Chapter 7 The Mole and Chemical Composition Table
Chapter 7 The Mole and Chemical Composition Table of Contents Section 1 Avogadro’s Number and Molar Conversions Section 2 Relative Atomic Mass and Chemical Formulas Section 3 Formulas and Percentage Composition Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Bellringer • List as many common counting units as you can. • Determine how many groups of each unit in your list are present in each of the following amounts: • 500 goldfish • 150 unicycles • 50 jet planes Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Objectives • Identify the mole as the unit used to count particles, whether atoms, ions, or molecules. • Use Avogadro’s number to convert between amount in moles and number of particles. • Solve problems converting between mass, amount in moles, and number of particles using Avogadro’s number and molar mass. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Avogadro’s Number and the Mole • The SI unit for amount is called the mole (mol). A mole is the number of atoms in exactly 12 grams of carbon-12. • Scientists use the mole to make counting large numbers of particles easier. • The number of particles in a mole is called Avogadro’s Number. Avogadro’s number is 6. 02214199 1023 units/mole. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Avogadro’s Number and the Mole, continued The Mole Is a Counting Unit • The mole is used to count out a given number of particles, whether they are atoms, molecules, formula units, ions, or electrons. • The mole is just one kind of counting unit: • 1 dozen = 12 objects • 1 hour = 3600 seconds • 1 mole = 6. 022 1023 particles Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts The Mole Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Avogadro’s Number and the Mole, continued Amount in Moles Can Be Converted to Number of Particles • Counting units are used to make conversion factors. • The definition of one mole is 6. 022 1023 particles = 1 mol • The conversion factor is Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
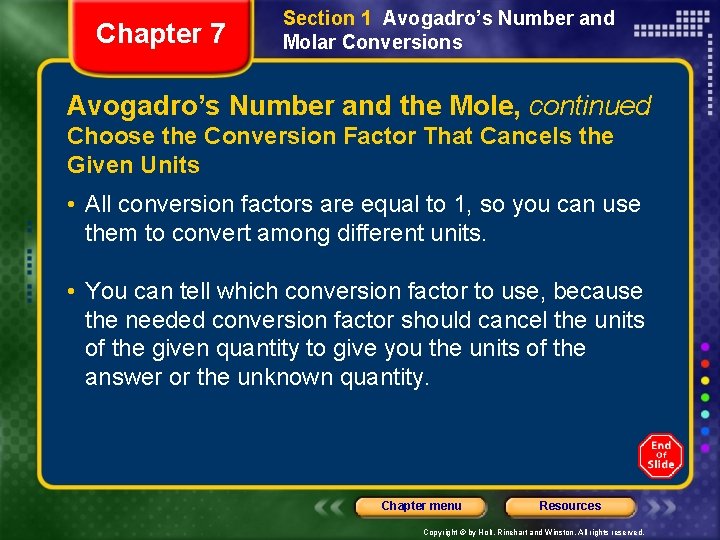
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Avogadro’s Number and the Mole, continued Choose the Conversion Factor That Cancels the Given Units • All conversion factors are equal to 1, so you can use them to convert among different units. • You can tell which conversion factor to use, because the needed conversion factor should cancel the units of the given quantity to give you the units of the answer or the unknown quantity. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Between Amount in Moles and Number of Particles Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
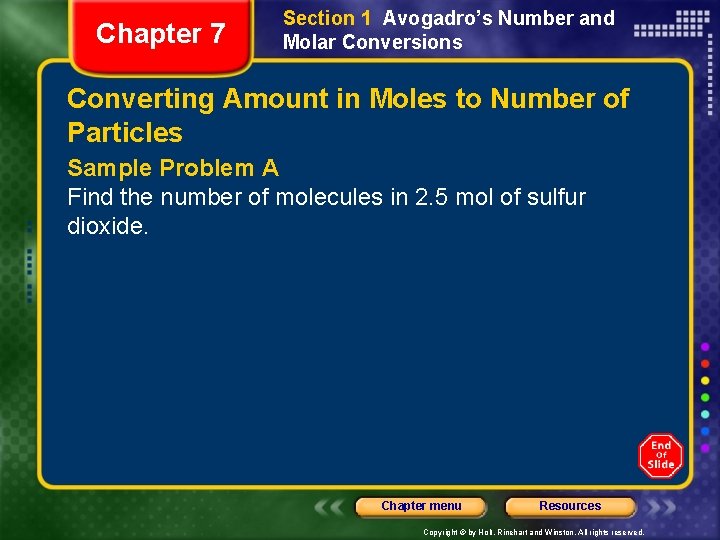
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Amount in Moles to Number of Particles Sample Problem A Find the number of molecules in 2. 5 mol of sulfur dioxide. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Amount in Moles to Number of Particles Sample Problem A Solution 2. 5 mol SO 2 ? = ? molecules SO 2 You are converting from the unit mol to the unit molecules. The conversion factor must have the units of molecules/mol. You use 6. 022 1023 molecules/1 mol. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Amount in Moles to Number of Particles Sample Problem A Solution, continued 2. 5 mol SO 2 ? = ? molecules SO 2 2. 5 mol SO 2 = 1. 5 1024 molecules SO 2 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
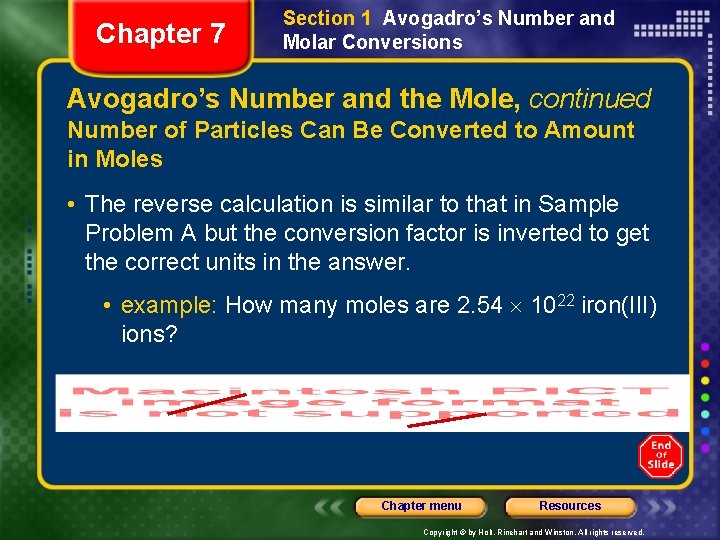
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Avogadro’s Number and the Mole, continued Number of Particles Can Be Converted to Amount in Moles • The reverse calculation is similar to that in Sample Problem A but the conversion factor is inverted to get the correct units in the answer. • example: How many moles are 2. 54 1022 iron(III) ions? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Amount in Moles Sample Problem B A sample contains 3. 01 1023 molecules of sulfur dioxide, SO 2. Determine the amount in moles. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Amount in Moles Sample Problem B Solution 3. 01 1023 molecules SO 2 ? = ? mol SO 2 You are converting from the unit molecules to the unit mol. The conversion factor must have the units of mol/molecules. You use 1 mol/6. 022 1023 molecules. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
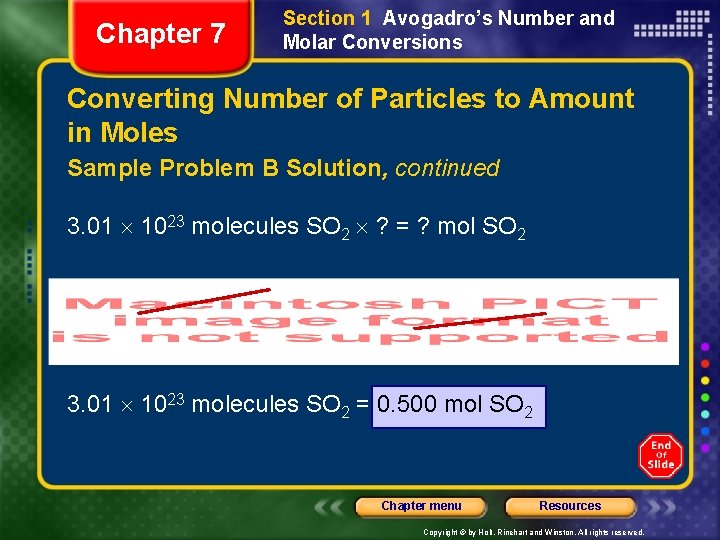
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Amount in Moles Sample Problem B Solution, continued 3. 01 1023 molecules SO 2 ? = ? mol SO 2 3. 01 1023 molecules SO 2 = 0. 500 mol SO 2 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Molar Mass Relates Moles to Grams Amount in Moles Can Be Converted to Mass • The molar mass is the mass in grams of one mole of an element or compound. • Molar mass is numerically equal to the atomic mass of monatomic elements and the formula mass of compounds and diatomic elements. • The units for molar mass are g/mol. • Molar mass can be used as a conversion factor in problems converting between mass and amount. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Molar Mass Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Molar Mass as a Conversion Factor Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Molar Mass Relates Moles to Grams, continued The Mole Plays a Central Part in Chemical Conversions • To convert from number of particles to mass, you must use a two-part process: • First, convert number of particles to amount in moles. • Second, convert amount in moles to mass in grams. • One step common to many problems in chemistry is converting to amount in moles. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
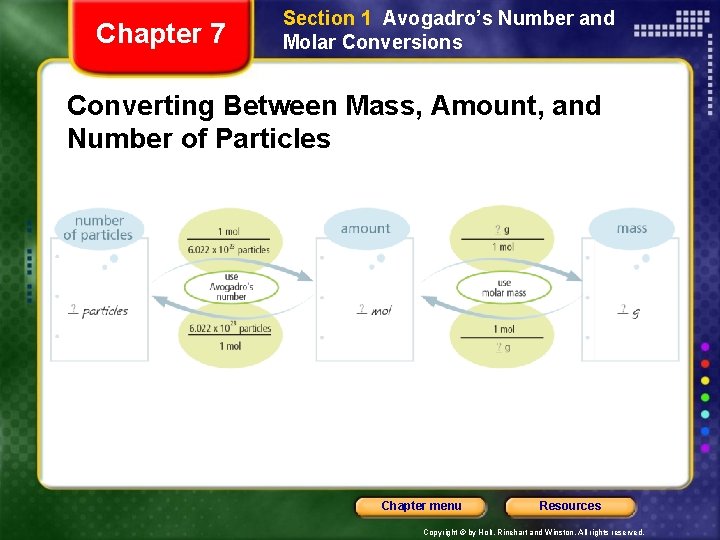
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Between Mass, Amount, and Number of Particles Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Mass Sample Problem C Find the mass in grams of 2. 44 1024 atoms of carbon, whose molar mass is 12. 01 g/mol. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Mass Sample Problem C Solution First part: 2. 44 1024 atoms ? = ? mol Select the conversion factor that will take you from number of atoms to amount in moles. You use 1 mol/6. 022 1023 atoms. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
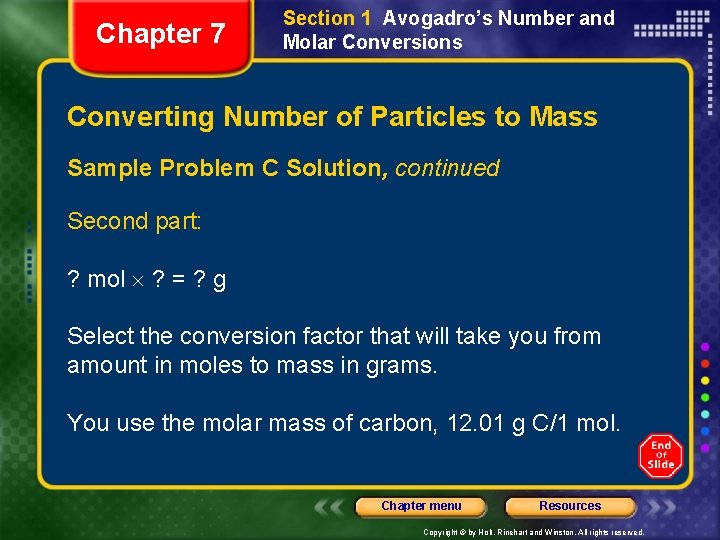
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Mass Sample Problem C Solution, continued Second part: ? mol ? = ? g Select the conversion factor that will take you from amount in moles to mass in grams. You use the molar mass of carbon, 12. 01 g C/1 mol. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
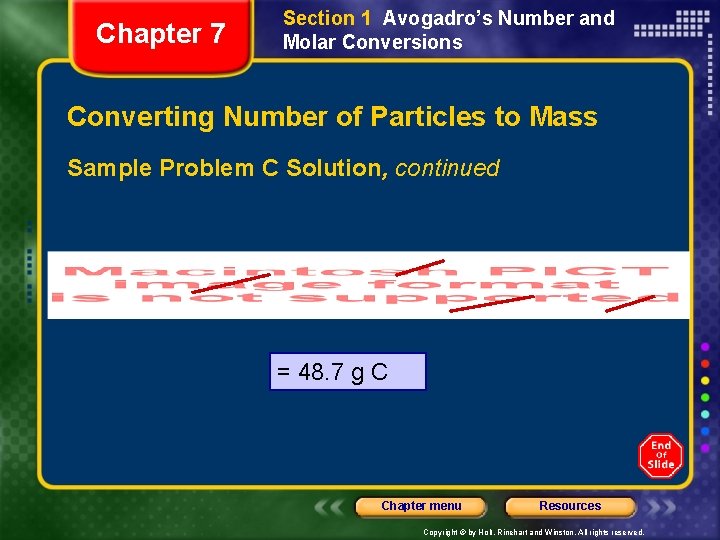
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Number of Particles to Mass Sample Problem C Solution, continued = 48. 7 g C Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Molar Mass Relates Moles to Grams, continued Mass Can Be Converted to Amount in Moles • Converting from mass to number of particles is the reverse of the operation in the previous problem. • To convert from mass to number of particles, you must use a two-part process: • First, convert mass in grams to amount in moles. • Second, convert amount in moles to number of particles. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Mass to Number of Particles Sample Problem D Find the number of molecules present in 47. 5 g of glycerol, C 3 H 8 O 3. The molar mass of glycerol is 92. 11 g/mol. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
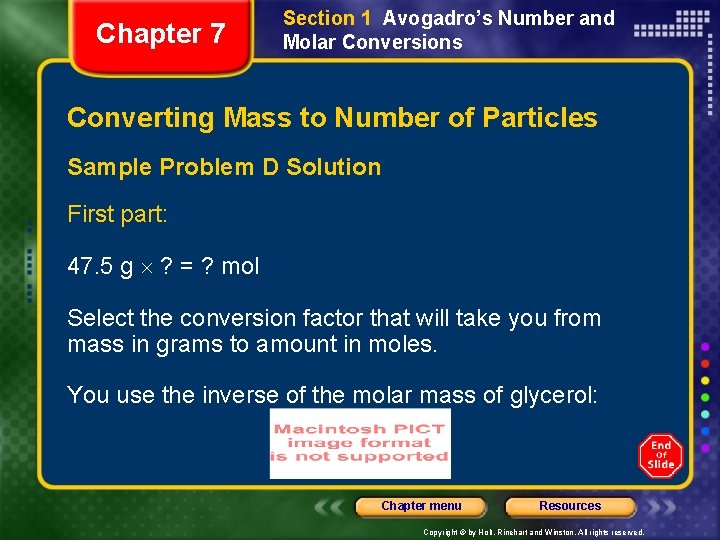
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Mass to Number of Particles Sample Problem D Solution First part: 47. 5 g ? = ? mol Select the conversion factor that will take you from mass in grams to amount in moles. You use the inverse of the molar mass of glycerol: Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Mass to Number of Particles Sample Problem D Solution, continued Second part: ? mol ? = ? molecules Select the conversion factor that will take you from amount in moles to number of particles. You use . Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
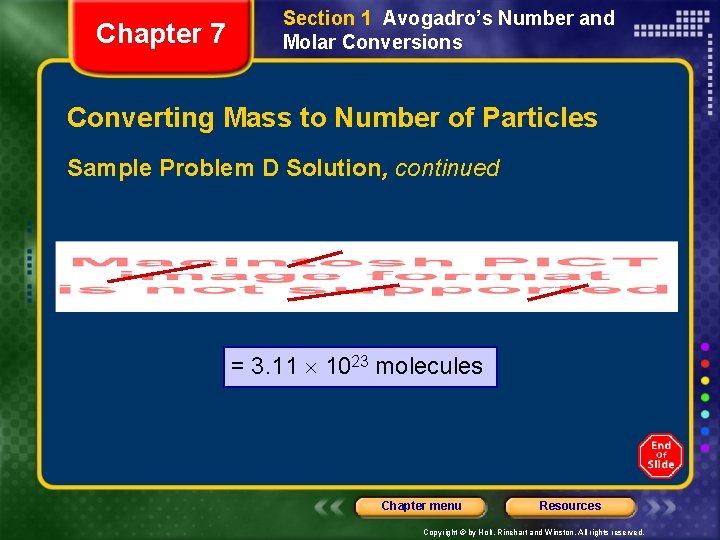
Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Converting Mass to Number of Particles Sample Problem D Solution, continued = 3. 11 1023 molecules Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 1 Avogadro’s Number and Molar Conversions Molar Mass Relates Moles to Grams, continued Remember to Round Consistently • Remember that an answer must never be given to more significant figures than is appropriate. • Round molar masses from the periodic table to two significant figures to the right of the decimal point. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Bellringer • Compare the masses of a roll of pennies and a roll of dimes. Both contain 50 coins. • Why are the masses of the rolls different when both rolls contain the same number of coins? • If given a roll of mixed coins, what information would you need to determine the mass of the roll? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Objectives • Use a periodic table or isotopic composition data to determine the average atomic masses of elements. • Infer information about a compound from its chemical formula. • Determine the molar mass of a compound from its formula. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Average Atomic Mass and the Periodic Table Most Elements are a Mixture of Isotopes • Isotopes are atoms that have different numbers of neutrons than other atoms of the same element do. • Average atomic mass is a weighted average of the atomic mass of an element’s isotopes. • If you know the abundance of each isotope, you can calculate the average atomic mass of an element. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Average Atomic Mass Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Average Atomic Mass Sample Problem E The mass of a Cu-63 atom is 62. 94 amu, and that of a Cu-65 atom is 64. 93 amu. Using the data below, find the average atomic mass of copper. • abundance of Cu-63 = 69. 17% • abundance of Cu-65 = 30. 83% Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Average Atomic Mass Sample Problem E Solution The contribution of each isotope is equal to its atomic mass multiplied by the fraction of that isotope. • contribution of Cu-63: • contribution of Cu-65: 62. 94 amu 0. 6917 64. 93 amu 0. 3083 Average atomic mass is the sum of the individual contributions: (62. 94 amu 0. 6917) + (64. 93 amu 0. 3083) = 63. 55 amu Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas and Moles Formulas Express Composition • A compound’s chemical formula tells you which elements, as well as how much of each, are present in a compound. • Formulas for covalent compounds show the elements and the number of atoms of each element in a molecule. • Formulas for ionic compounds show the simplest ratio of cations and anions in any pure sample. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas and Moles, continued Formulas Express Composition, continued • Any sample of compound has many atoms and ions, and the formula gives a ratio of those atoms or ions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
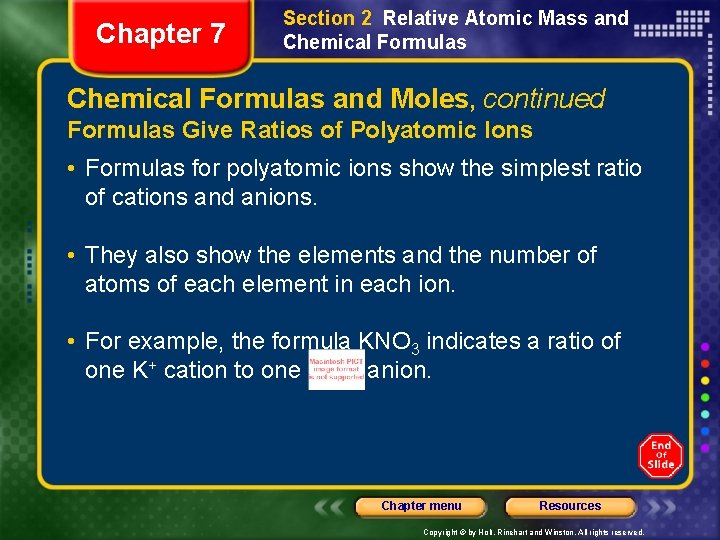
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas and Moles, continued Formulas Give Ratios of Polyatomic Ions • Formulas for polyatomic ions show the simplest ratio of cations and anions. • They also show the elements and the number of atoms of each element in each ion. • For example, the formula KNO 3 indicates a ratio of one K+ cation to one anion. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
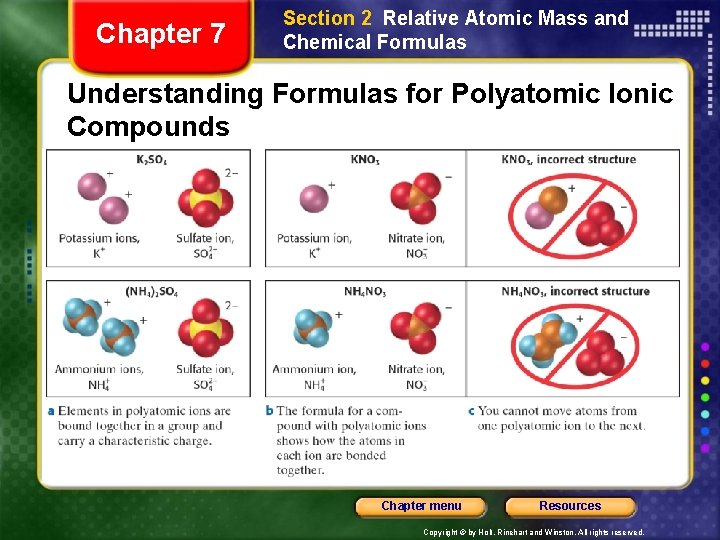
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Understanding Formulas for Polyatomic Ionic Compounds Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas and Moles, continued Formulas Are Used to Calculate Molar Masses • The molar mass of a molecular compound is the sum of the masses of all the atoms in the formula expressed in g/mol. • The molar mass of an ionic compound is the sum of the masses of all the atoms in the formula expressed in g/mol. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Formula Mass Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
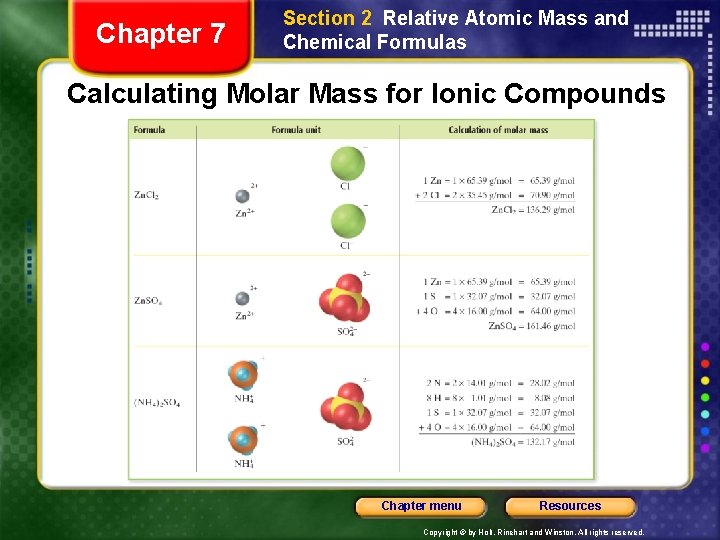
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Molar Mass for Ionic Compounds Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Molar Mass of Compounds Sample Problem F Find the molar mass of barium nitrate, Ba(NO 3)2. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
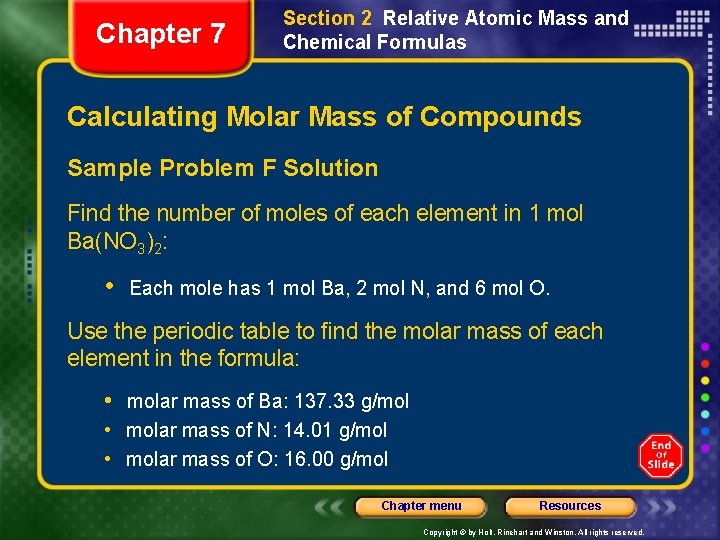
Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Molar Mass of Compounds Sample Problem F Solution Find the number of moles of each element in 1 mol Ba(NO 3)2: • Each mole has 1 mol Ba, 2 mol N, and 6 mol O. Use the periodic table to find the molar mass of each element in the formula: • molar mass of Ba: 137. 33 g/mol • molar mass of N: 14. 01 g/mol • molar mass of O: 16. 00 g/mol Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 2 Relative Atomic Mass and Chemical Formulas Calculating Molar Mass of Compounds Sample Problem F Solution, continued Multiply the molar mass of each element by the number of moles of each element. Add these masses to get the total molar mass of Ba(NO 3)2. mass of 1 mol Ba = 1 137. 33 g/mol = 137. 33 g/mol mass of 2 mol N = 2 14. 01 g/mol = 28. 02 g/mol + mass of 6 mol O = 6 16. 00 g/mol = 96. 00 g/mol molar mass of Ba(NO 3)2 = 261. 35 g/mol Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Bellringer • Brainstorm a list of what you know about percentages. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Objectives • Determine a compound’s empirical formula from its percentage composition. • Determine the molecular formula or formula unit of a compound from its empirical formula and its formula mass. • Calculate percentage composition of a compound from its molecular formula or formula unit. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data • The percentage composition is the percentage by mass of each element in a compound. • Percentage composition helps verify a substance’s identity. • Percentage composition also can be used to compare the ratio of masses contributed by the elements in two different substances. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
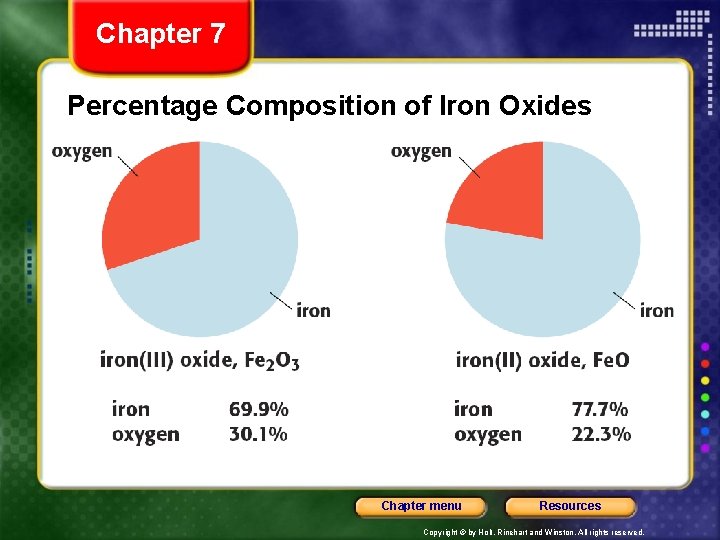
Chapter 7 Percentage Composition of Iron Oxides Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data, continued Determining Empirical Formulas • An empirical formula is a chemical formula that shows the simplest ratio for the relative numbers and kinds of atoms in a compound. • An actual formula shows the actual ratio of elements or ions in a single unit of a compound. • For example, the empirical formula for ammonium nitrate is NH 2 O, while the actual formula is NH 4 NO 2. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
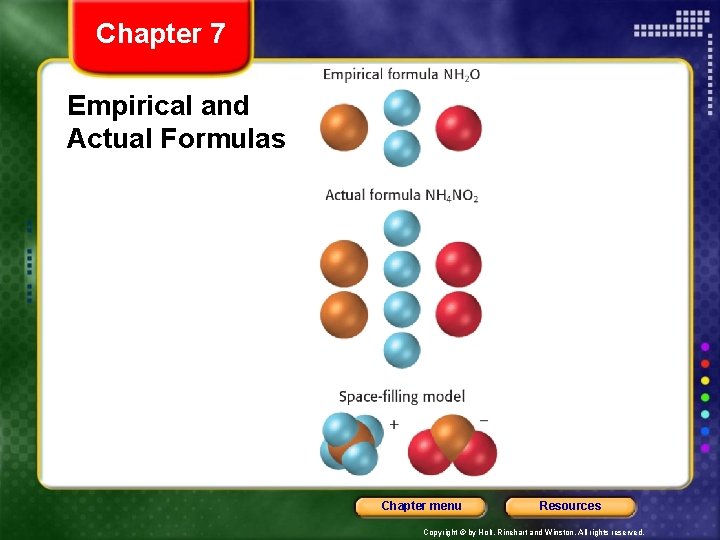
Chapter 7 Empirical and Actual Formulas Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data, continued Determining Empirical Formulas, continued • You can use the percentage composition for a compound to determine its empirical formula. 1. Convert the percentage of each element to g. 2. Convert from g to mol using the molar mass of each element as a conversion factor. 3. Compare these amounts in mol to find the simplest whole-number ratio among the elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Percentage Composition Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 3 Formulas and Percentage Composition Determining an Empirical Formula from Percentage Composition Sample Problem G Chemical analysis of a liquid shows that it is 60. 0% C, 13. 4% H, and 26. 6% O by mass. Calculate the empirical formula of this substance. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
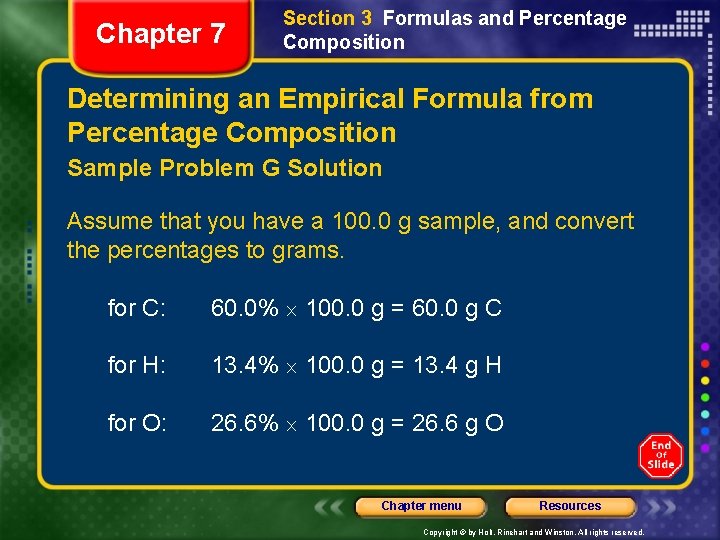
Chapter 7 Section 3 Formulas and Percentage Composition Determining an Empirical Formula from Percentage Composition Sample Problem G Solution Assume that you have a 100. 0 g sample, and convert the percentages to grams. for C: 60. 0% 100. 0 g = 60. 0 g C for H: 13. 4% 100. 0 g = 13. 4 g H for O: 26. 6% 100. 0 g = 26. 6 g O Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 3 Formulas and Percentage Composition Determining an Empirical Formula from Percentage Composition Sample Problem G Solution, continued Convert the mass of each element into the amount in moles, using the reciprocal of the molar mass. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
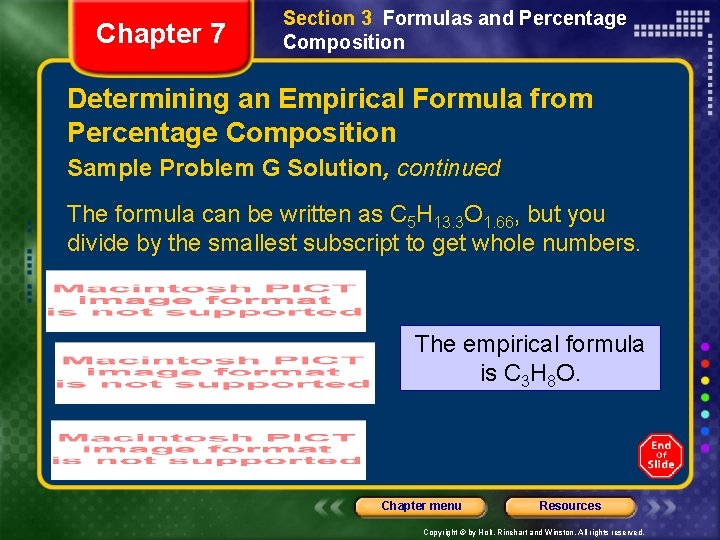
Chapter 7 Section 3 Formulas and Percentage Composition Determining an Empirical Formula from Percentage Composition Sample Problem G Solution, continued The formula can be written as C 5 H 13. 3 O 1. 66, but you divide by the smallest subscript to get whole numbers. The empirical formula is C 3 H 8 O. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data, continued Molecular Formulas Are Multiples of Empirical Formulas • The formula for an ionic compound shows the simplest whole-number ratio of the large numbers of ions in a crystal of the compound. • A molecular formula is a whole-number multiple of the empirical formula. • The molar mass of any compound is equal to the molar mass of the empirical formula times a whole number, n. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Visual Concepts Comparing Molecular and Empirical Formulas Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Determining a Molecular Formula from an Empirical Formula Sample Problem H The empirical formula for a compound is P 2 O 5. Its experimental molar mass is 284 g/mol. Determine the molecular formula of the compound. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
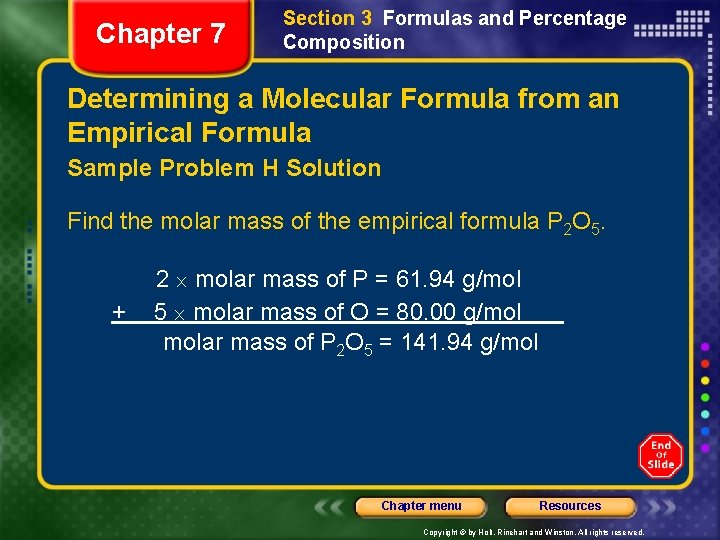
Chapter 7 Section 3 Formulas and Percentage Composition Determining a Molecular Formula from an Empirical Formula Sample Problem H Solution Find the molar mass of the empirical formula P 2 O 5. + 2 molar mass of P = 61. 94 g/mol 5 molar mass of O = 80. 00 g/mol molar mass of P 2 O 5 = 141. 94 g/mol Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
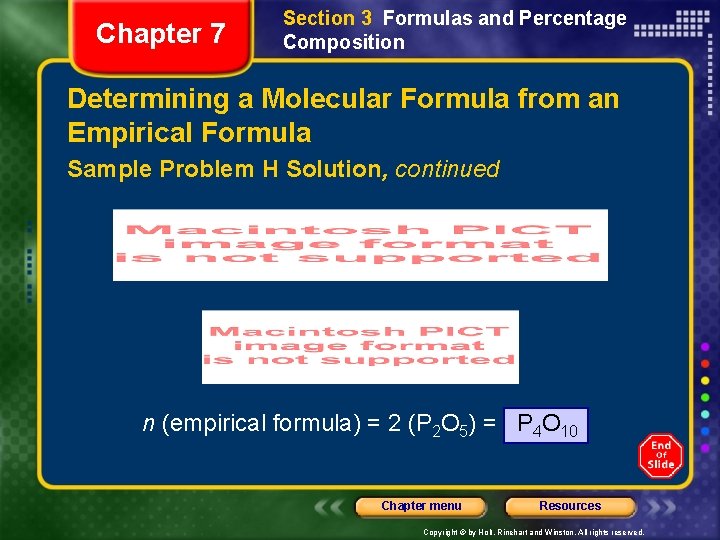
Chapter 7 Section 3 Formulas and Percentage Composition Determining a Molecular Formula from an Empirical Formula Sample Problem H Solution, continued n (empirical formula) = 2 (P 2 O 5) = P 4 O 10 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data, continued Chemical Formulas Can Give Percentage Composition • If you know the chemical formula of any compound, then you can calculate the percentage composition. • From the subscripts, determine the mass contributed by each element and add these to get molar mass. • Divide the mass of each element by the molar mass. • Multiply by 100 to find the percentage composition of that element. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
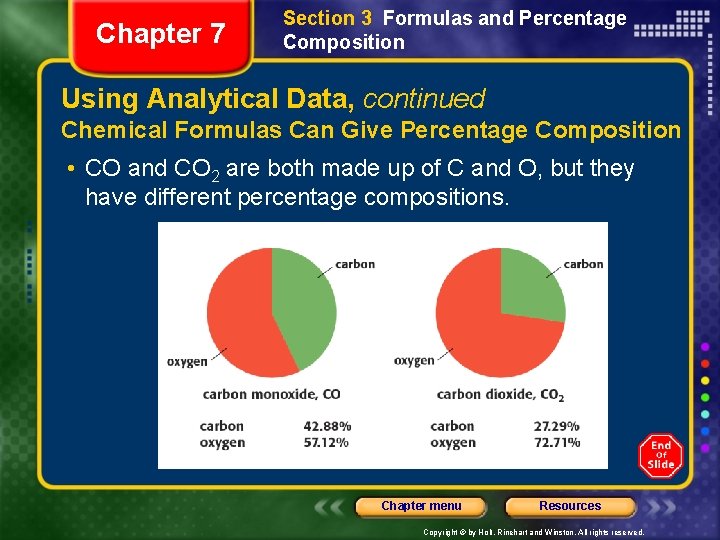
Chapter 7 Section 3 Formulas and Percentage Composition Using Analytical Data, continued Chemical Formulas Can Give Percentage Composition • CO and CO 2 are both made up of C and O, but they have different percentage compositions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
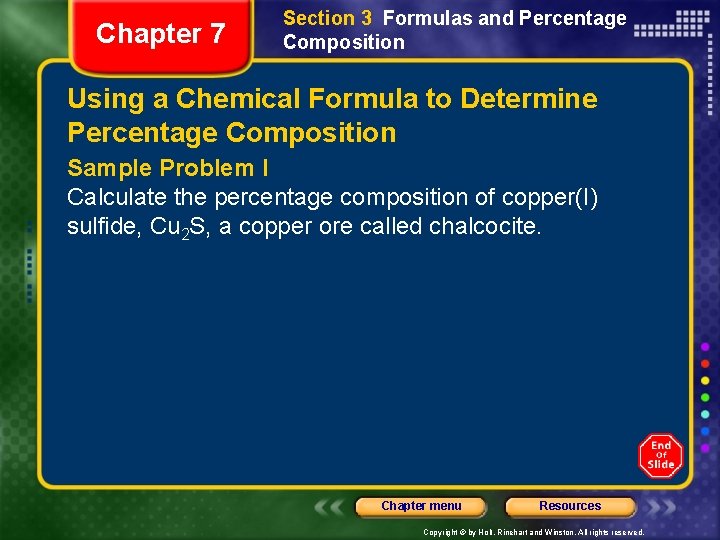
Chapter 7 Section 3 Formulas and Percentage Composition Using a Chemical Formula to Determine Percentage Composition Sample Problem I Calculate the percentage composition of copper(I) sulfide, Cu 2 S, a copper ore called chalcocite. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
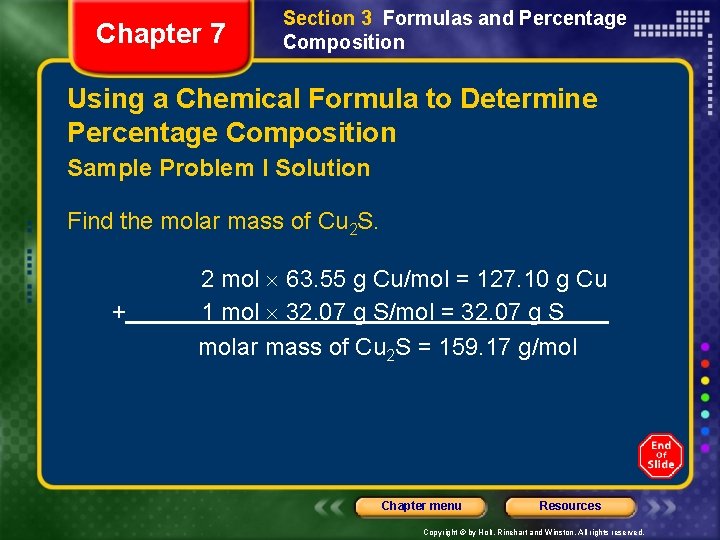
Chapter 7 Section 3 Formulas and Percentage Composition Using a Chemical Formula to Determine Percentage Composition Sample Problem I Solution Find the molar mass of Cu 2 S. + 2 mol 63. 55 g Cu/mol = 127. 10 g Cu 1 mol 32. 07 g S/mol = 32. 07 g S molar mass of Cu 2 S = 159. 17 g/mol Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 3 Formulas and Percentage Composition Using a Chemical Formula to Determine Percentage Composition Sample Problem I Solution, continued Calculate the fraction that each element contributes to the total mass by substituting the masses into the equations below and rounding correctly. 79. 852% Cu Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Section 3 Formulas and Percentage Composition Using a Chemical Formula to Determine Percentage Composition Sample Problem I Solution, continued 20. 15% S Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Understanding Concepts 1. Element A has two isotopes. One has an atomic mass of 120 and constitutes 60%; the other has an atomic mass of 122 and constitutes 40%. Which range below includes the average atomic mass of Element A? A. B. C. D. less than 120 between 120 and 121 between 121 and 122 greater than 122 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Understanding Concepts 2. Which of the following can be determined from the empirical formula of a compound alone? F. G. H. I. the true formula of the compound the molecular mass of the compound the percentage composition of the compound the arrangement of atoms within a molecule of the compound Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Understanding Concepts 3. How many atoms are in 0. 5 moles of Na. Cl? A. B. C. D. 1. 204 1023 3. 011 1023 6. 022 1023 9. 033 1023 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Understanding Concepts 4. How many moles of calcium (mass = 40. 1) are in a serving of milk containing 290 mg of calcium? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Understanding Concepts 5. How is Avogadro's number related to moles? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Understanding Concepts 6. Antimony has two isotopes. One, amounting to 57. 3% of the atoms, has a mass of 120. 9 amu. The other, 42. 7% of the atoms, has a mass of 122. 9 amu. What is the average atomic mass of antimony? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Reading Skills Read the passage below. Then answer the questions. In 1800 two English chemists, Nicholson and Carlisle, discovered that when an electric current is passed through water, hydrogen and oxygen were produced in a 2: 1 volume ratio and a 1: 8 mass ratio. This evidence helped to support John Dalton's theory that matter consisted of atoms, demonstrating that water consists of the two elements in a constant proportion. If the same number of moles of each gas occupy the same volume, then each molecule of water must consist of twice as much hydrogen as oxygen, even though the mass of hydrogen is only one-eighth that of oxygen. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Reading Skills 7. Based on this experiment, what is the empirical formula of water? F. G. H. I. HO H 2 O 8 O 8 H Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Reading Skills 8. How would the experimental result have been different if hydrogen gas existed as individual atoms while oxygen formed molecules with two atoms bound by a covalent bond? A. B. C. D. The result would be the same. The ratio of hydrogen to oxygen would be 1: 1. The ratio of hydrogen to oxygen would be 1: 4. The ratio of hydrogen to oxygen would be 4: 1. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Reading Skills 9. How does the empirical formula for water compare to its molecular formula? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
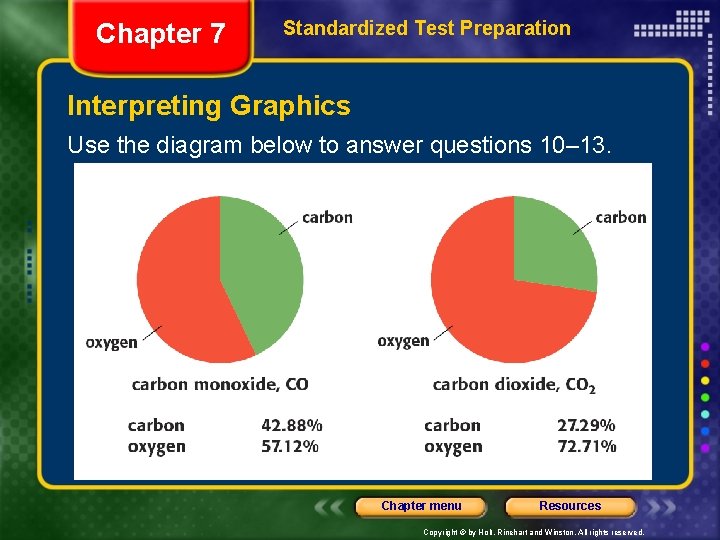
Chapter 7 Standardized Test Preparation Interpreting Graphics Use the diagram below to answer questions 10– 13. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Interpreting Graphics 10. How many moles of oxygen atoms are there in 100. 0 moles of carbon dioxide? F. G. H. I. 66. 7 72. 7 100. 0 200. 0 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Interpreting Graphics 11. Explain why the percentage of oxygen in carbon dioxide is not twice the percentage of oxygen in carbon monoxide, if there are twice as many oxygen atoms. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 7 Standardized Test Preparation Interpreting Graphics 12. If you did not know the true formulas for carbon monoxide and carbon dioxide, what information would you need beyond what is provided in the illustration in order to calculate them? A. B. C. D. the percentage compositions the atomic masses of carbon and oxygen the melting and boiling points of each compound the number of atoms of each element in the compound Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 7 Standardized Test Preparation Interpreting Graphics 13. How many grams of carbon are contained in 200. 0 grams of carbon dioxide? F. G. H. I. 27. 29 42. 88 54. 58 85. 76 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
- Slides: 85