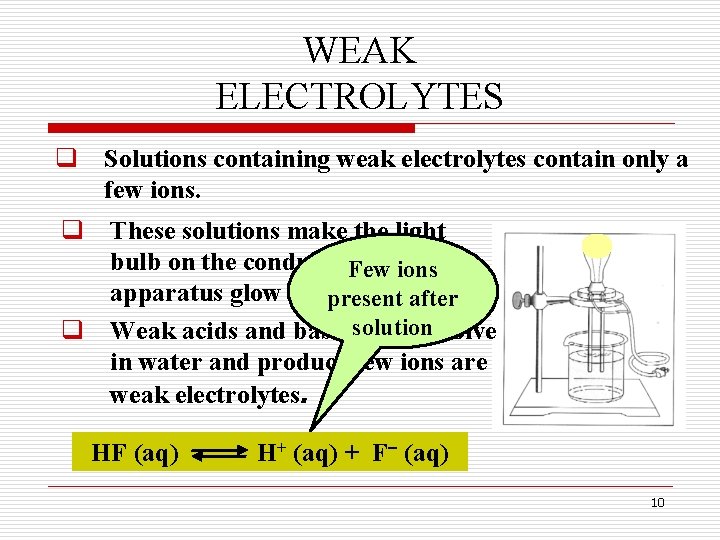
WEAK ELECTROLYTES q Solutions containing weak electrolytes contain only a few ions. q These solutions make the light bulb on the conductivity Few ions apparatus glow dimly. present after q Weak acids and basessolution that dissolve in water and produce few ions are weak electrolytes. HF (aq) H+ (aq) + F (aq) 10

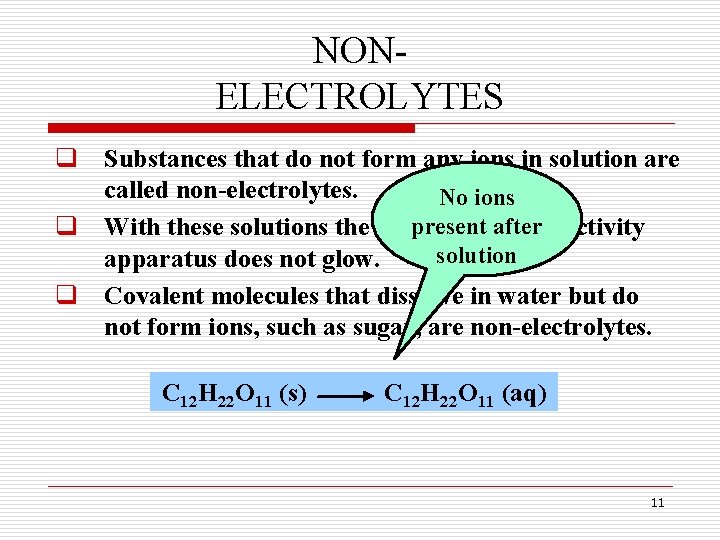
NONELECTROLYTES q Substances that do not form any ions in solution are called non-electrolytes. No ions present q With these solutions the bulb on theafter conductivity solution apparatus does not glow. q Covalent molecules that dissolve in water but do not form ions, such as sugar, are non-electrolytes. C 12 H 22 O 11 (s) C 12 H 22 O 11 (aq) 11

ELECTROLYTES & NON-ELECTROLYTES 12

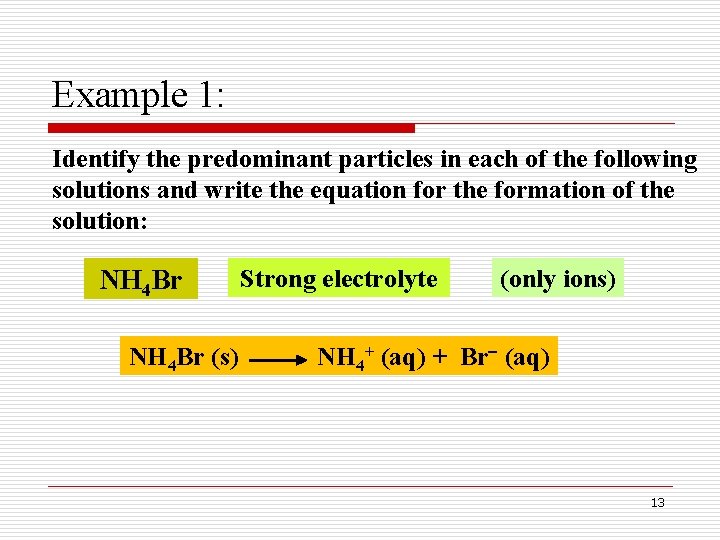
Example 1: Identify the predominant particles in each of the following solutions and write the equation for the formation of the solution: NH 4 Br (s) Strong electrolyte (only ions) NH 4+ (aq) + Br (aq) 13

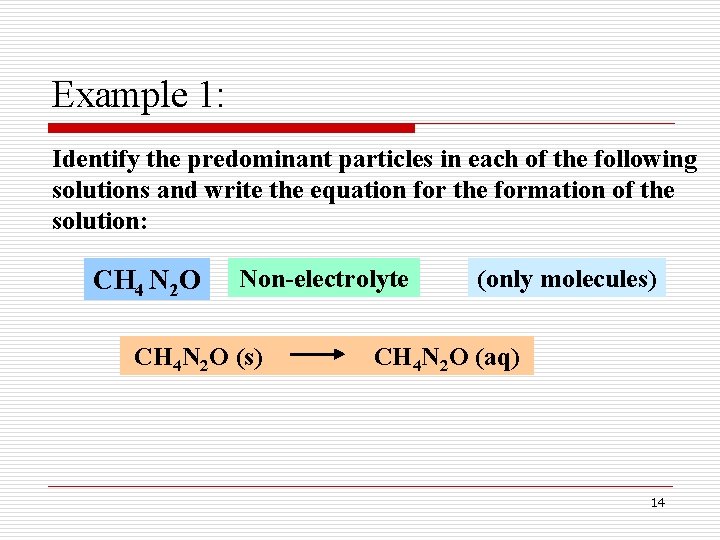
Example 1: Identify the predominant particles in each of the following solutions and write the equation for the formation of the solution: CH 4 N 2 O Non-electrolyte CH 4 N 2 O (s) (only molecules) CH 4 N 2 O (aq) 14

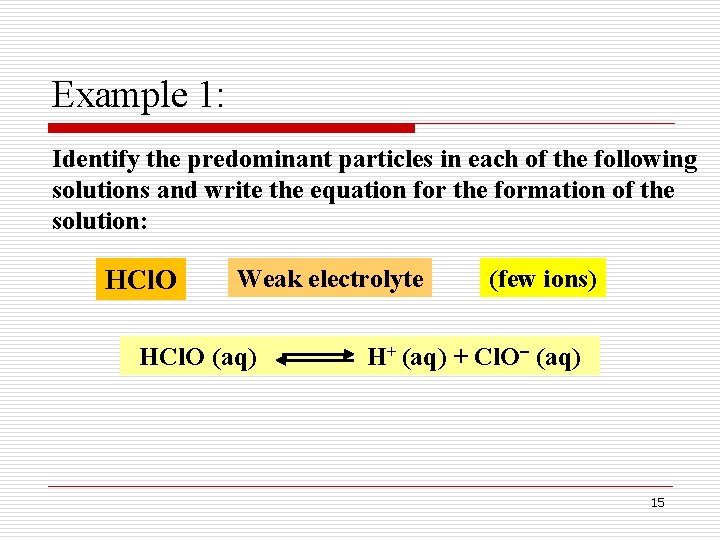
Example 1: Identify the predominant particles in each of the following solutions and write the equation for the formation of the solution: HCl. O Weak electrolyte HCl. O (aq) (few ions) H+ (aq) + Cl. O (aq) 15

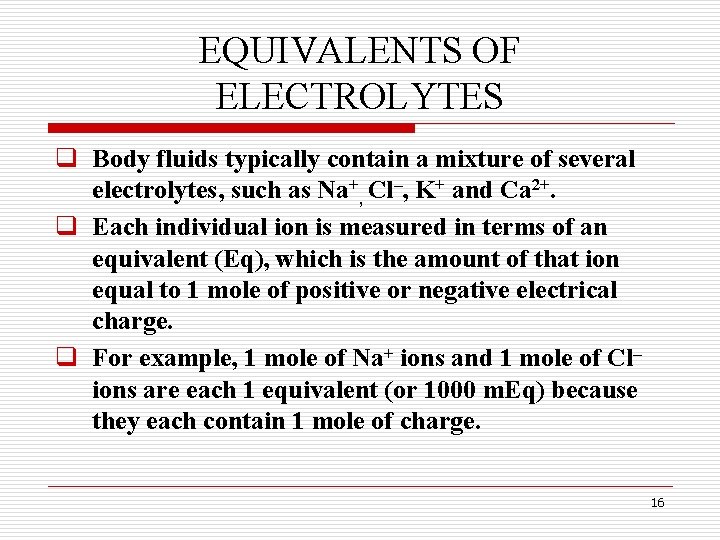
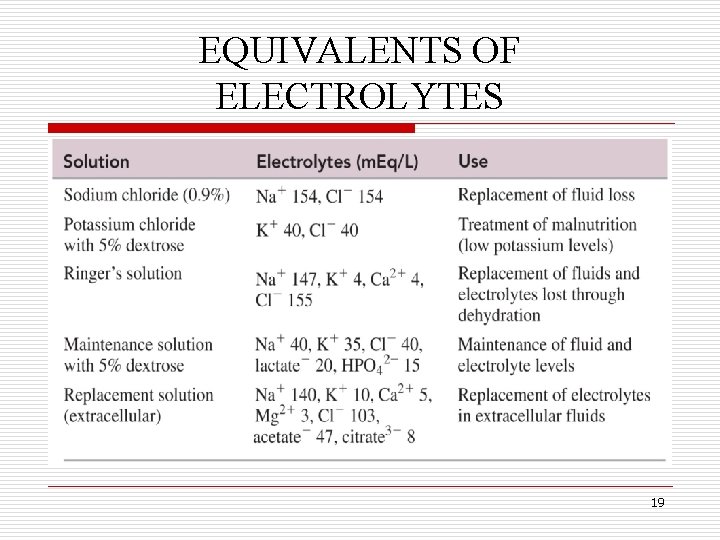
EQUIVALENTS OF ELECTROLYTES q Body fluids typically contain a mixture of several electrolytes, such as Na+, Cl–, K+ and Ca 2+. q Each individual ion is measured in terms of an equivalent (Eq), which is the amount of that ion equal to 1 mole of positive or negative electrical charge. q For example, 1 mole of Na+ ions and 1 mole of Cl– ions are each 1 equivalent (or 1000 m. Eq) because they each contain 1 mole of charge. 16

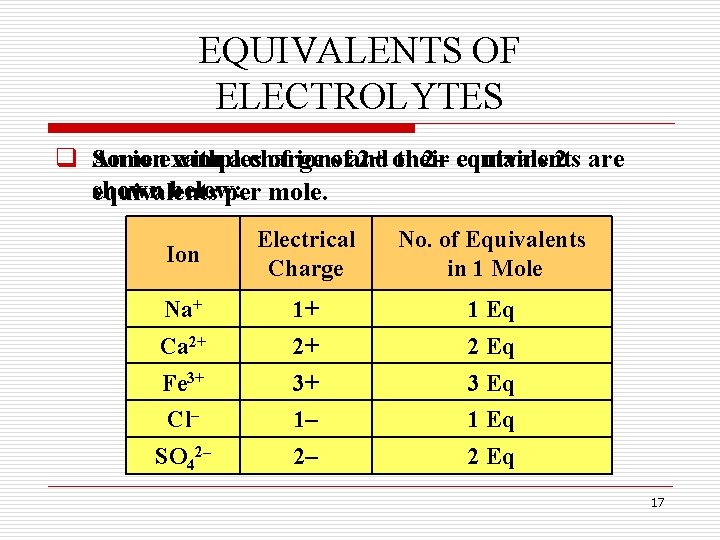
EQUIVALENTS OF ELECTROLYTES q Some An ionexamples with a charge of ionsofand 2+ or their 2– contains equivalents 2 are shown below: equivalents per mole. Ion Electrical Charge No. of Equivalents in 1 Mole Na+ 1+ 1 Eq Ca 2+ 2+ 2 Eq Fe 3+ 3+ 3 Eq Cl– 1– 1 Eq SO 42– 2– 2 Eq 17

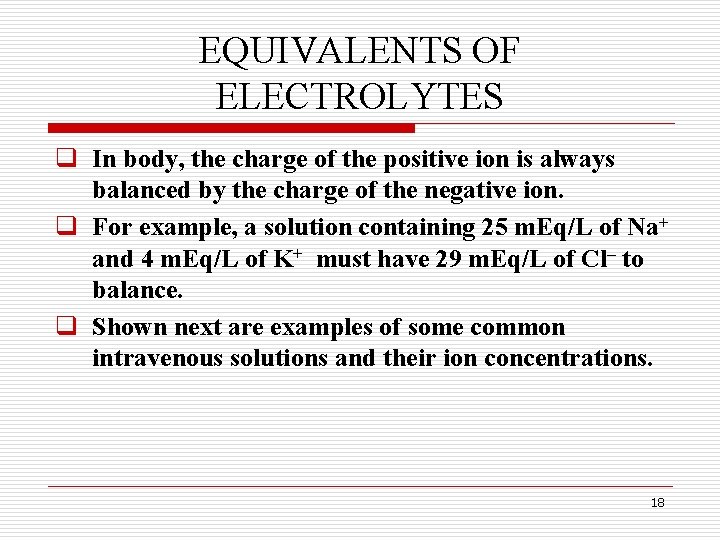
EQUIVALENTS OF ELECTROLYTES q In body, the charge of the positive ion is always balanced by the charge of the negative ion. q For example, a solution containing 25 m. Eq/L of Na+ and 4 m. Eq/L of K+ must have 29 m. Eq/L of Cl– to balance. q Shown next are examples of some common intravenous solutions and their ion concentrations. 18

EQUIVALENTS OF ELECTROLYTES 19

Example 1: Indicate the number of equivalents in each of the following: 2 mol K+ 1 Eq 2 mol x 1 mol = 2 Eq Mg 2+ 2 Eq 0. 5 mol x 1 mol = 1 Eq 3 mol CO 32 2 Eq 3 mol x 1 mol = 6 Eq 0. 5 mol 20

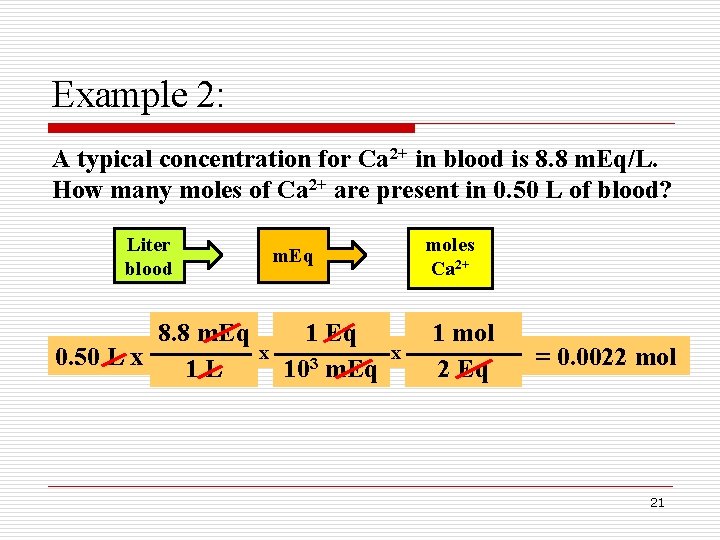
Example 2: A typical concentration for Ca 2+ in blood is 8. 8 m. Eq/L. How many moles of Ca 2+ are present in 0. 50 L of blood? Liter blood 8. 8 m. Eq 0. 50 L x 1 L moles Ca 2+ m. Eq x 1 Eq 103 m. Eq x 1 mol 2 Eq = 0. 0022 mol 21

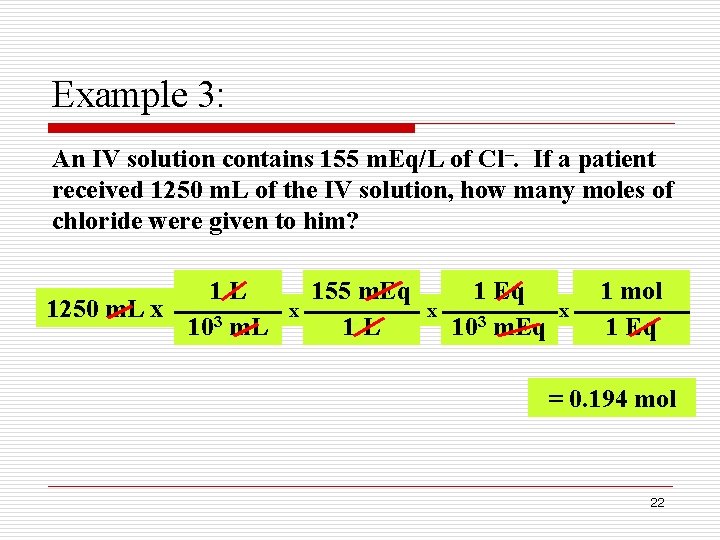
Example 3: An IV solution contains 155 m. Eq/L of Cl–. If a patient received 1250 m. L of the IV solution, how many moles of chloride were given to him? 1 L 1250 m. L x 103 m. L x 155 m. Eq 1 L x 1 Eq 103 m. Eq x 1 mol 1 Eq = 0. 194 mol 22

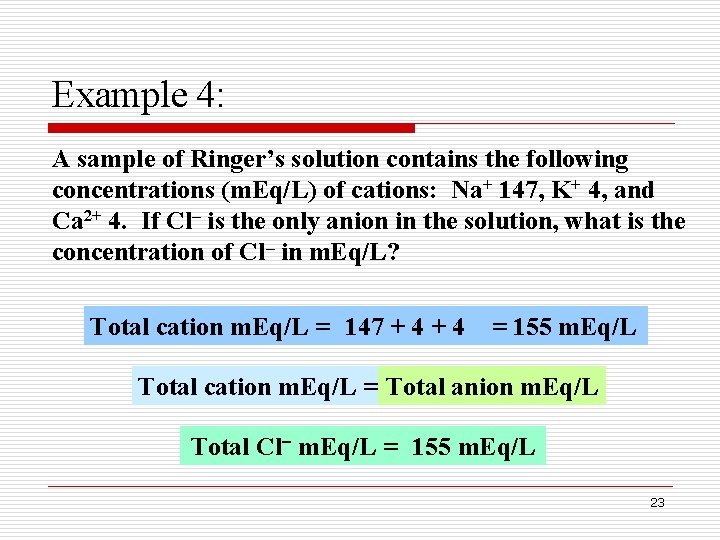
Example 4: A sample of Ringer’s solution contains the following concentrations (m. Eq/L) of cations: Na+ 147, K+ 4, and Ca 2+ 4. If Cl– is the only anion in the solution, what is the concentration of Cl– in m. Eq/L? Total cation m. Eq/L = 147 + 4 = 155 m. Eq/L Total cation m. Eq/L = Total anion m. Eq/L Total Cl m. Eq/L = 155 m. Eq/L 23

SOLUBILITY q Solubility refers to the maximum amount of solute that can be dissolved in a given amount of solvent. q Many factors affect the solubility of a solute in a solution. Type of solute Type of solvent Temperature Solubility is measured in grams of solute per 100 grams of solvent at a given temperature. 24

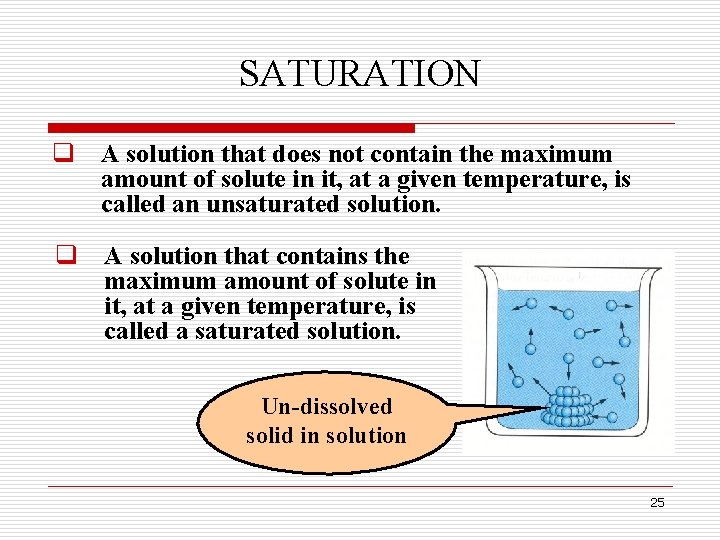
SATURATION q A solution that does not contain the maximum amount of solute in it, at a given temperature, is called an unsaturated solution. q A solution that contains the maximum amount of solute in it, at a given temperature, is called a saturated solution. Un-dissolved solid in solution 25

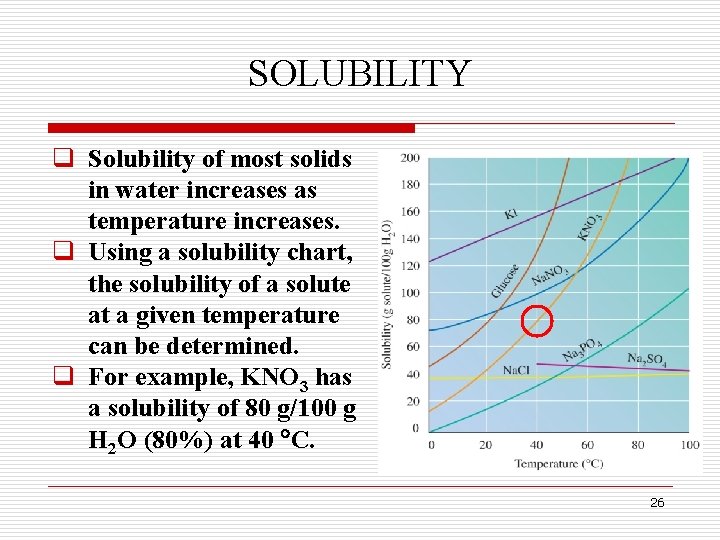
SOLUBILITY q Solubility of most solids in water increases as temperature increases. q Using a solubility chart, the solubility of a solute at a given temperature can be determined. q For example, KNO 3 has a solubility of 80 g/100 g H 2 O (80%) at 40 C. 26

SOLUBILITY OF GASES q Solubility of gases in water decreases as temperature increases. q At higher temperatures more gas molecules have the energy to escape from solution. q Henry’s law states that the solubility of a gas is directly proportional to the pressure above the liquid. q For example, a can of soda is carbonated at high pressures in order to increase the solubility of CO 2. Once the can is opened, the pressure is reduced and the excess gas escapes from the solution. 27

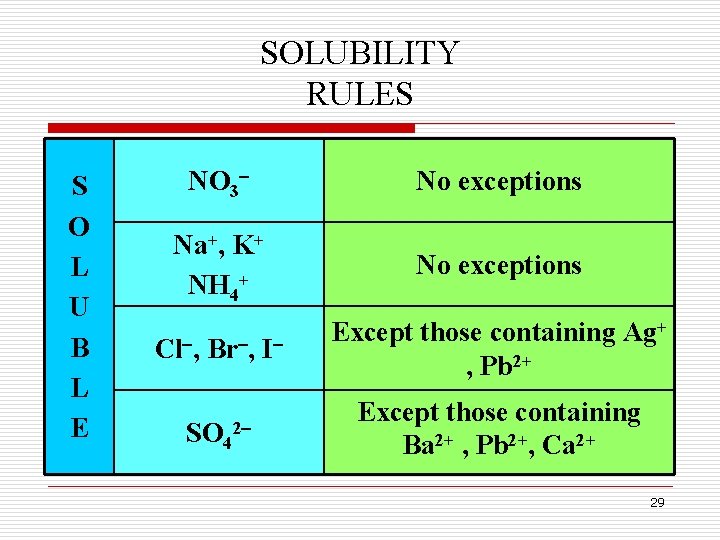
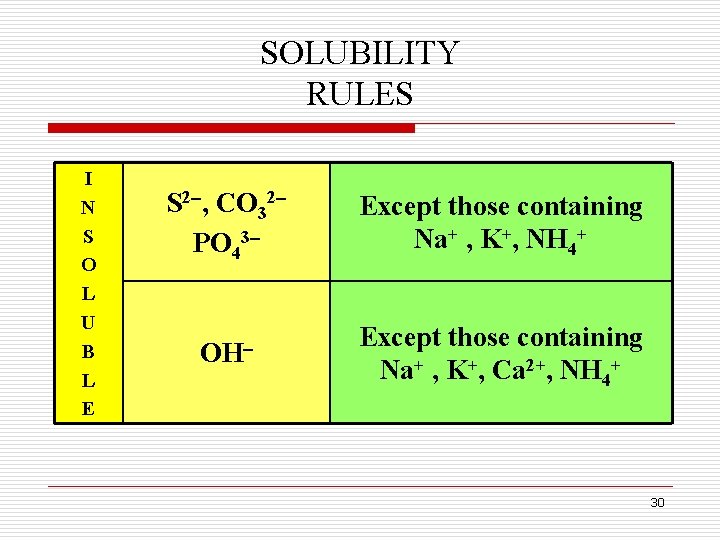
SOLUBLE & INSOLUBLE SALTS q Many ionic solids dissolve in water and are called soluble. q However, some ionic salts do not dissolve in water and do not form ions in solution. These salts are called insoluble salts and remain solid in solution. q Chemists use a set of solubility rules to predict whether a salt is soluble or insoluble. q These rules are summarized next. 28

SOLUBILITY RULES S O L U B L E NO 3 No exceptions Na+, K+ NH 4+ No exceptions Cl , Br , I Except those containing Ag+ , Pb 2+ SO 42 Except those containing Ba 2+ , Pb 2+, Ca 2+ 29

SOLUBILITY RULES I N S O L U B L E S 2 , CO 32 PO 43 Except those containing Na+ , K+, NH 4+ OH Except those containing Na+ , K+, Ca 2+, NH 4+ 30

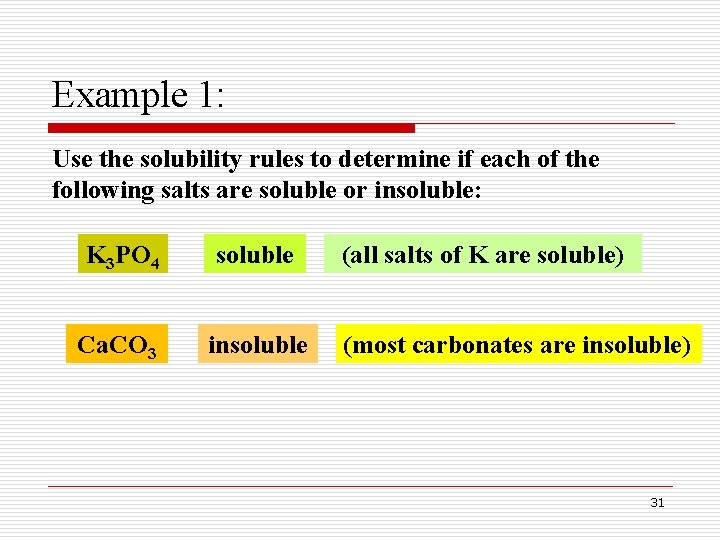
Example 1: Use the solubility rules to determine if each of the following salts are soluble or insoluble: K 3 PO 4 soluble Ca. CO 3 insoluble (all salts of K are soluble) (most carbonates are insoluble) 31

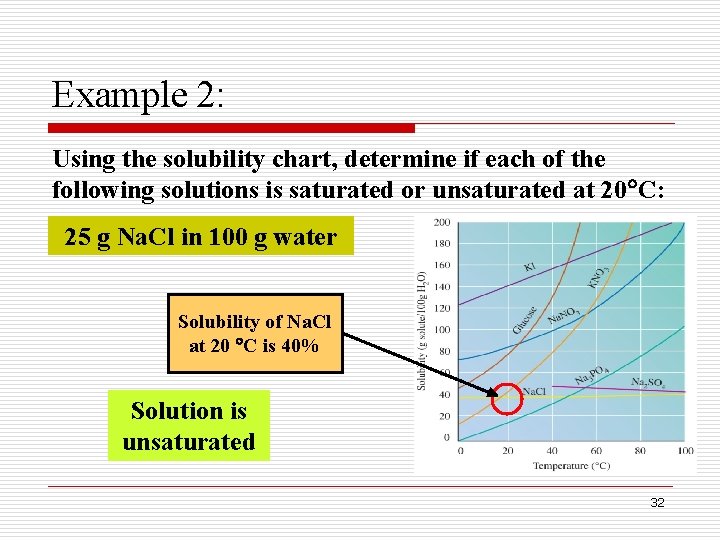
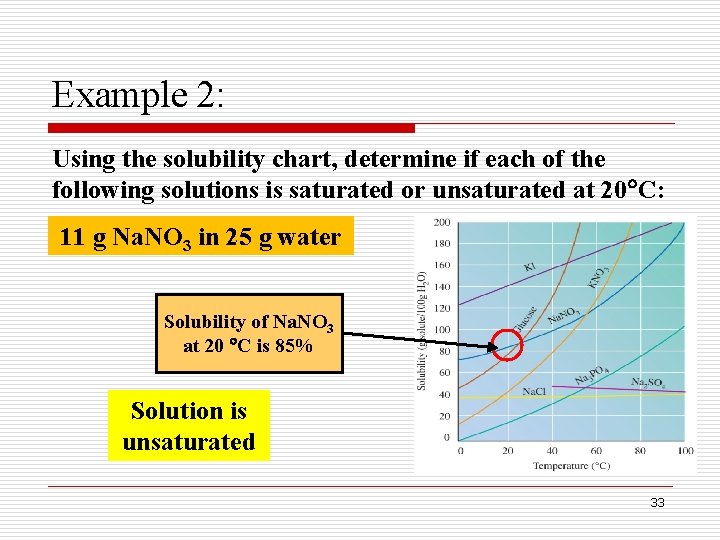
Example 2: Using the solubility chart, determine if each of the following solutions is saturated or unsaturated at 20 C: 25 g Na. Cl in 100 g water Solubility of Na. Cl at 20 C is 40% Solution is unsaturated 32

Example 2: Using the solubility chart, determine if each of the following solutions is saturated or unsaturated at 20 C: 11 g Na. NO 3 in 25 g water Solubility of Na. NO 3 at 20 C is 85% Solution is unsaturated 33

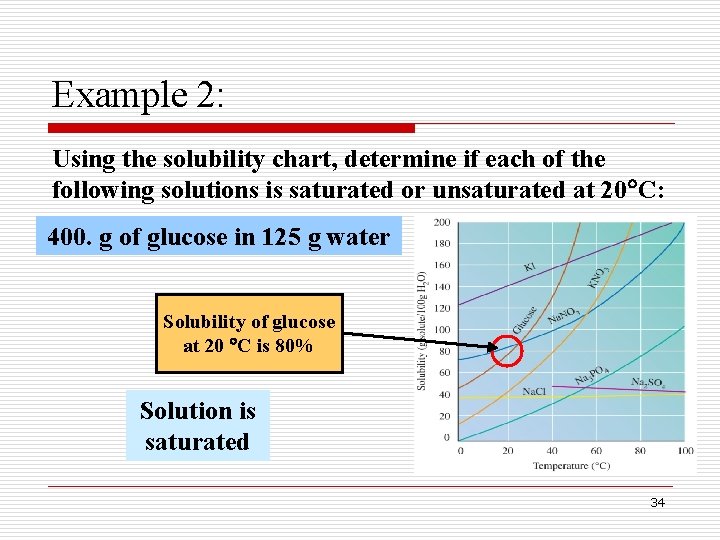
Example 2: Using the solubility chart, determine if each of the following solutions is saturated or unsaturated at 20 C: 400. g of glucose in 125 g water Solubility of glucose at 20 C is 80% Solution is saturated 34

FORMATION OF A SOLID q Solubility rules can be used to predict whether a solid, called a precipitate, can be formed when two solutions of ionic compounds are mixed. q A solid is formed when two ions of an insoluble salt come in contact with one another. q For example, when a solution of K 2 Cr. O 4 is mixed with a solution of Ba(NO 3)2 a yellow insoluble salt Ba. Cr. O 4 is produced. 35

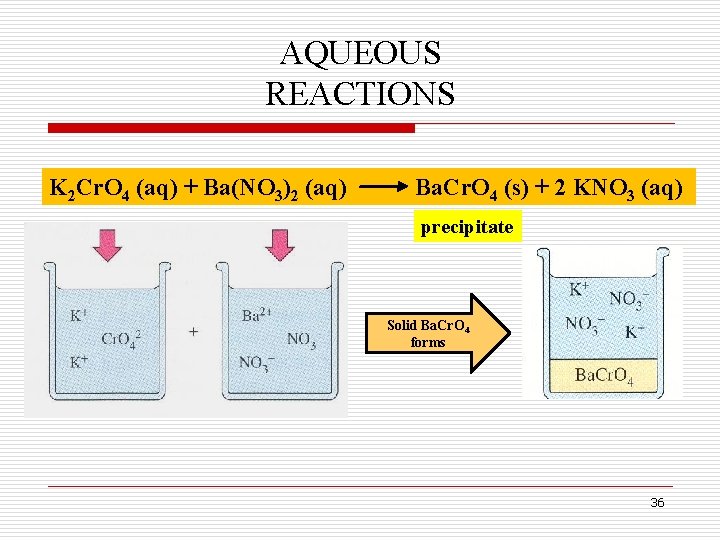
AQUEOUS REACTIONS K 2 Cr. O 4 (aq) + Ba(NO 3)2 (aq) Ba. Cr. O 4 (s) + 2 KNO 3 (aq) precipitate Solid Ba. Cr. O 4 forms 36

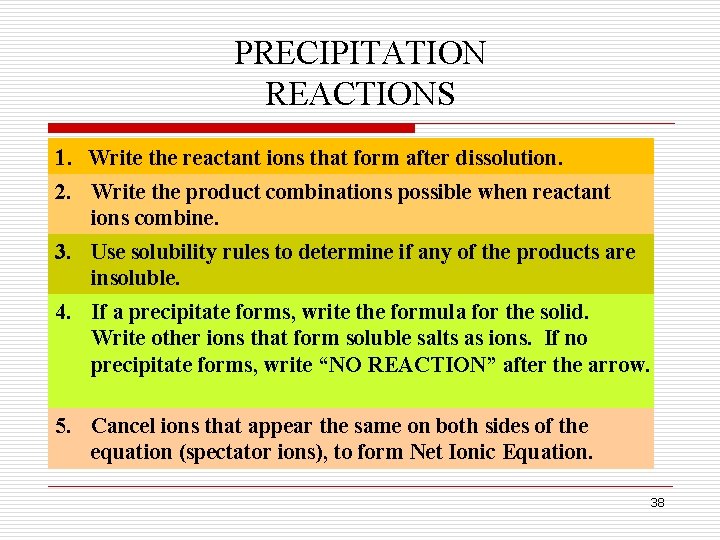
PRECIPITATION REACTIONS q Double replacement reactions in which a precipitate is formed are called precipitation reactions. q To predict a precipitate, follow the steps outlined next. 37

PRECIPITATION REACTIONS 1. Write the reactant ions that form after dissolution. 2. Write the product combinations possible when reactant ions combine. 3. Use solubility rules to determine if any of the products are insoluble. 4. If a precipitate forms, write the formula for the solid. Write other ions that form soluble salts as ions. If no precipitate forms, write “NO REACTION” after the arrow. 5. Cancel ions that appear the same on both sides of the equation (spectator ions), to form Net Ionic Equation. 38

PRECIPITATION REACTIONS The reaction of K 2 Cr. O 4 and Ba(NO 3)2 can be predicted as shown below ? ? ? Step 1: 2 K+ +Cr. O 42 + Ba 2+ + 2 NO 3 Step 2: 2 K+ +Cr. O 42 +Ba 2+ + 2 NO 3 Ba. Cr. O 4(? ) + 2 K+NO 3 (? ) Net Ionic Step 3: 2 K+ +Cr. O 42 +Ba 2+ + 2 NO Equation Ba. Cr. O 4(s) + 2 K+NO 3 (aq) 3 Step 4: 2 K+ +Cr. O 42 +Ba 2+ + 2 NO 3 Ba. Cr. O 4(s) + 2 K+ +2 NO 3 Step 5: Ba 2+ + Cr. O 42 Ba. Cr. O 4 (s) spectator ions precipitate 39

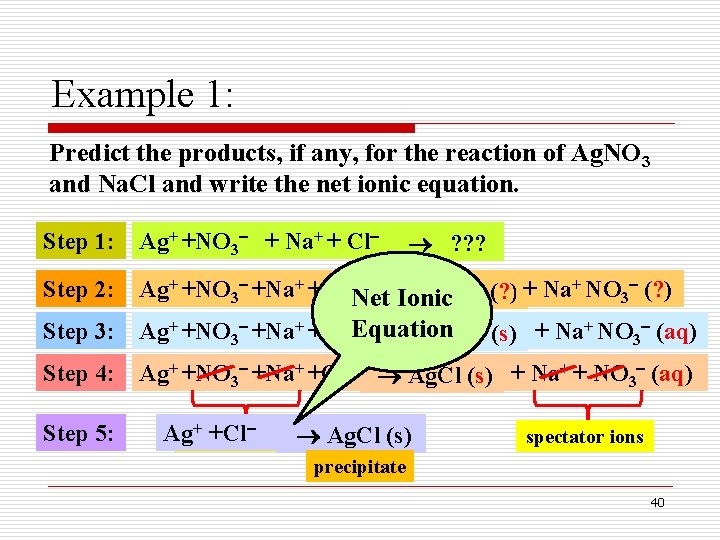
Example 1: Predict the products, if any, for the reaction of Ag. NO 3 and Na. Cl and write the net ionic equation. Step 1: Ag+ +NO 3 + Na+ + Cl Step 2: Ag+ +NO 3 +Na+ +Cl Net Ionic Ag+Cl (? ) + Na+ NO 3 (? ) Step 3: Ag+ +NO 3 +Na+ +Cl Equation Ag+Cl (s) + Na+ NO 3 (aq) Step 4: Ag+ +NO 3 +Na+ +Cl Ag. Cl (s) + Na+ + NO 3 (aq) Step 5: ? ? ? + +Cl Agspectator ions Ag. Cl (s) spectator ions precipitate 40

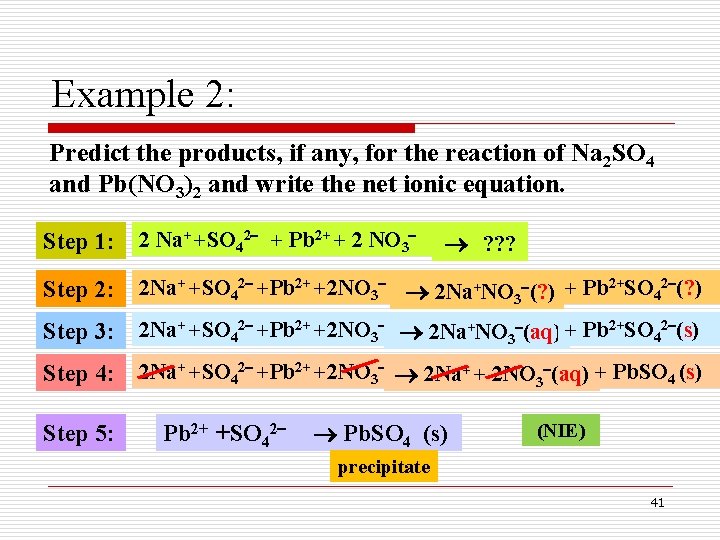
Example 2: Predict the products, if any, for the reaction of Na 2 SO 4 and Pb(NO 3)2 and write the net ionic equation. Step 1: 2 Na+ +SO 42 + Pb 2+ + 2 NO 3 Step 2: 2 Na+ +SO 42 +Pb 2+ +2 NO 3 2 Na+NO 3 (? ) + Pb 2+SO 42 (? ) Step 3: 2 Na+ +SO 42 +Pb 2+ +2 NO 3 2 Na+NO 3 (aq) + Pb 2+SO 42 (s) Step 4: 2 Na+ +SO 42 +Pb 2+ +2 NO 3 2 Na+ + 2 NO 3 (aq) + Pb. SO 4 (s) Step 5: Pb 2+ +SO 42 ? ? ? Pb. SO 4 (s) (NIE) precipitate 41

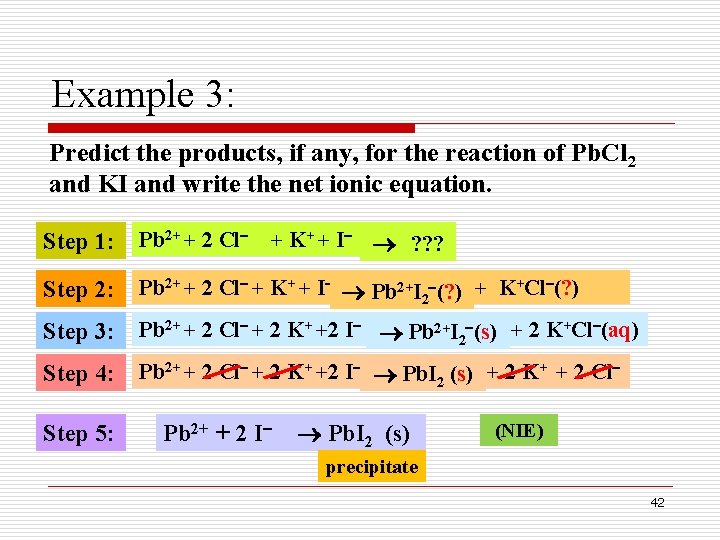
Example 3: Predict the products, if any, for the reaction of Pb. Cl 2 and KI and write the net ionic equation. Step 1: Pb 2+ + 2 Cl Step 2: Pb 2+ + 2 Cl + K+ + I Pb 2+I 2 (? ) + K+Cl (? ) Step 3: Pb 2+ + 2 Cl + 2 K+ +2 I Pb 2+I 2 (s) + 2 K+Cl (aq) Step 4: Pb 2+ + 2 Cl + 2 K+ +2 I Pb. I 2 (s) + 2 K+ + 2 Cl Step 5: + K+ + I ? ? ? Pb 2+ + 2 I Pb. I 2 (s) (NIE) precipitate 42

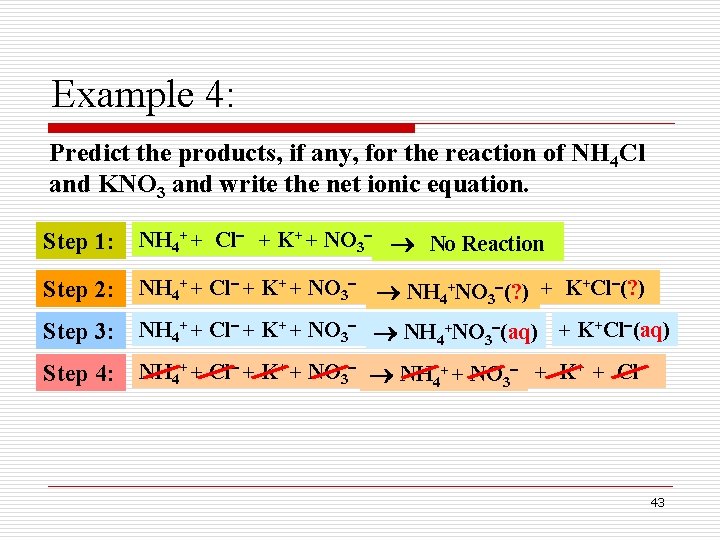
Example 4: Predict the products, if any, for the reaction of NH 4 Cl and KNO 3 and write the net ionic equation. Step 1: NH 4+ + Cl + K+ + NO 3 ? ? ? No Reaction Step 2: NH 4+ + Cl + K+ + NO 3 NH 4+NO 3 (? ) + K+Cl (? ) Step 3: NH 4+ + Cl + K+ + NO 3 NH 4+NO 3 (aq) + K+Cl (aq) Step 4: NH 4+ + Cl + K+ + NO 3 NH 4+ + NO 3 + K+ + Cl 43

THE END 44