Chapter 8 3 8 4 Intermolecular Forces CHM


- Slides: 16

Chapter 8. 3 -8. 4 – Intermolecular Forces CHM 1111 Section 04 Instructor: Dr. Jules Carlson Class Time: M/W/F 1: 30 -2: 20 Wednesday, November 16 th

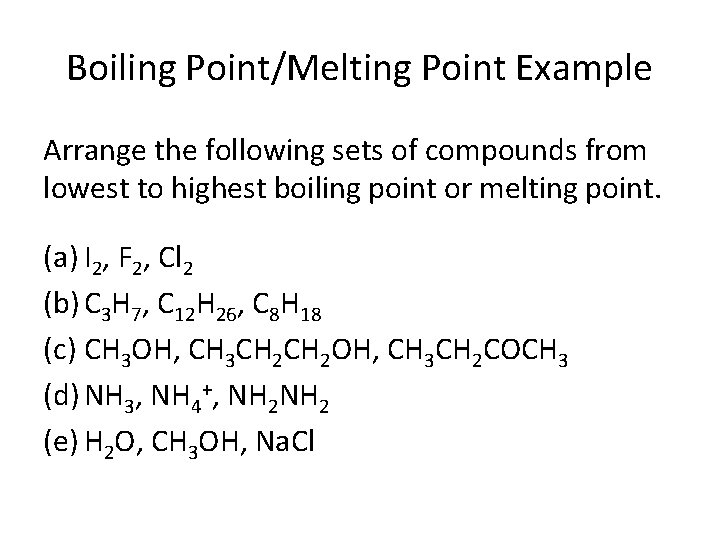
Boiling Point/Melting Point Example Arrange the following sets of compounds from lowest to highest boiling point or melting point. (a) I 2, F 2, Cl 2 (b) C 3 H 7, C 12 H 26, C 8 H 18 (c) CH 3 OH, CH 3 CH 2 OH, CH 3 CH 2 COCH 3 (d) NH 3, NH 4+, NH 2 (e) H 2 O, CH 3 OH, Na. Cl

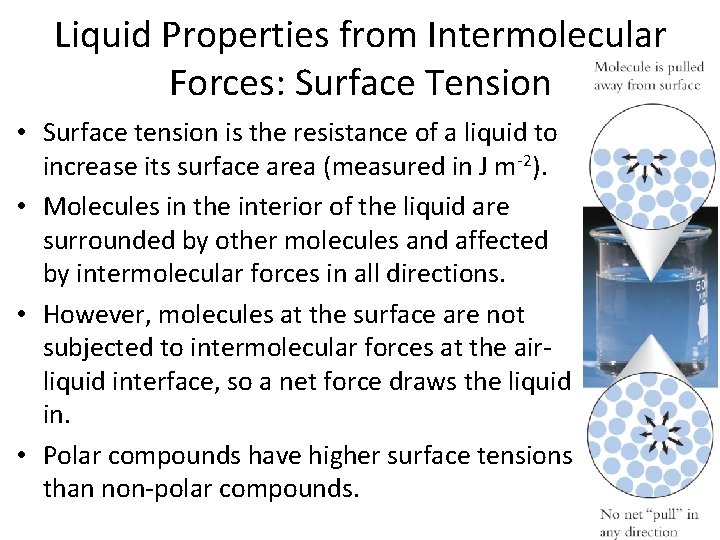
Liquid Properties from Intermolecular Forces: Surface Tension • Surface tension is the resistance of a liquid to increase its surface area (measured in J m-2). • Molecules in the interior of the liquid are surrounded by other molecules and affected by intermolecular forces in all directions. • However, molecules at the surface are not subjected to intermolecular forces at the airliquid interface, so a net force draws the liquid in. • Polar compounds have higher surface tensions than non-polar compounds.

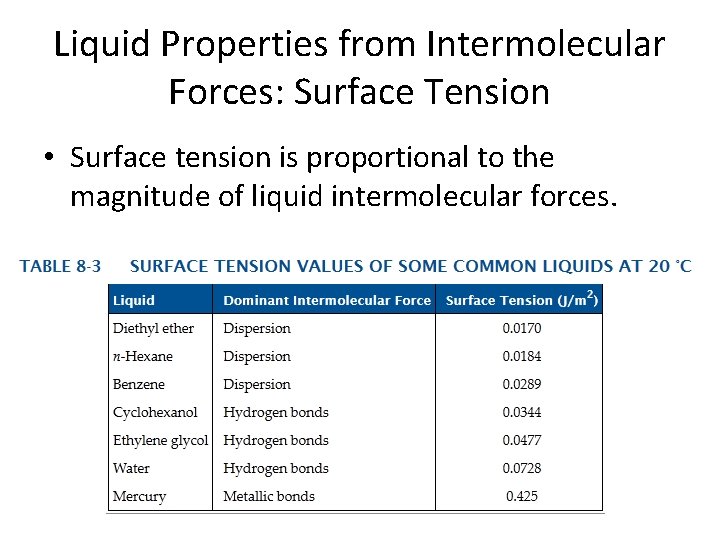
Liquid Properties from Intermolecular Forces: Surface Tension • Surface tension is proportional to the magnitude of liquid intermolecular forces.

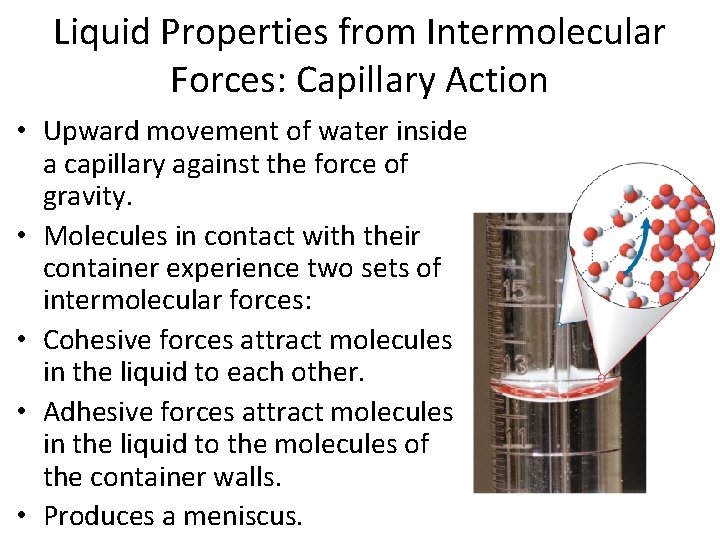
Liquid Properties from Intermolecular Forces: Capillary Action • Upward movement of water inside a capillary against the force of gravity. • Molecules in contact with their container experience two sets of intermolecular forces: • Cohesive forces attract molecules in the liquid to each other. • Adhesive forces attract molecules in the liquid to the molecules of the container walls. • Produces a meniscus.

Liquid Properties from Intermolecular Forces: Viscosity • Viscosity – a liquid’s resistance to flow. • Viscosity depends upon molecular shapes and sizes. Small molecules like water and acetone have lower viscosity while sugars (i. e. honey) and oils have higher viscosity. • Viscosity decreases with increasing temperature.

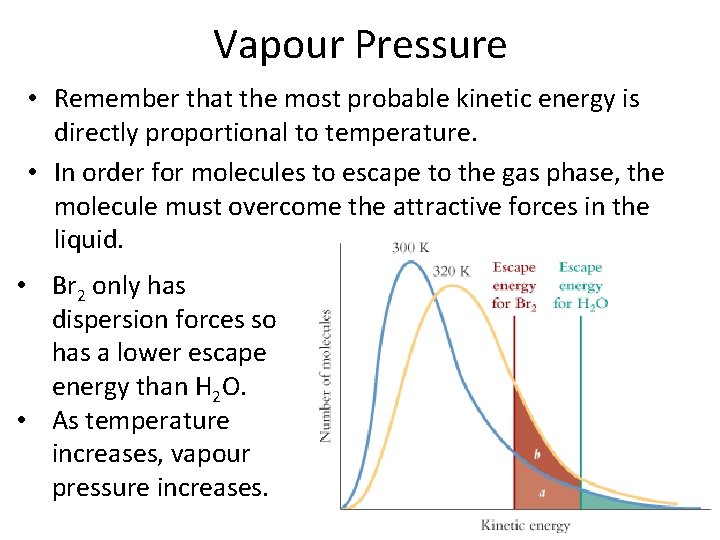
Vapour Pressure • Remember that the most probable kinetic energy is directly proportional to temperature. • In order for molecules to escape to the gas phase, the molecule must overcome the attractive forces in the liquid. • Br 2 only has dispersion forces so has a lower escape energy than H 2 O. • As temperature increases, vapour pressure increases.

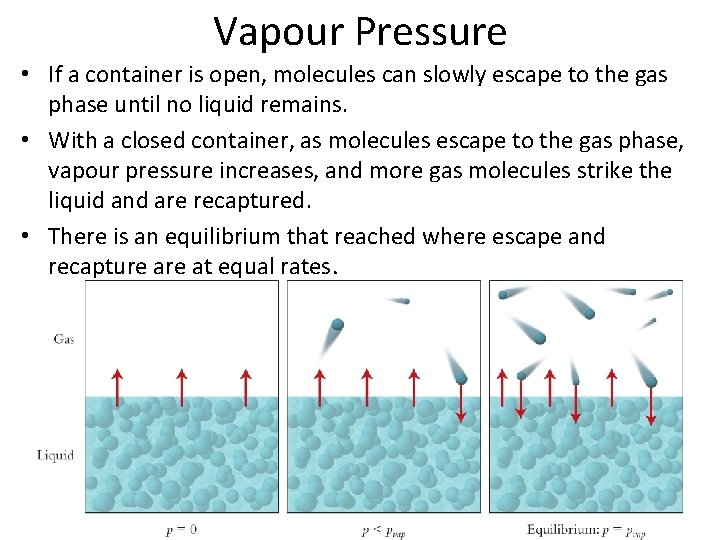
Vapour Pressure • If a container is open, molecules can slowly escape to the gas phase until no liquid remains. • With a closed container, as molecules escape to the gas phase, vapour pressure increases, and more gas molecules strike the liquid and are recaptured. • There is an equilibrium that reached where escape and recapture at equal rates.

Vapour Pressure Problem • For the following pairs of liquids, which has the lower vapour pressure at room temperature and why? a) Water (H 2 O) or Methanol (Me. OH) b) 1 -pentanol (C 5 H 12 OH) or 1 -hexanol (C 6 H 13 OH) c) Methyl chloride (CH 3 Cl) or chloroform (CHCl 3)

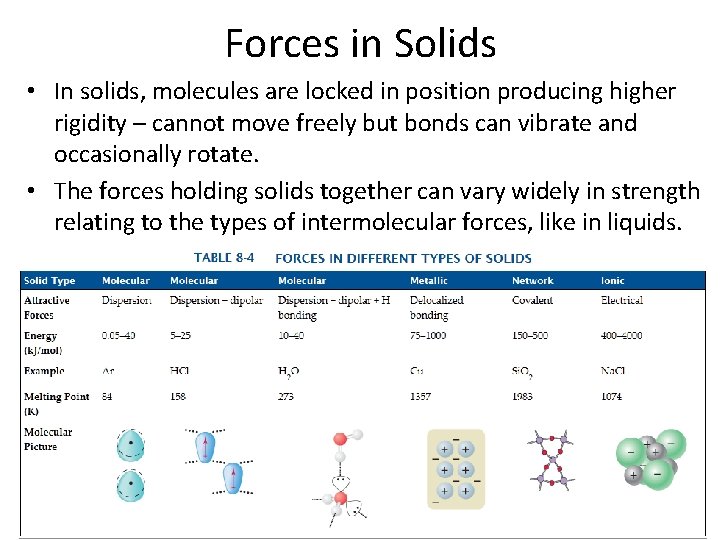
Forces in Solids • In solids, molecules are locked in position producing higher rigidity – cannot move freely but bonds can vibrate and occasionally rotate. • The forces holding solids together can vary widely in strength relating to the types of intermolecular forces, like in liquids.

Types of Solids Four types of solids: 1. Molecular solids – aggregates of molecules held together by dispersion forces, dipolar forces, and/or hydrogen bonding. 2. Network Solids – molecules in array of covalent bonds 3. Metallic Solids – metals bonding from electrons in highly delocalized valence electrons. 4. Ionic Solids – have anions and cations strongly attracted to each other by electrical forces.

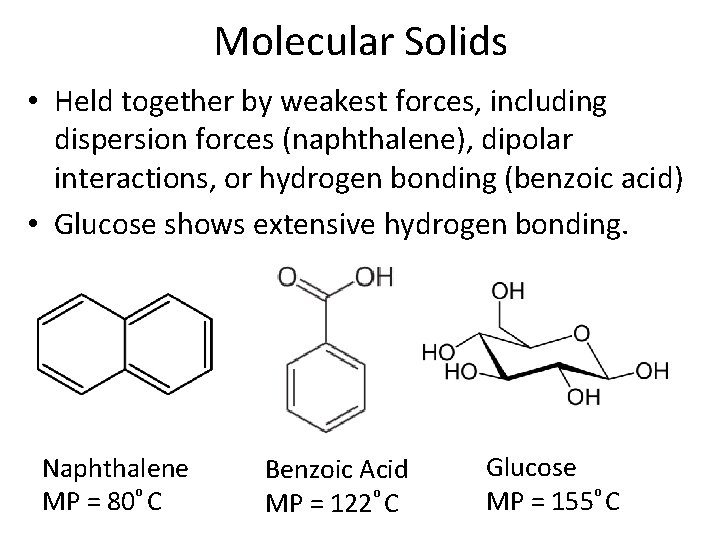
Molecular Solids • Held together by weakest forces, including dispersion forces (naphthalene), dipolar interactions, or hydrogen bonding (benzoic acid) • Glucose shows extensive hydrogen bonding. Naphthalene MP = 80⁰ C Benzoic Acid MP = 122⁰ C Glucose MP = 155⁰ C

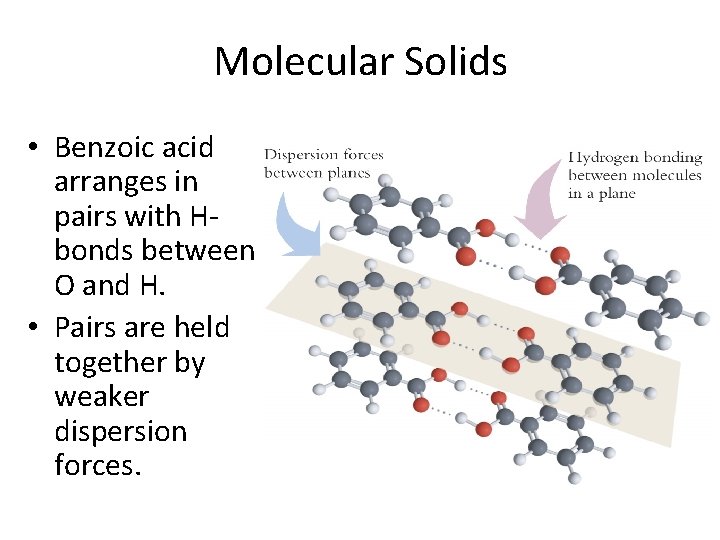
Molecular Solids • Benzoic acid arranges in pairs with Hbonds between O and H. • Pairs are held together by weaker dispersion forces.

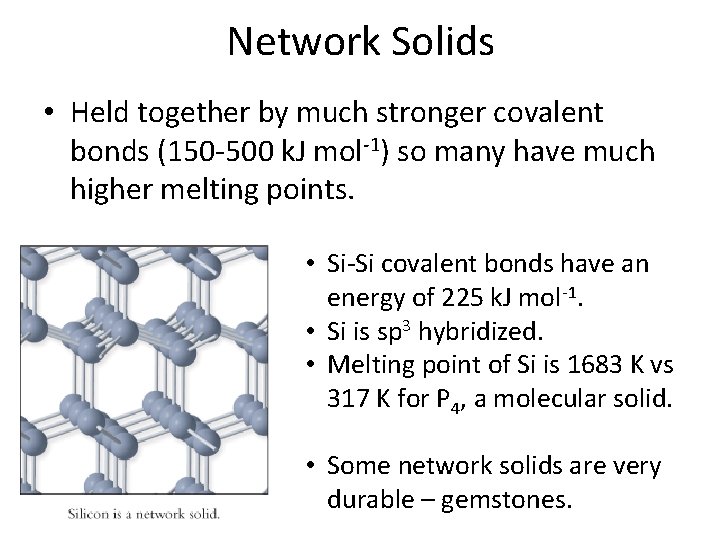
Network Solids • Held together by much stronger covalent bonds (150 -500 k. J mol-1) so many have much higher melting points. • Si-Si covalent bonds have an energy of 225 k. J mol-1. • Si is sp 3 hybridized. • Melting point of Si is 1683 K vs 317 K for P 4, a molecular solid. • Some network solids are very durable – gemstones.

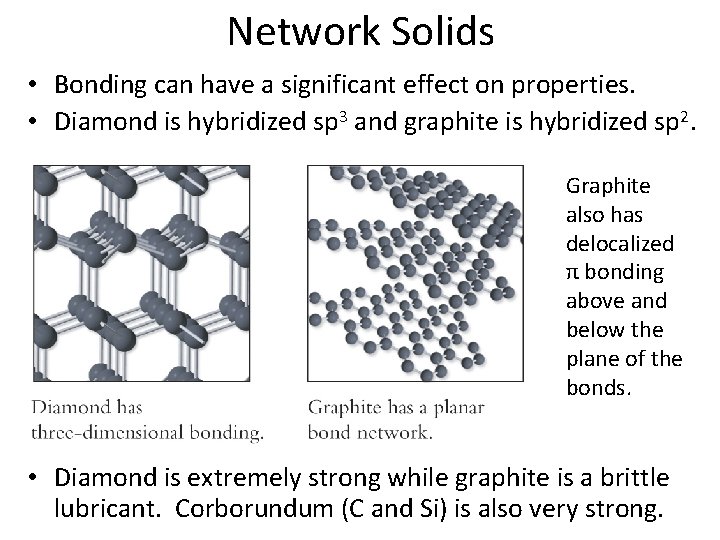
Network Solids • Bonding can have a significant effect on properties. • Diamond is hybridized sp 3 and graphite is hybridized sp 2. Graphite also has delocalized π bonding above and below the plane of the bonds. • Diamond is extremely strong while graphite is a brittle lubricant. Corborundum (C and Si) is also very strong.

Metallic Solids • Metallic solids have highly delocalized valence orbitals. Produces a “sea” of mobile valence electrons. • Metals in group 1 are soft, have low melting points, and have poor electrical conductivity. • Metals are ductile and malleable. • Metals in the middle of the d-block are very strong and have the highest melting points. (Tungsten’s MP = 3407 ⁰C). Have high numbers of valence electrons that can occupy bonding orbitals in the metal lattice. • Metals in group 11 (Copper, Silver, Gold) are have high electrical conductivity.