Chapter 7 Trends in the Periodic Table 5

Chapter 7: Trends in the Periodic Table 5 th Year Chemistry Ms. Kelly
Trends in atomic radii n n Not possible to measure distance between the nucleus and the outermost electrons of an atom……. (Heisenberg Uncertainty Principle) This distance is known as the bond length
Bond Length • measured by techniques such as X-ray diffraction and electron diffraction n Hydrogen molecule = distance between 2 nuclei is 0. 074 nanometres (1 nm = 1 x 10 -9 m) Therefore the covalent radius of a hydrogen atom is half this – 0. 037 nm. Note no values of covalent radii for noble gases – don’t form covalent bonds with one another
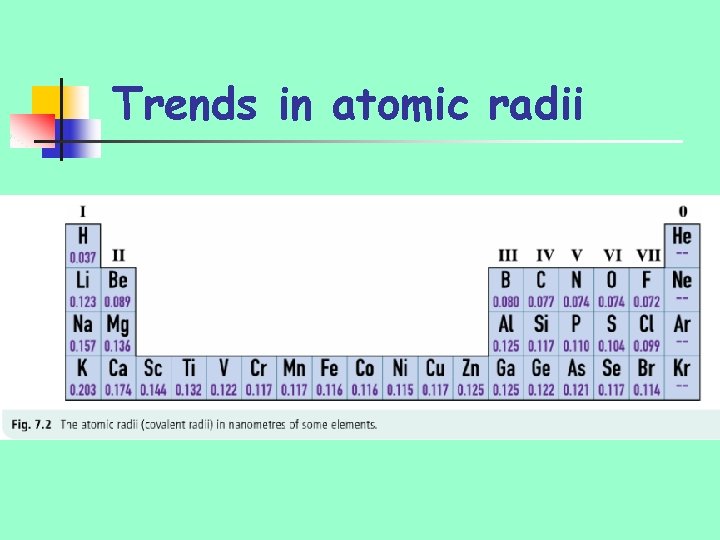
Trends in atomic radii
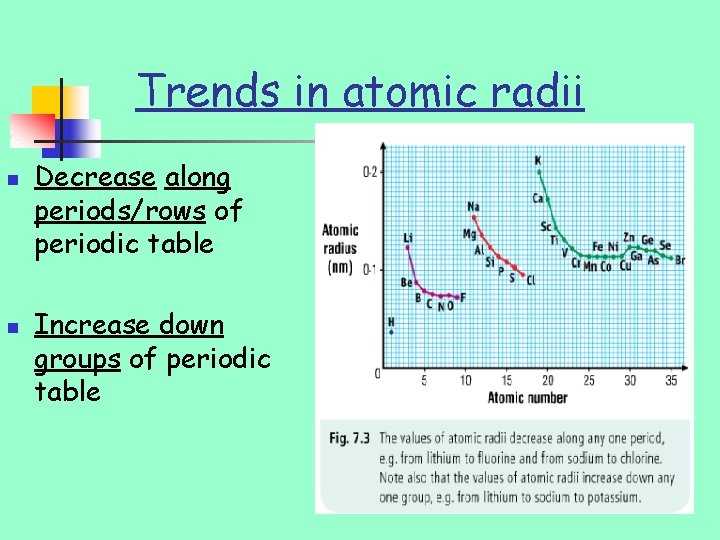
Trends in atomic radii n n Decrease along periods/rows of periodic table Increase down groups of periodic table

Size of an atom is governed by the electrostatic attraction between the positively charged protons and the negatively charged electrons n n If this attraction is large, the positive protons will pull the outer electrons closer to the nucleus giving a smaller atomic radius If this attraction is small, the electrons will be further from the nucleus giving a larger atomic radius

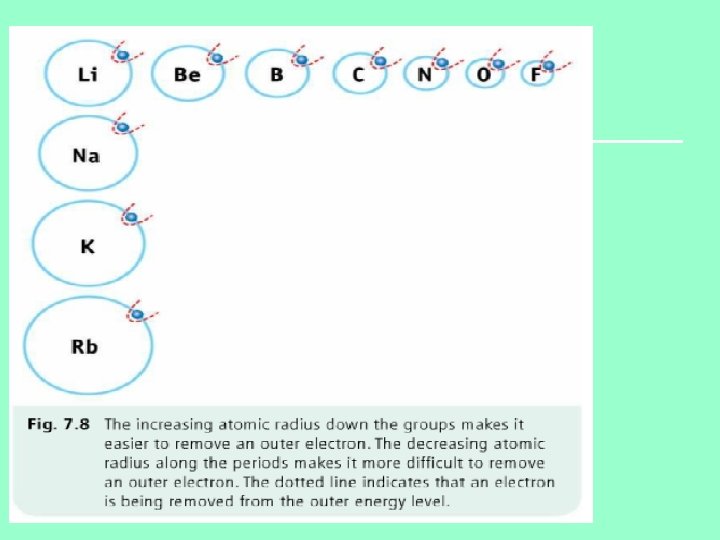
Reason 1 n As you go down a group additional electrons go into a new energy level/shells n Since the outer electrons are becoming further away from nucleus, the atomic radius increases Reason 2 n Screening effect of inner electrons n Electrons in inner energy levels shield/screen outer electrons from positive charge of nucleus.
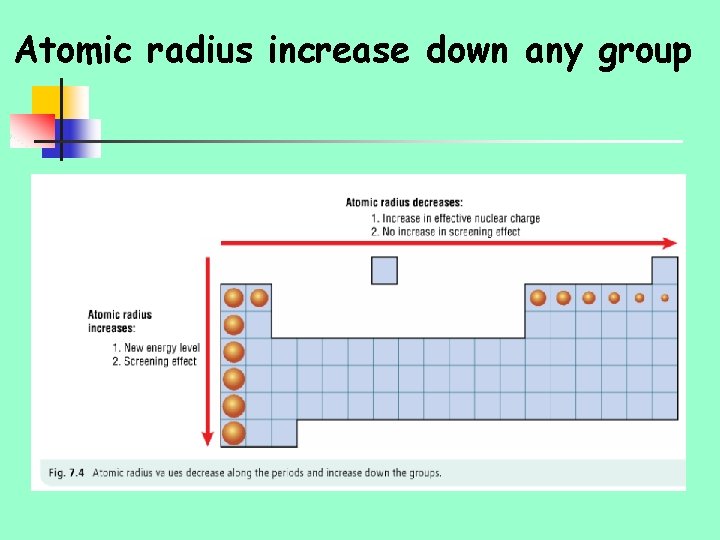
Atomic radius increase down any group
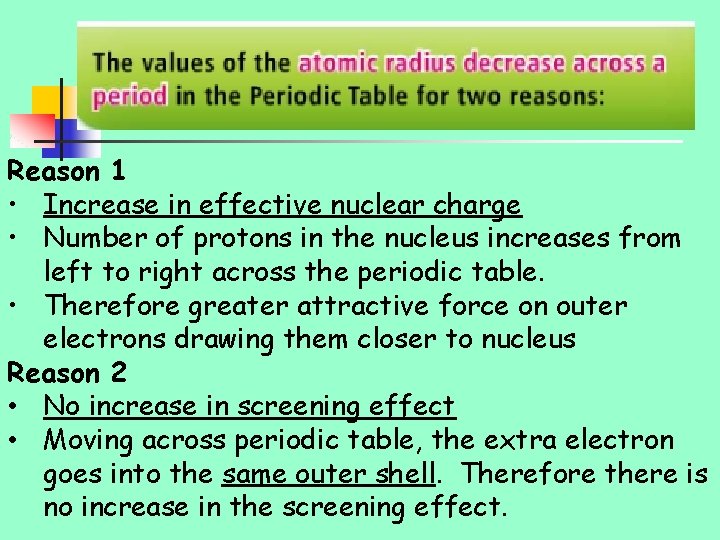
Reason 1 • Increase in effective nuclear charge • Number of protons in the nucleus increases from left to right across the periodic table. • Therefore greater attractive force on outer electrons drawing them closer to nucleus Reason 2 • No increase in screening effect • Moving across periodic table, the extra electron goes into the same outer shell. Therefore there is no increase in the screening effect.

To measure the tendency for an atom to lose electrons chemists define a term called the first ionisation energy.
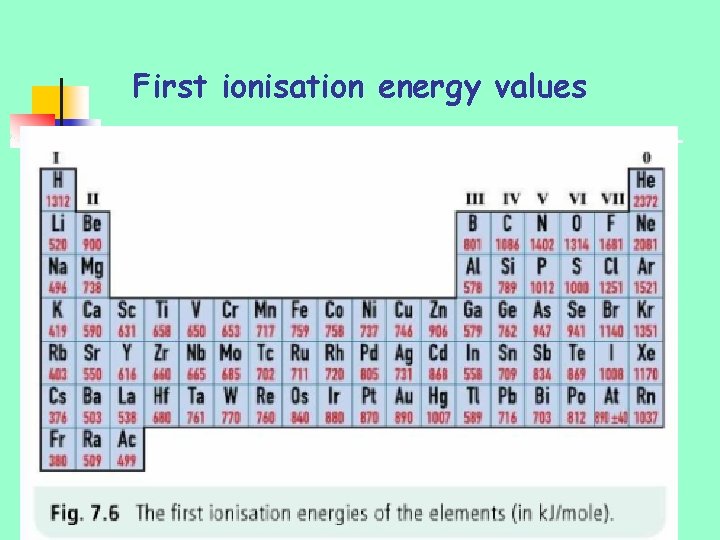
First ionisation energy values
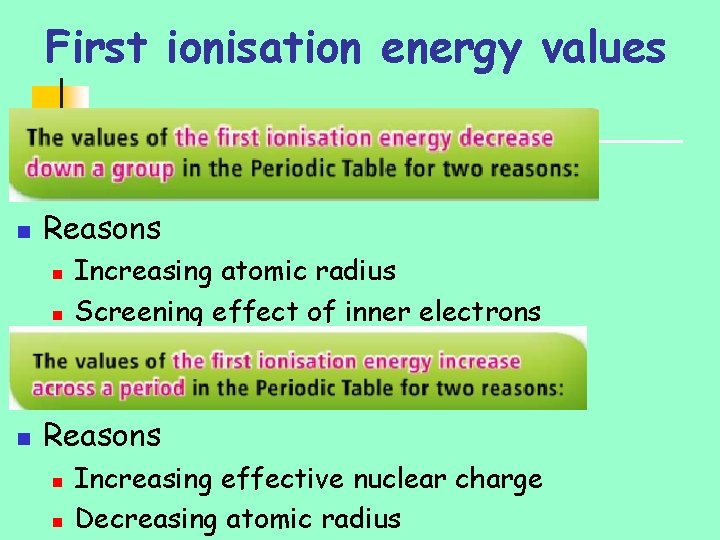
First ionisation energy values n Reasons n n n Increasing atomic radius Screening effect of inner electrons Reasons n n Increasing effective nuclear charge Decreasing atomic radius
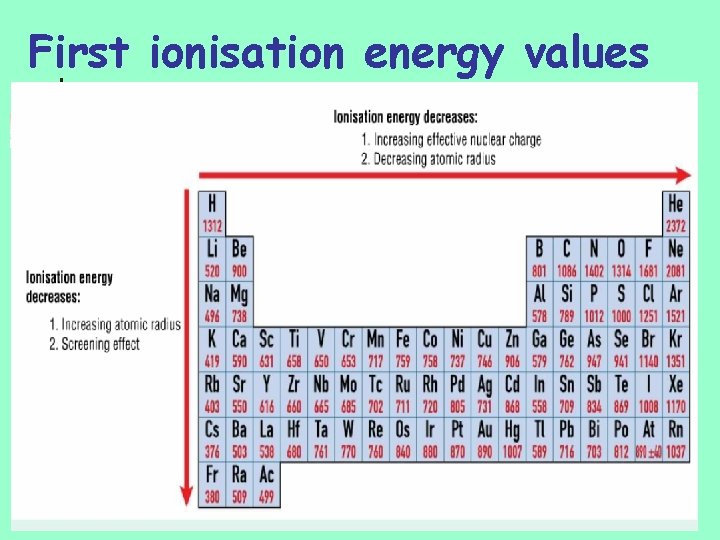
First ionisation energy values n n increase across a period in periodic table…. . i. e. more difficult to remove most loosely bound electron as you go across periodic table Reasons
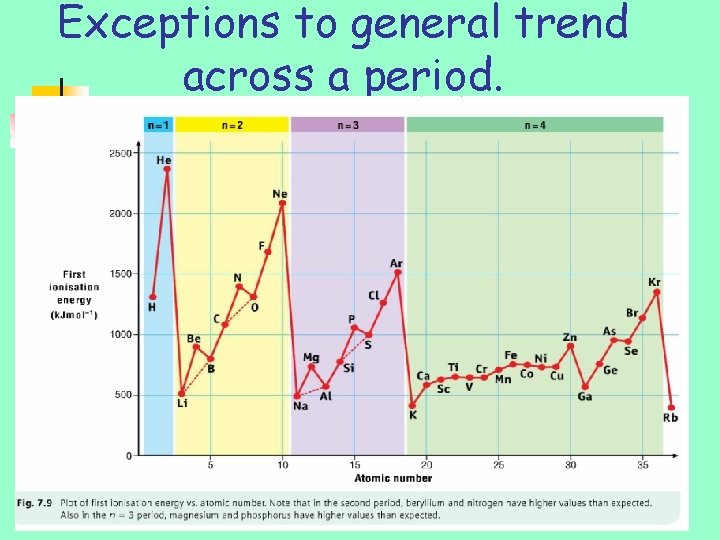
Exceptions to general trend across a period.

Exceptions to general trend across a period. • Higher than expected ionisation energies • Extra stability – filled or half filled sublevels
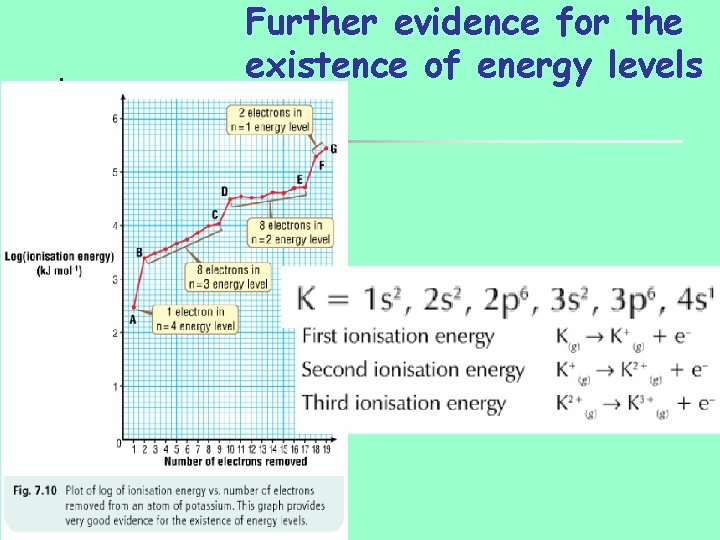
Further evidence for the existence of energy levels

Second ionisation energy

General points on ionization energies n Steady increase as electrons removed n n Ion become more positive, grater attraction on remaining electrons Positive ion smaller, remaining electrons closer to nucleus Large increase in ionisation energy when electron removed from new energy level/shell Substantial increase when e removed from new sublevel
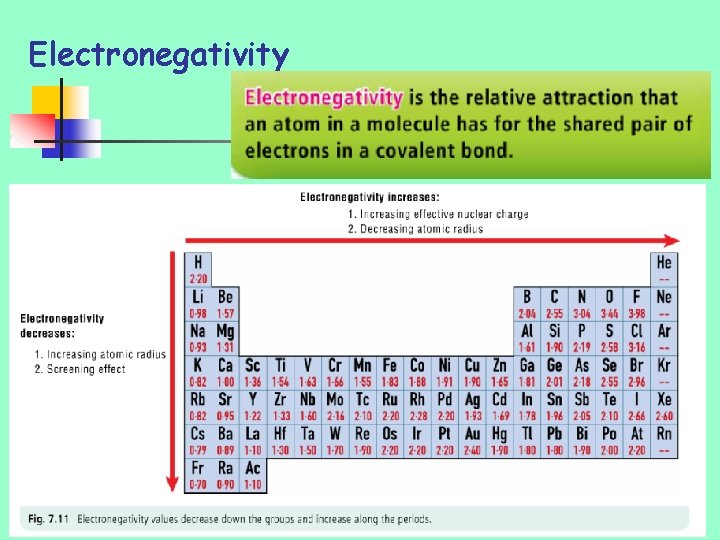
Electronegativity
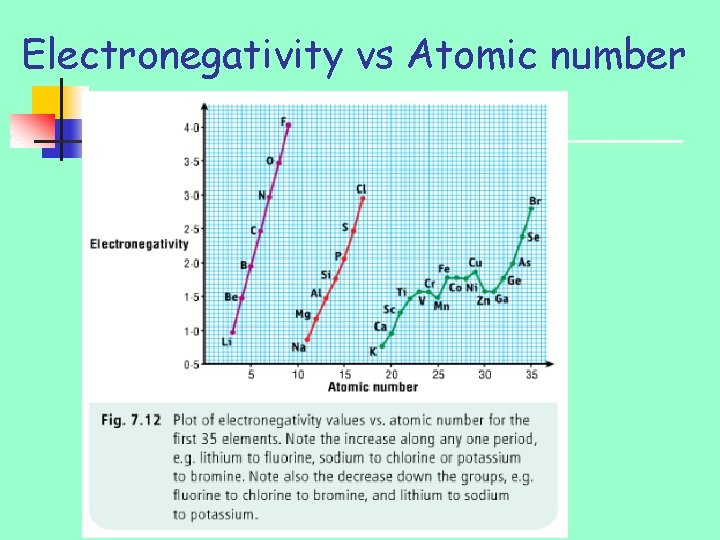
Electronegativity vs Atomic number

Values of electronegativities decrease down the groups in the Periodic table Reason 1 n n Increasing atomic radius Atomic radii increase down any group. The outer electrons are becoming further away from the attractive force of the nucleus. Therefore there is a smaller attraction between the nucleus and the shared pair of electrons…. . ie electronegativity decreases
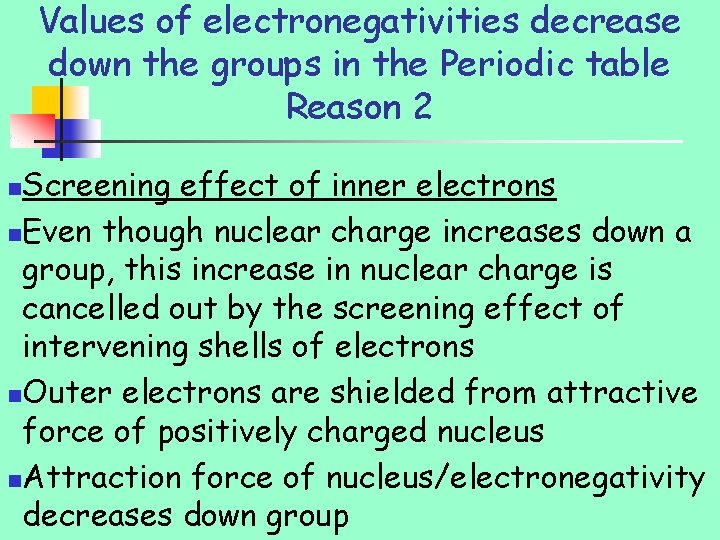
Values of electronegativities decrease down the groups in the Periodic table Reason 2 Screening effect of inner electrons n. Even though nuclear charge increases down a group, this increase in nuclear charge is cancelled out by the screening effect of intervening shells of electrons n. Outer electrons are shielded from attractive force of positively charged nucleus n. Attraction force of nucleus/electronegativity decreases down group n

Values of electronegativities increase across the periods in the Periodic table Reason 1 n n n Increasing nuclear charge Moving from left to right across periodic table, number protons increasing in nucleus Therefore attraction between nucleus and outer electrons is also increasing Therefore the electrons involved in binding are being more strongly attracted to the nucleus The electronegativity increases

Values of electronegativities increase across the periods in the Periodic table Reason 2 n n n Decreasing atomic radius Within any row the atomic radius decreases from left to right Therefore outer electrons are becoming closer to the nucleus Thus there is greater attraction between nucleus and these outer electrons Electronegativity increases

Trends within groups Group 1 – Alkali metals n n n Very reactive, low first ionization values So reactive, none occur free in nature Readily lose single outer electron to form ionic compounds Low melting points So reactive stored under oil or in glass container with air removed (Rb, Cs)
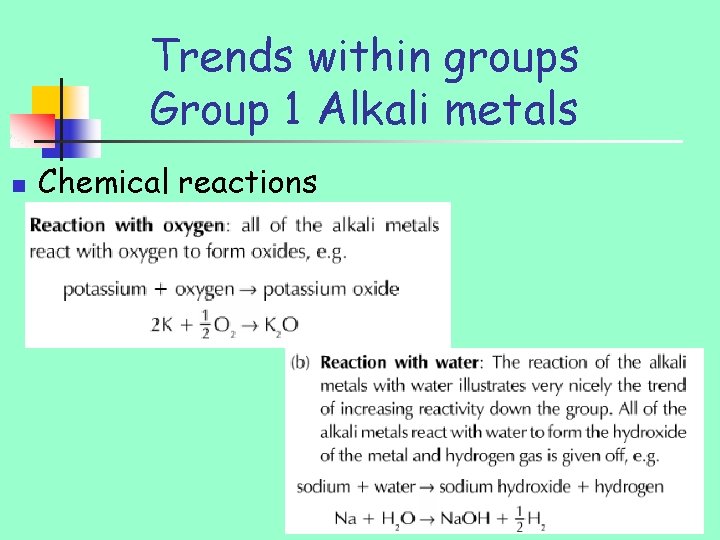
Trends within groups Group 1 Alkali metals n Chemical reactions

Alkalis -Reaction with Acid n Too dangerous, explosive !!!

Halogens (Group 7) – Trends in chemical reactivity n Properties n n n Most electronegative elements in periodic table Fluorine most electronegative element, electronegativity decreases down group Quite reactive, Fluorine most reactive Don’t exist free in nature Remove electrons from other substances easily – oxidising agents

Trends in chemical reactivity of halogens – group 7 n n n If chlorine gas bubbles through solution of bromide ions, chlorine takes electron form bromide Cl – EN 3. 0 Br – EN 2. 8
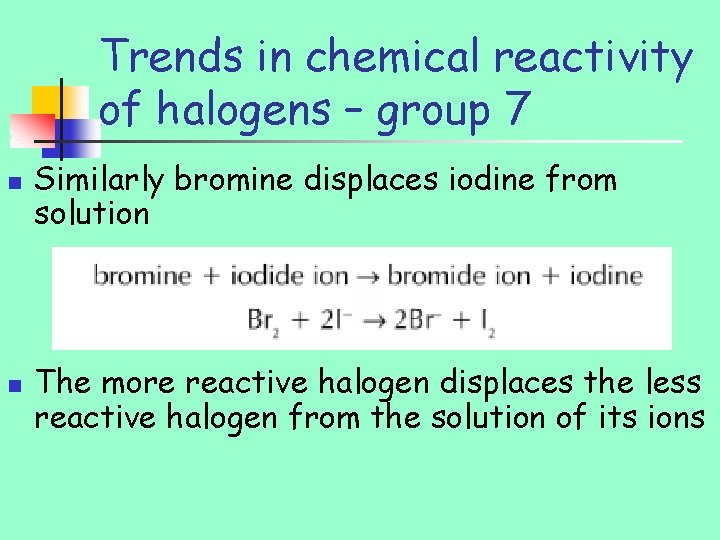
Trends in chemical reactivity of halogens – group 7 n n Similarly bromine displaces iodine from solution The more reactive halogen displaces the less reactive halogen from the solution of its ions

Trends – group 2 n n n Group 2 – alkaline earth metals Be does not react with water Magnesium reacts very slowly with water Calcium undergoes a steady reaction with water Reason – ionisation energy decreases down the group Strontium and barium react more vigorously with water

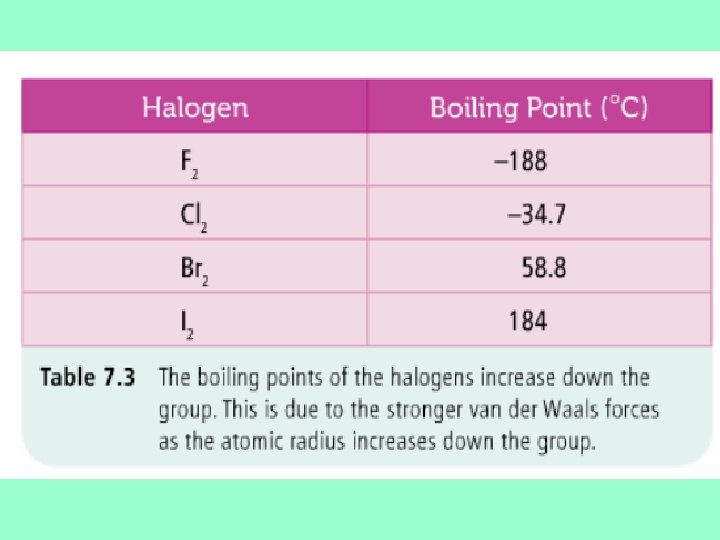
Trends – Physical properties n n Noble inert gases – form practically no compounds, unreactive Steady increase in boiling points down the group from helium to radon This is related to the increasing atomic radius …more Van der Waals forces…. . Similar trend observed in the halogens
- Slides: 34