Chapter 7 Using Chemical Formulas Formula Mass Formula




- Slides: 15

Chapter 7 Using Chemical Formulas

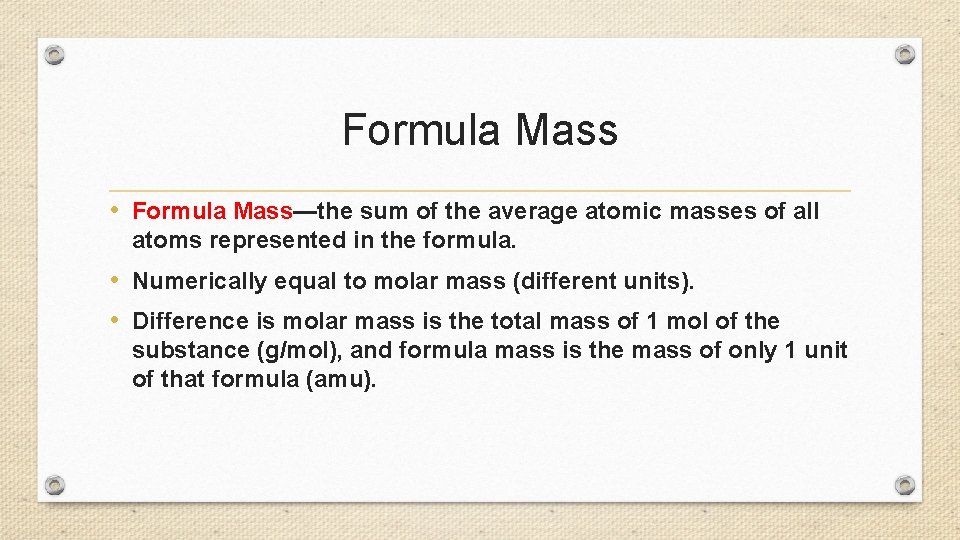
Formula Mass • Formula Mass—the sum of the average atomic masses of all atoms represented in the formula. • Numerically equal to molar mass (different units). • Difference is molar mass is the total mass of 1 mol of the substance (g/mol), and formula mass is the mass of only 1 unit of that formula (amu).

Formulas • Subscript numbers tell how many moles of each atom are in 1 mol of the compound or molecule. • Example: Al 2 S 3 shows there are 2 mol of Aluminum and 3 mol of Sulfur in every 1 mol of Al 2 S 3

Practice • Determine the number of moles of each atom present in the following formulas: • Mg(OH)2 • H 2 SO 4 • Ca(NO 3)2 • Determine the molar mass for each compound

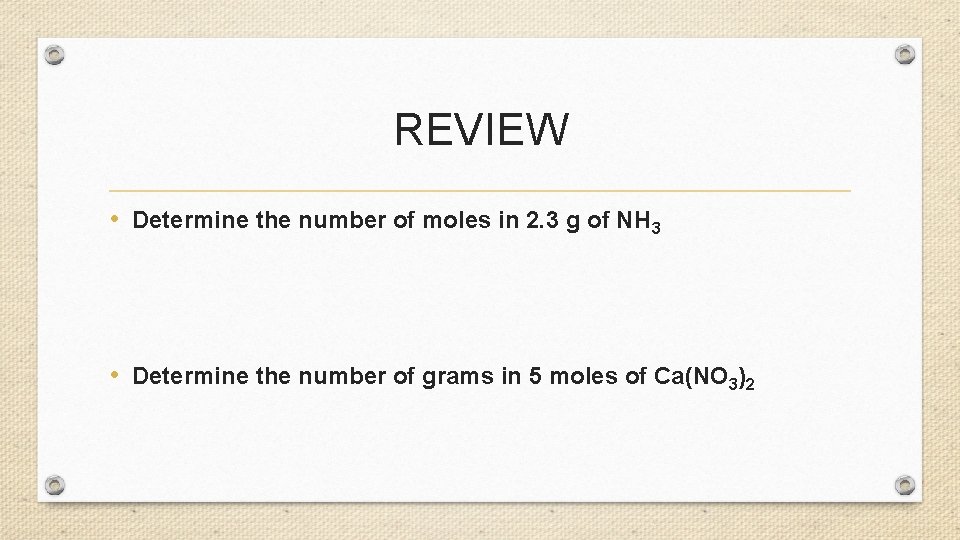
REVIEW • Determine the number of moles in 2. 3 g of NH 3 • Determine the number of grams in 5 moles of Ca(NO 3)2

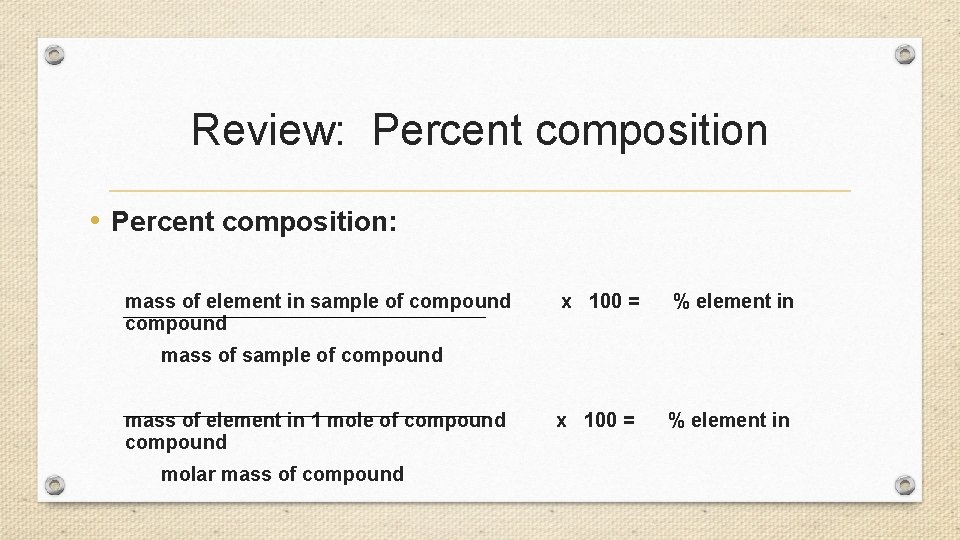
Review: Percent composition • Percent composition: mass of element in sample of compound x 100 = % element in mass of sample of compound mass of element in 1 mole of compound molar mass of compound

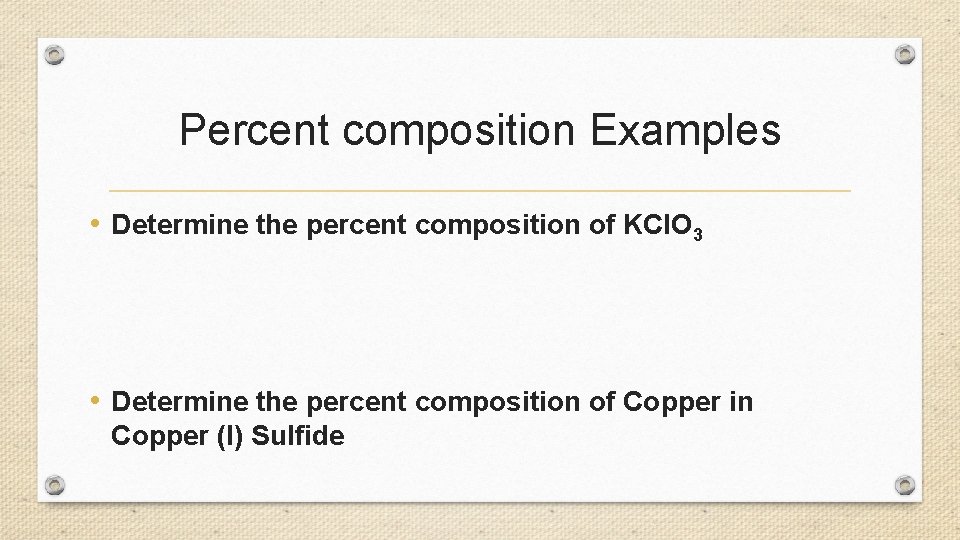
Percent composition Examples • Determine the percent composition of KCl. O 3 • Determine the percent composition of Copper in Copper (I) Sulfide

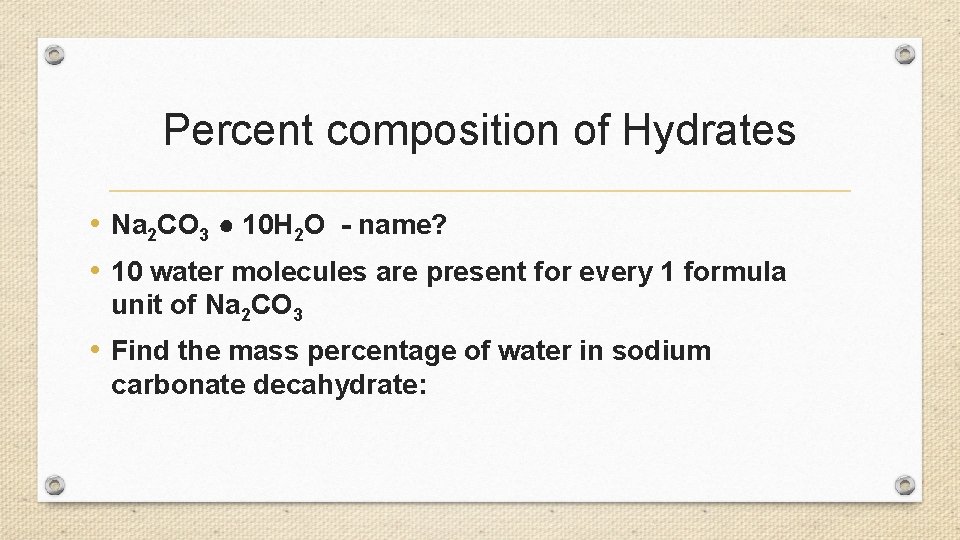
Percent composition of Hydrates • Na 2 CO 3 ● 10 H 2 O - name? • 10 water molecules are present for every 1 formula unit of Na 2 CO 3 • Find the mass percentage of water in sodium carbonate decahydrate:

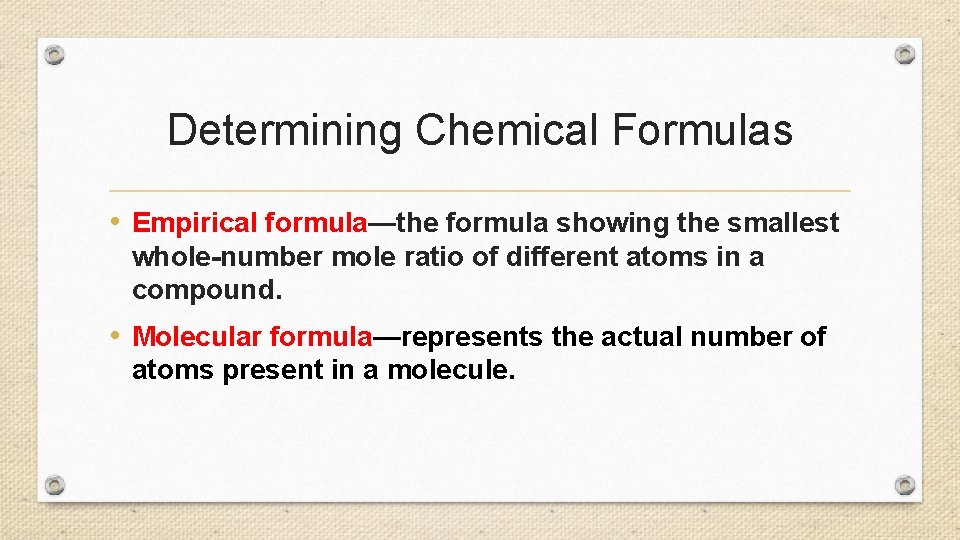
Determining Chemical Formulas • Empirical formula—the formula showing the smallest whole-number mole ratio of different atoms in a compound. • Molecular formula—represents the actual number of atoms present in a molecule.

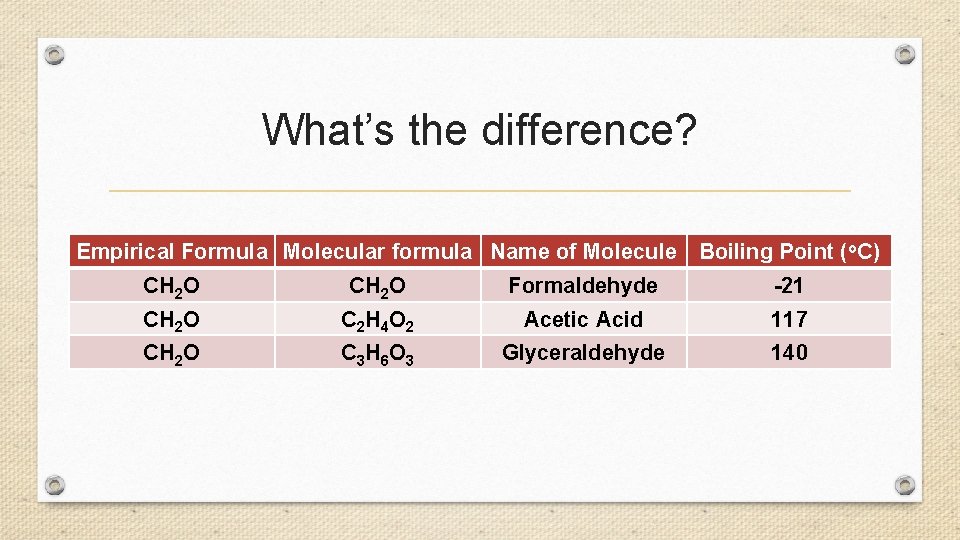
What’s the difference? Empirical Formula Molecular formula Name of Molecule Boiling Point (o. C) CH 2 O Formaldehyde -21 CH 2 O C 2 H 4 O 2 Acetic Acid 117 CH 2 O C 3 H 6 O 3 Glyceraldehyde 140

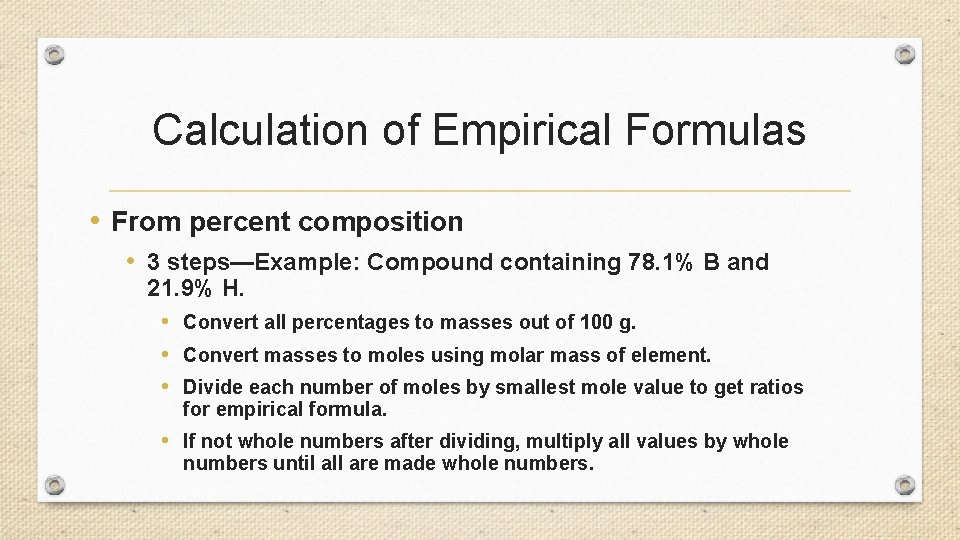
Calculation of Empirical Formulas • From percent composition • 3 steps—Example: Compound containing 78. 1% B and 21. 9% H. • Convert all percentages to masses out of 100 g. • Convert masses to moles using molar mass of element. • Divide each number of moles by smallest mole value to get ratios for empirical formula. • If not whole numbers after dividing, multiply all values by whole numbers until all are made whole numbers.

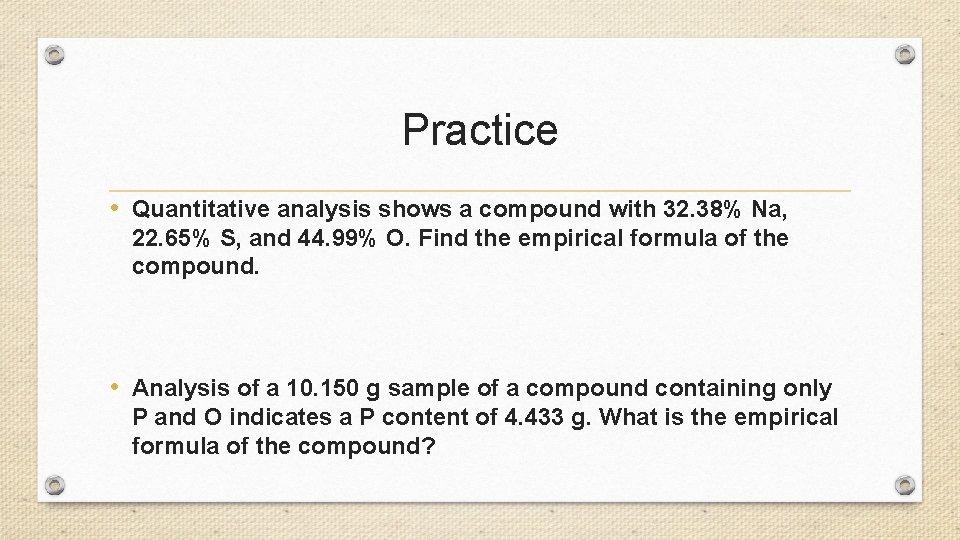
Practice • Quantitative analysis shows a compound with 32. 38% Na, 22. 65% S, and 44. 99% O. Find the empirical formula of the compound. • Analysis of a 10. 150 g sample of a compound containing only P and O indicates a P content of 4. 433 g. What is the empirical formula of the compound?

Empirical Formula of Hydrates • A 5. 018 gram sample of a certain hydrate of magnesium sulfate, Mg. SO 4 • x. H 2 O, is heated until all the water is driven off. The resulting anhydrous compound weighs 2. 449 grams. What is the formula of the hydrate?

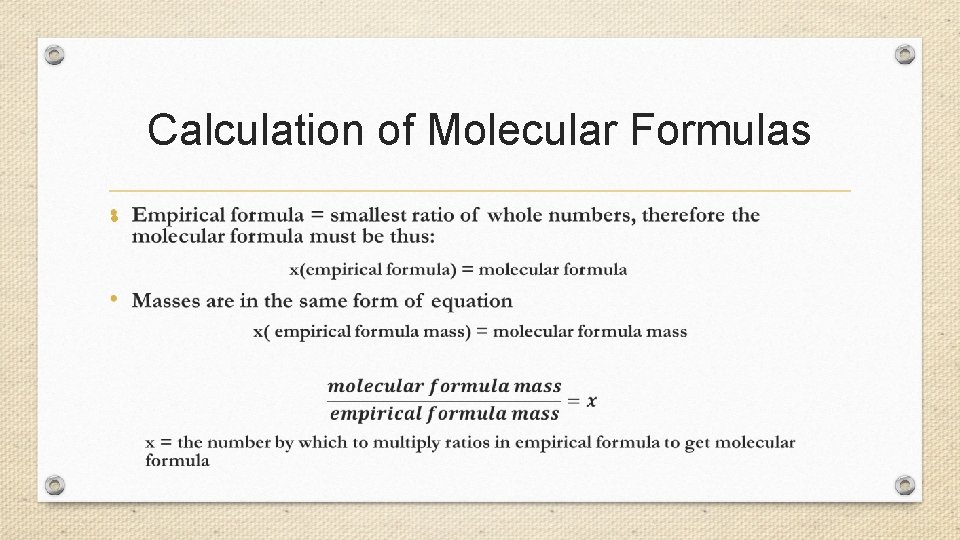
Calculation of Molecular Formulas •

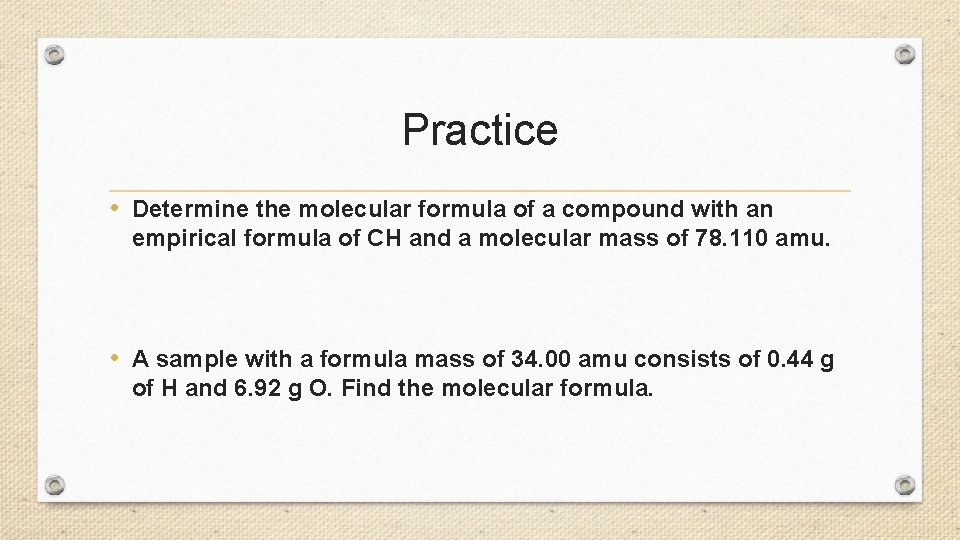
Practice • Determine the molecular formula of a compound with an empirical formula of CH and a molecular mass of 78. 110 amu. • A sample with a formula mass of 34. 00 amu consists of 0. 44 g of H and 6. 92 g O. Find the molecular formula.