Chapter 8 8 2 Solubility and Concentration Solubility

Chapter 8 8. 2 Solubility and Concentration

Solubility �The maximum amount of a solute that dissolves in a given amount of solvent at a constant temperature is called solubility.

Solubility �Solubility is given as grams of solute per 100 grams of solvent at a specific temperature. �Example: �The solubility of table salt or sodium chloride is 36. 0. �That means that 36. 0 grams of sodium chloride will dissolve in 100 grams of water at 20 degrees Celsius.

Types of Solutions �Depending on the amount of solute in solution, solutions can be: �Saturated �Unsaturated �Supersaturated

Saturated Solutions �A saturated solution contains as much solute as the solvent can hold at a given temperature. �If you add more solute to a saturated solution, it will NOT dissolve.

Unsaturated Solutions �Unsaturated solutions have less than the maximum amount of solute that can be dissolved in the solvent.

Supersaturated Solutions �Supersaturated solutions contain more solute than they can normally hold at a given temperature. �These are very unstable. �The solvent must be heated above normal temperature to make the extra solute dissolve and then carefully cooled.

Solutions �The solubility of sugar in water is 203. 9 grams at 20 degrees Celsius. �If 50 grams of sugar are dissolved in water, what type of solution is it? �What if 203. 9 grams of sugar are dissolved? �What if 210 grams of sugar are dissolved?

Solutions Answers �Unsaturated �Supersaturated
What Affects Solubility? �Solubility is affected by: �The polarity of the solvent �Temperature �Pressure

Polar and Nonpolar Solvents Solvent Type Solute Type Will Solution Form? Polar (or ionic) More likely Polar Nonpolar Not likely Nonpolar Polar (or ionic) Not likely Nonpolar More likely
Temperature �For solid solutes, solubility increases as temperature increases. �For gas solutes, solubility decreases as temperature increases.

Pressure �For gas solutes, solubility increases as pressure increases.

Concentration of Solutions �Concentration can be expressed as percent by volume, percent by mass, and molarity.
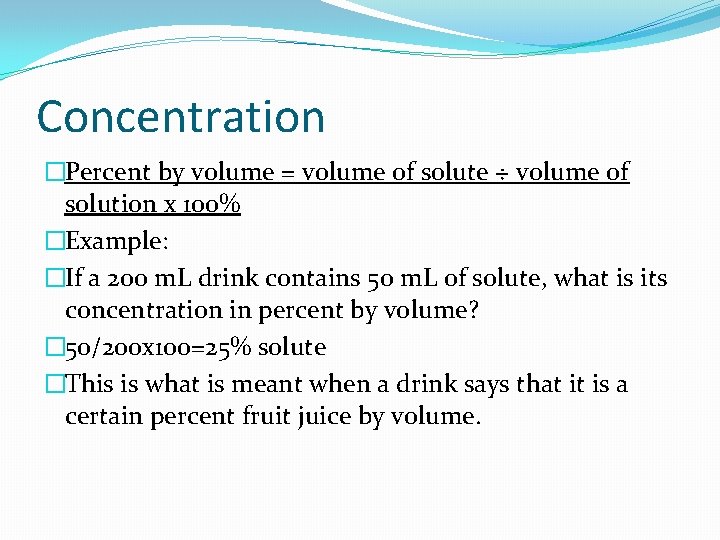
Concentration �Percent by volume = volume of solute ÷ volume of solution x 100% �Example: �If a 200 m. L drink contains 50 m. L of solute, what is its concentration in percent by volume? � 50/200 x 100=25% solute �This is what is meant when a drink says that it is a certain percent fruit juice by volume.

Percent by Volume �A 200 m. L drink is 27% fruit juice by volume. What volume of fruit juice does it contain in milliliters? �Fruit juice/200 x 100=27 �Fruit juice/200=27/100 �Fruit juice=(27/100)x 200 �Fruit juice =54 m. L
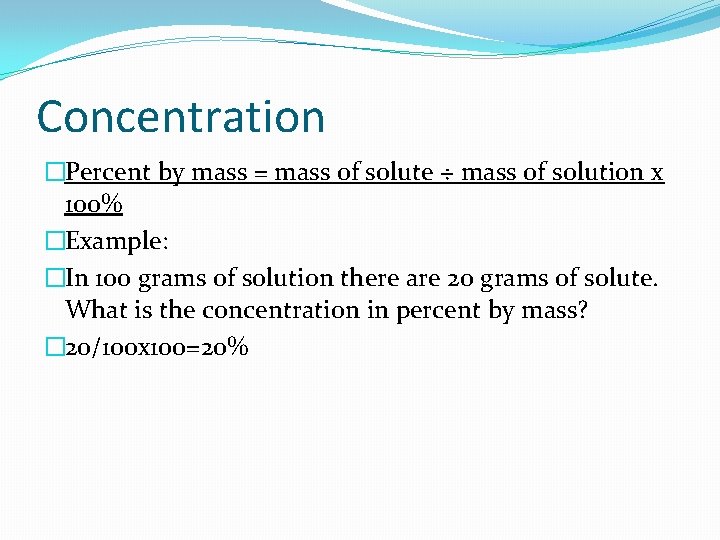
Concentration �Percent by mass = mass of solute ÷ mass of solution x 100% �Example: �In 100 grams of solution there are 20 grams of solute. What is the concentration in percent by mass? � 20/100 x 100=20%

Percent by Mass �A 200 g solution is allowed to evaporate. After the solvent is gone, 20 grams of solute remain. What was the solution’s concentration in percent by mass? � 20/200 x 100=10%

Concentration �Molarity = moles of solute/liters of solution �Molarity is the number of moles of a solute dissolved per liter of solution.
Molarity �To make a 1 -molar (1 M) solution of sodium chloride in water: �Calculate the molar mass of the solute. �Na. Cl has a molar mass of 58. 5 grams. �If 58. 5 grams of Na. Cl is mixed with enough water to make 1 liter of solution, the resulting solution is 1 molar.

Molarity �How would you make a 1 M solution of table sugar (C 12 H 22 O 11)? �Find the molar mass of table sugar. � 12 x 12. 011+22 x 1. 0079+11 x 15. 999= � 144. 132+22. 1738+175. 989= � 342. 295 g/mol �Add enough water to 342 grams of table sugar to make 1 liter of solution.
- Slides: 21