CHAPTER 7 Structure and Properties of Materials Defects












![◎About Vacancies Ion vacancies occur in pairs [as with Schottky defects] They form in ◎About Vacancies Ion vacancies occur in pairs [as with Schottky defects] They form in](https://slidetodoc.com/presentation_image_h/bc5c023fc843d9cd6cbad553f3f847a9/image-34.jpg)

- Slides: 35

CHAPTER 7 Structure and Properties of Materials Defects and Properties: Point Defects and Diffusion 1

7 -I. Introduction : Defect and Properties u Many material properties are determined or related to defects of structures: • Diffusion point defects • Electrical conductivity of ceramics defects (vacancies) point • Mechanical strength size) microstructure (grain • Plastic deformation dislocation • Flexural strength of ceramics defects (pores and cracks) volume 2

7 -II. Diffusion: General Principles and Diffusion in Metallic Crystals A. Introduction ◎ Diffusion : self-movement of an atom or a molecule F 5. 1 F 5. 2 ◎ movement of an atom or a molecule : (A) bulk flow : e. g. , a flowing fluid, local flow of fluid by convection (due to density difference) occur only in fluids, i. e. , gases and liquids. (B) diffusion : movement of single atoms or molecules occur in all materials, i. e. , gases, liquids and solids. 3

◎ example of movement of atoms or molecules, (1) spreading of a gas in air • air with convection : bulk flow + diffusion • air is still : diffusion (only) (2) spreading of a drop of ink in water • water with convection or stirring : bulk flow + diffusion • still water : diffusion (only) (3) solution of salt or sugar in water (4) diffusion of oxygen into jet engine components : diffusion severe degradation in mechanical properties. 4

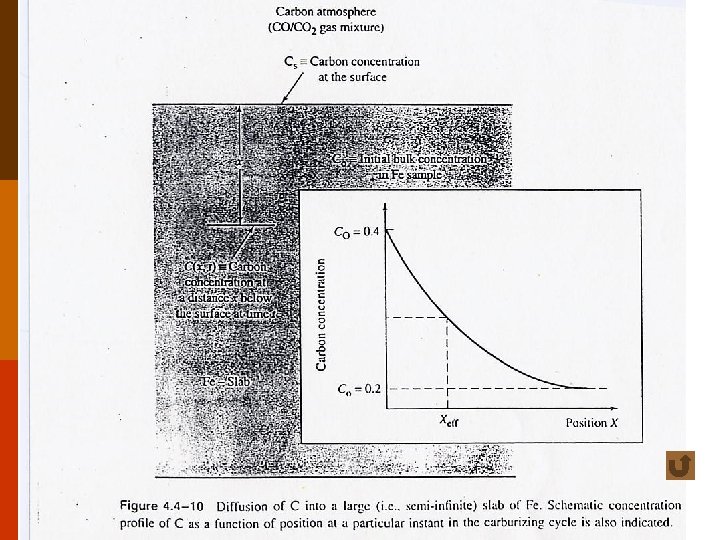
◎ Practical uses of diffusion (1) microelectronics industries : doping of P, B or other dopants into Si wafers. (2) heart-lung machine : diffusion of O 2 + CO 2 through silicon-based rubber membrane. (3) ceramic gases senors : diffusion of O 2 through Zr. O 2 ceramics. (4) carburization of steels : to improve wear resistance. f 4. 4 -10 (5) coating of turbine blades : to improve oxidation resistance. 5

◎ diffusion and point effects • diffusing species is a type of point defect F 5. 3 • diffusion processes are related to movement of point defects. 6

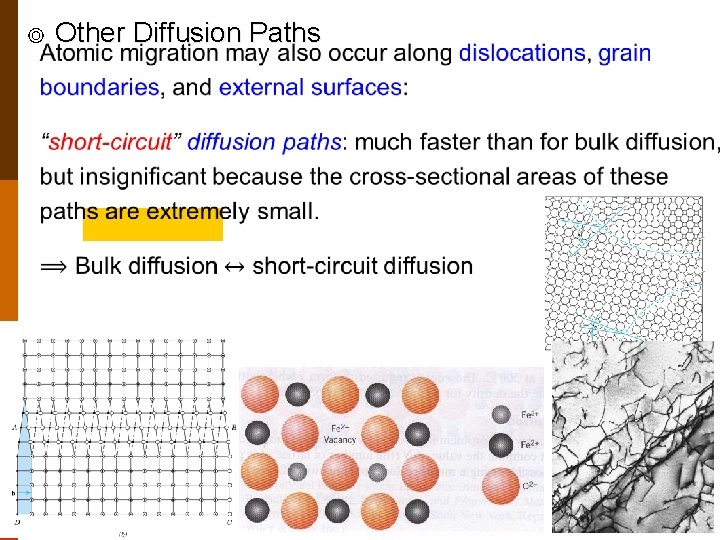
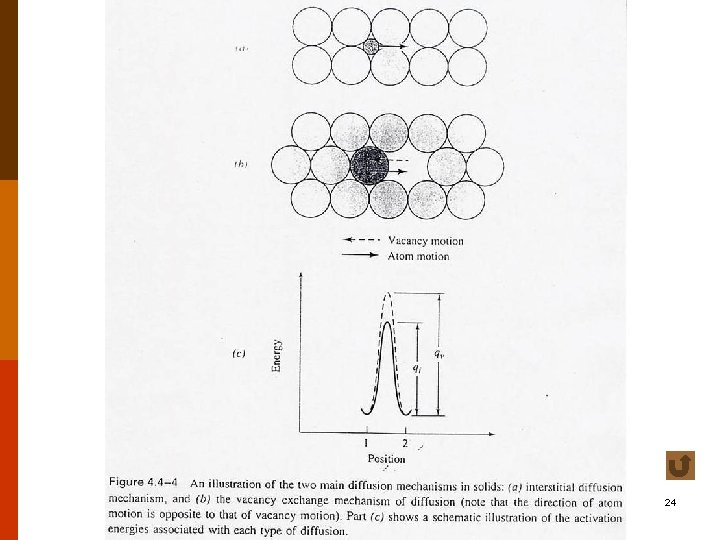
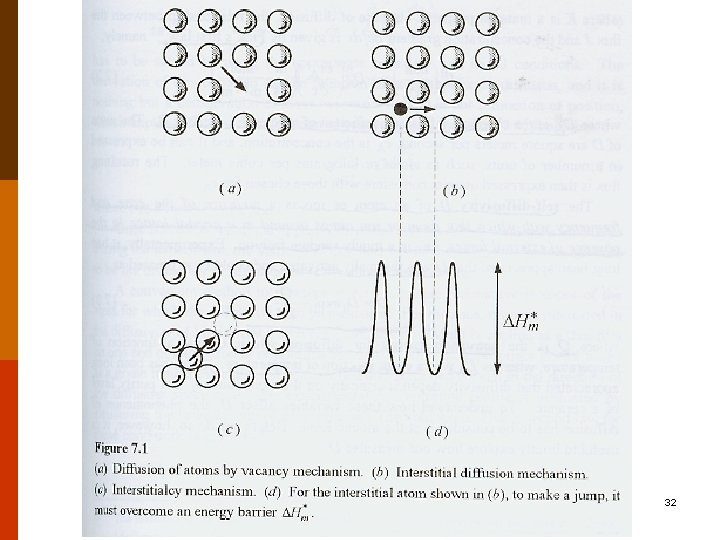
B. Mechanisms of Diffusion in (Covalent and Metallic) Crystals ◎ Two main mechanisms : (bulk diffusion) F 5. 3 (1) interstitial diffusion • activation energy : qi(or Qi) (2) vacancy (exchange) diffusion • occur only when there is a vacant lattice site adjacent to it. • activation energy : qv(or Qv) (both to form a vacancy and to move the atom) • usually, qv > qi. f 4. 4 -4 7

◎ Other Diffusion Paths 8

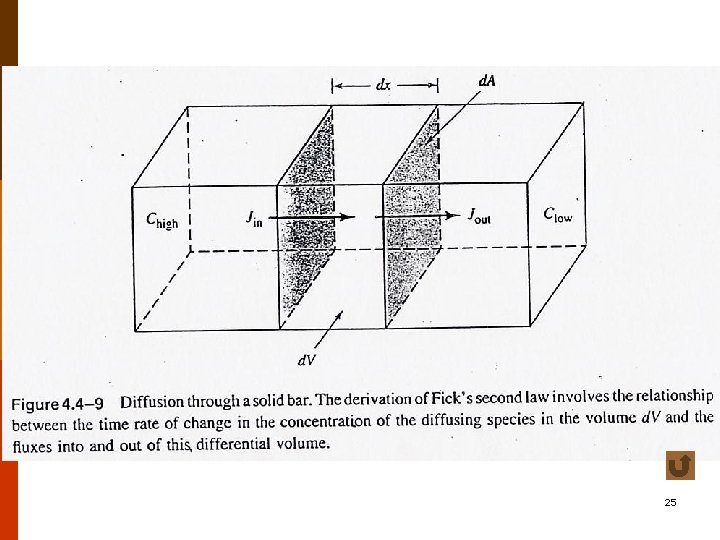
C. A Physical Description of Diffusion (Fick’s First Law) ◎ rate of diffusion J : net number of atoms(or mass) moving across a plane in a particular direction per unit area per unit time ( J ≡ flux, 流通量, atoms/cm 2 –s or Kg/m 2 -s) f 4. 4 -9 (5. 1 a) (5. 1 b) 9

◎ diffusion : random movement of an atom or a molecule or more precisely for solids, a random jump of an atom. ◎ Other driving forces for diffusion : – Thermal gradient : thermophoresis – potential (electrical) gradient : for charged species 10

◎ Steady State Diffusion (steady state: unchanged with time; constant with time) 11 F 5. 7 T 5. 2

◎ Nonsteady State Diffusion: Fick’s Second Law 12

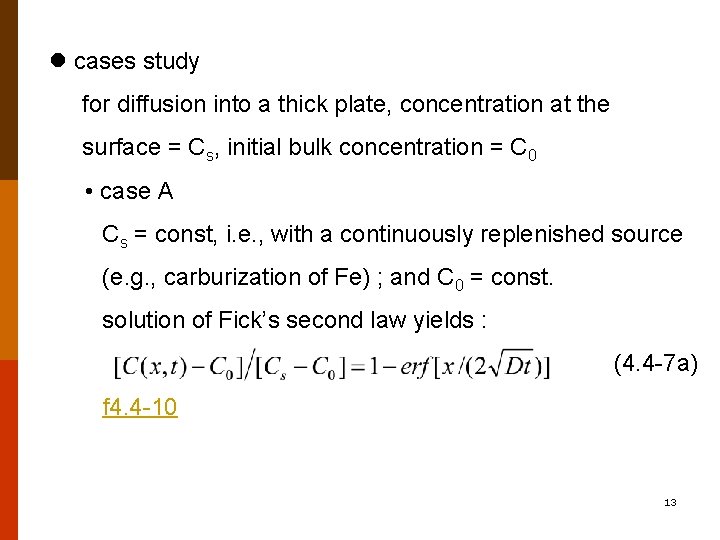
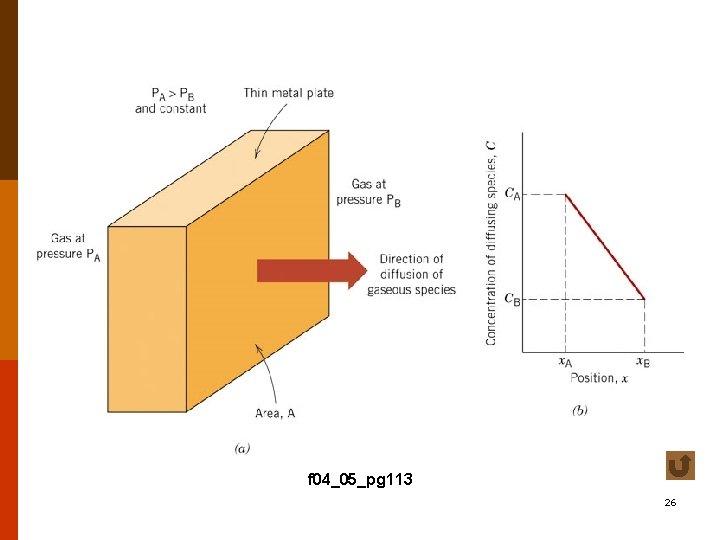
cases study for diffusion into a thick plate, concentration at the surface = Cs, initial bulk concentration = C 0 • case A Cs = const, i. e. , with a continuously replenished source (e. g. , carburization of Fe) ; and C 0 = const. solution of Fick’s second law yields : (4. 4 -7 a) f 4. 4 -10 13

• Case B C 0 = 0, initial Cs = , i. e. , not continuously replenished (e. g. , doping in semiconductor industry) (4. 4 -7 b) Effective Penetration Distance Xeff , defined as the point where (4. 4 -8) 14

substituting into Eq. (4. 4 -7) and using Fig. 4. 4 -11 (4. 4 -10) or (4. 4 -11) : geometry factor (=1 for a plate and 2 for cylinders) D~T (D = D 0 exp (-Q/RT) (D = const, i. e. , T = const) (t = const) 15

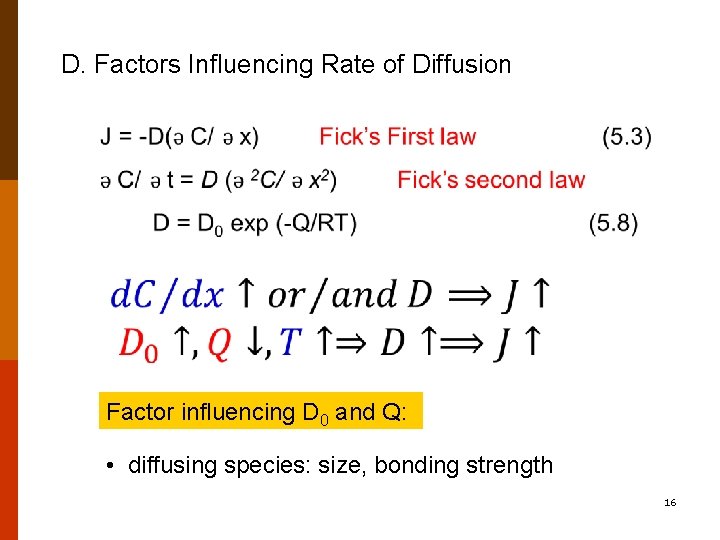
D. Factors Influencing Rate of Diffusion Factor influencing D 0 and Q: • diffusing species: size, bonding strength 16

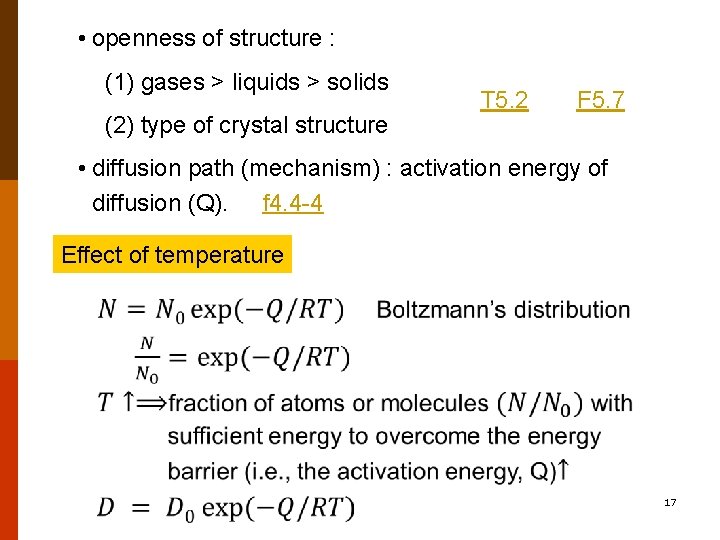
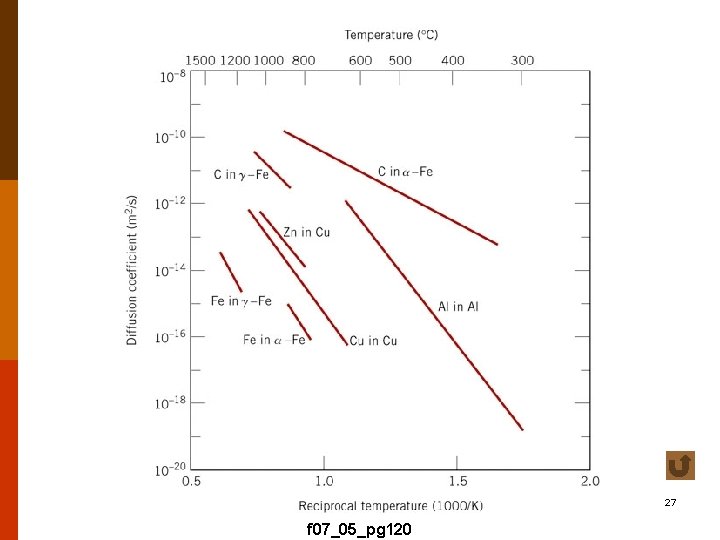
• openness of structure : (1) gases > liquids > solids (2) type of crystal structure T 5. 2 F 5. 7 • diffusion path (mechanism) : activation energy of diffusion (Q). f 4. 4 -4 Effect of temperature 17

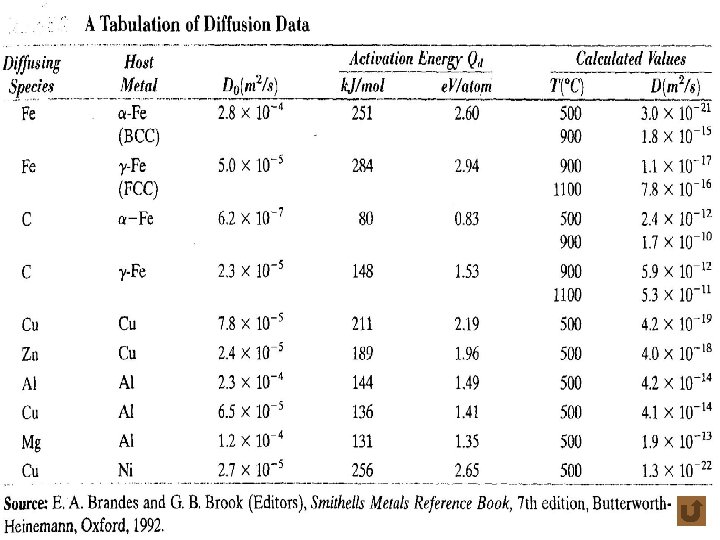
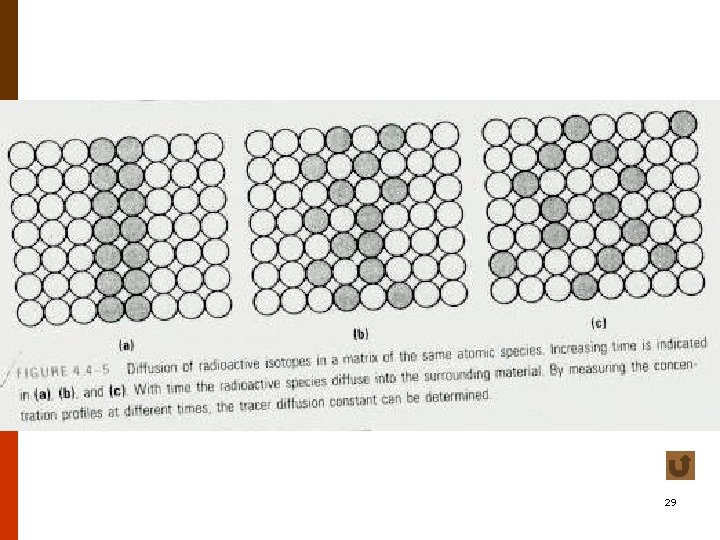
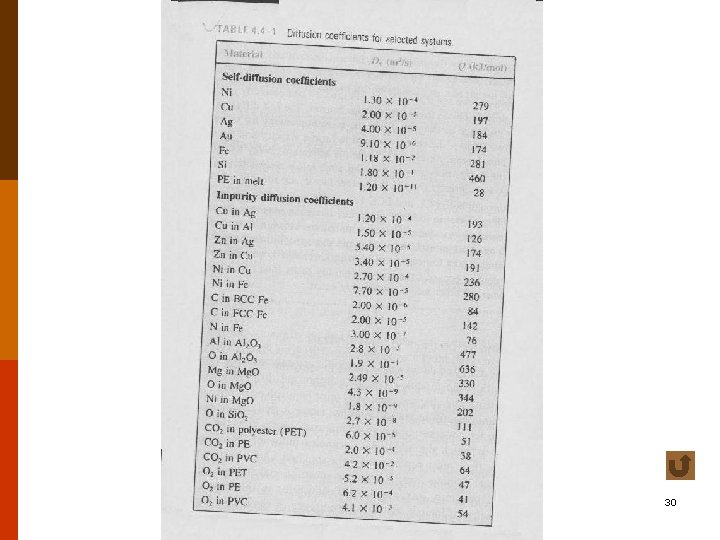
Diffusion for Different Levels of Concentration • self-diffusion coefficient or tracer diffusion coefficient • impurity diffusion (interdiffusion) impurity diffusion coefficient • chemical diffusion coefficient f 4. 4 -5 t 4. 4 -1 18

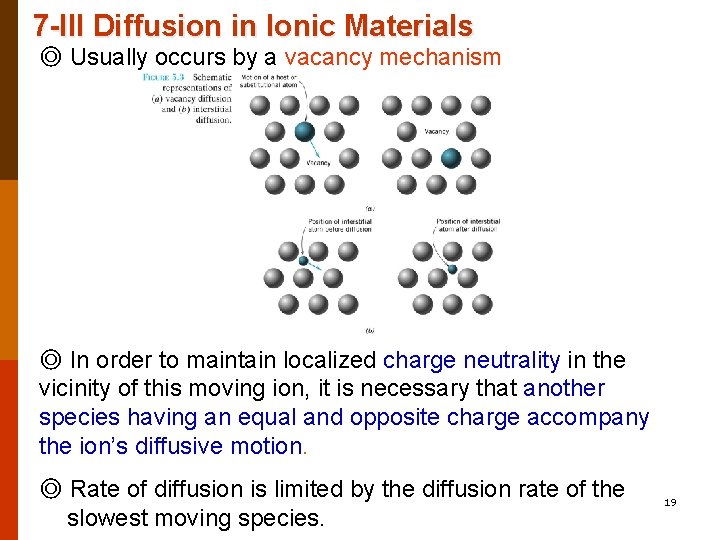
7 -III Diffusion in Ionic Materials ◎ Usually occurs by a vacancy mechanism ◎ In order to maintain localized charge neutrality in the vicinity of this moving ion, it is necessary that another species having an equal and opposite charge accompany the ion’s diffusive motion. ◎ Rate of diffusion is limited by the diffusion rate of the slowest moving species. 19

20

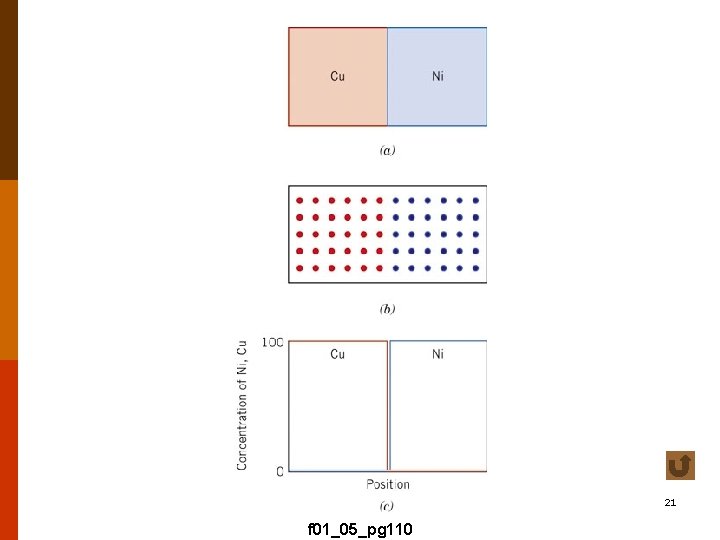
21 f 01_05_pg 110

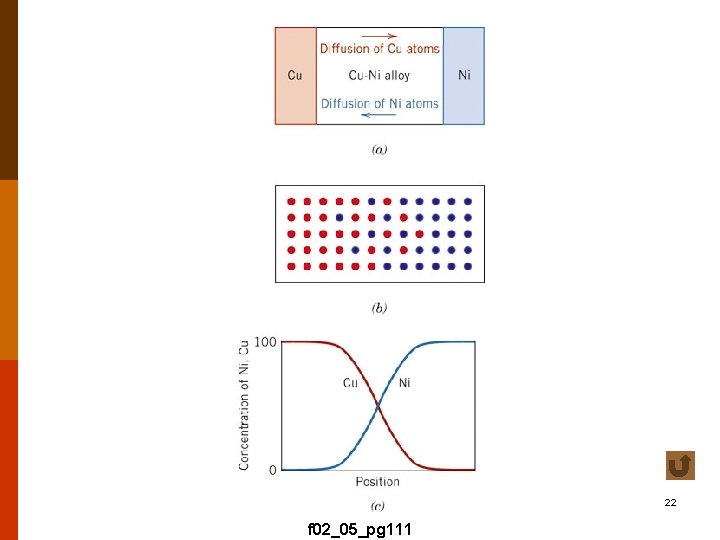
22 f 02_05_pg 111

23

24

25

f 04_05_pg 113 26

27 f 07_05_pg 120

28

29

30

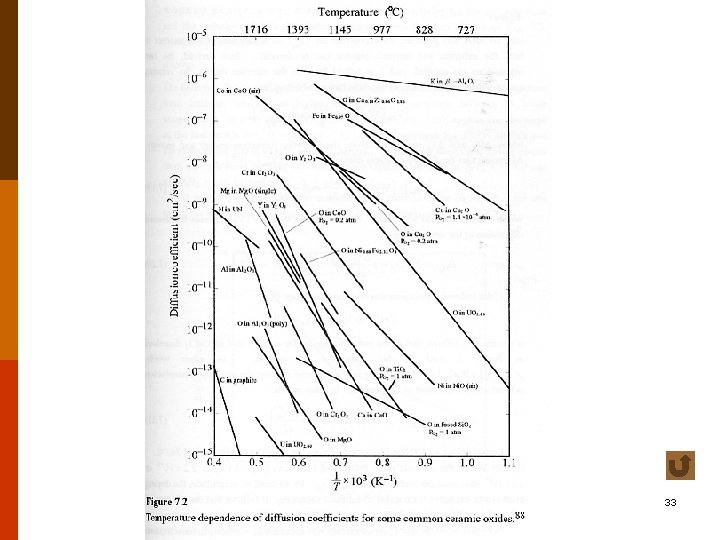
31

32

33
![About Vacancies Ion vacancies occur in pairs as with Schottky defects They form in ◎About Vacancies Ion vacancies occur in pairs [as with Schottky defects] They form in](https://slidetodoc.com/presentation_image_h/bc5c023fc843d9cd6cbad553f3f847a9/image-34.jpg)
◎About Vacancies Ion vacancies occur in pairs [as with Schottky defects] They form in nonstoichiometric compounds. They are created by substitutional impurity ions having different charge states than the host ions. 34

Example for ion diffusion : Electrolyte Fuel Cell Over all reaction H 2 + 1/2 O 2 H 2 O(g) + electrical energy Fuel (H 2 or CO) Anode (porous) load Electrolyte (dense) O-2 Cathode (porous) O 2 Oxidizer (air or O 2) Electrolyte u Materials :YSZ (8% Y 2 O 3 -Zr. O 2) an oxygen vacancy is created for every mole of the dopant Y 2 O 3: oxygen-ion conductivity. 35