Chapter 7 Solutions Copyright 2005 by Pearson Education

Chapter 7 Solutions Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1
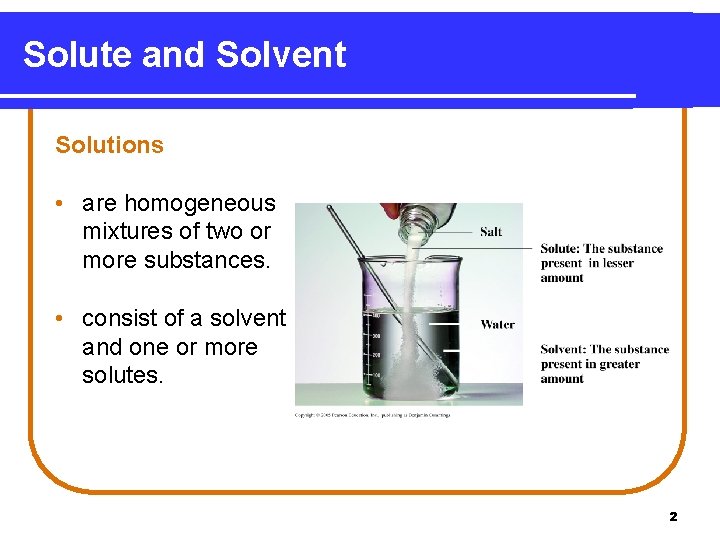
Solute and Solvent Solutions • are homogeneous mixtures of two or more substances. • consist of a solvent and one or more solutes. 2
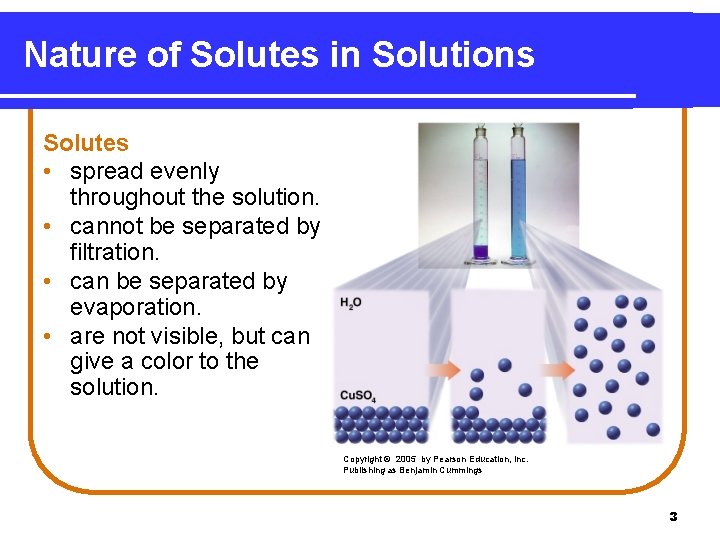
Nature of Solutes in Solutions Solutes • spread evenly throughout the solution. • cannot be separated by filtration. • can be separated by evaporation. • are not visible, but can give a color to the solution. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3

Examples of Solutions The solute and solvent in a solution can be a solid, liquid, and/or a gas. 4

Learning Check Identify the solute in each of the following solutions. A. 2 g sugar and 100 m. L water B. 60. 0 m. L of ethyl alcohol and 30. 0 m. L of methyl alcohol C. 55. 0 m. L water and 1. 50 g Na. Cl D. Air: 200 m. L O 2 and 800 m. L N 2 5

Solution Identify the solute in each of the following solutions. A. 2 g sugar B. 30. 0 m. L of methyl alcohol C. 1. 5 g Na. Cl D. 200 m. L O 2 6
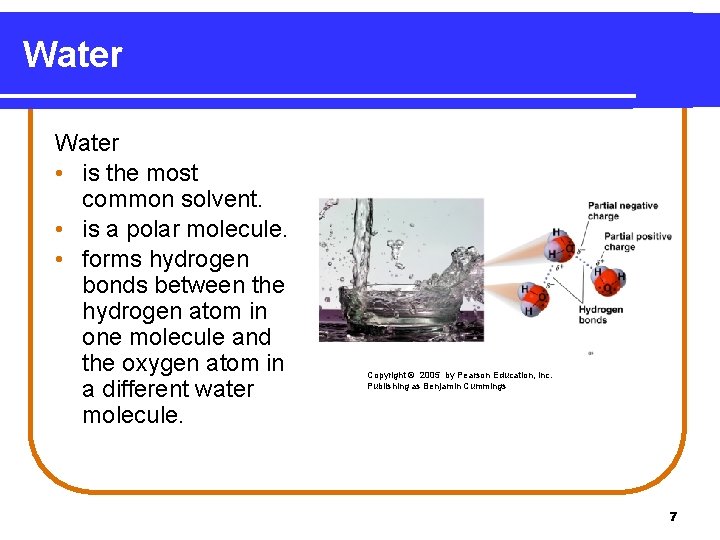
Water • is the most common solvent. • is a polar molecule. • forms hydrogen bonds between the hydrogen atom in one molecule and the oxygen atom in a different water molecule. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7
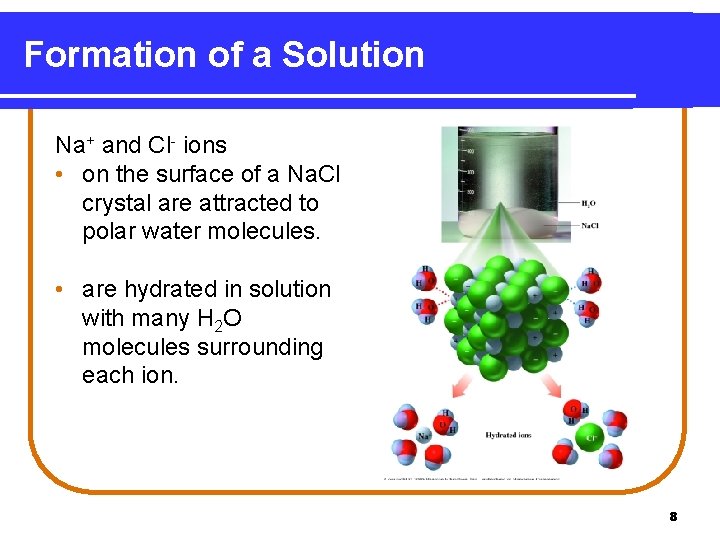
Formation of a Solution Na+ and Cl- ions • on the surface of a Na. Cl crystal are attracted to polar water molecules. • are hydrated in solution with many H 2 O molecules surrounding each ion. 8
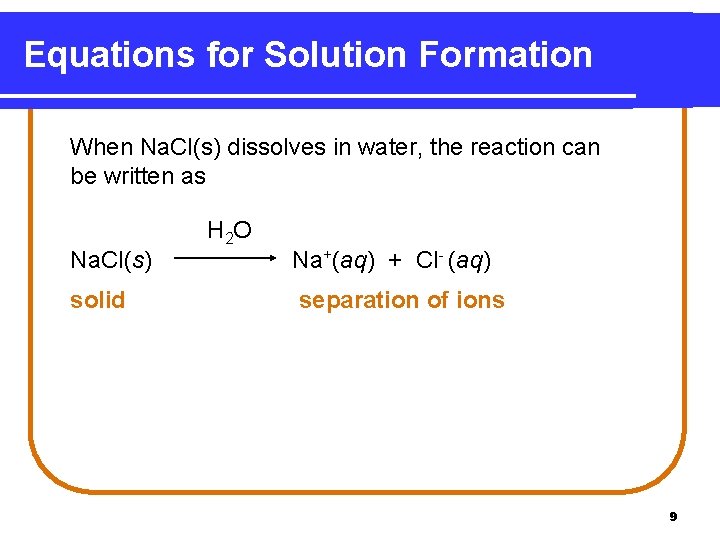
Equations for Solution Formation When Na. Cl(s) dissolves in water, the reaction can be written as Na. Cl(s) solid H 2 O Na+(aq) + Cl- (aq) separation of ions 9
Learning Check Solid Li. Cl is added to water. It dissolves because A. the Li+ ions are attracted to the 1) oxygen atom ( -) of water. 2) hydrogen atom ( +) of water. B. the Cl- ions are attracted to the 1) oxygen atom ( -) of water. 2) hydrogen atom ( +) of water. 10
Solution Solid Li. Cl is added to water. It dissolves because A. the Li+ ions are attracted to the 1) oxygen atom ( -) of water. B. the Cl- ions are attracted to the 2) hydrogen atom ( +) of water. 11

Like Dissolves Like Two substances form a solution • when there is an attraction between the particles of the solute and solvent. • when a polar solvent such as water dissolves polar solutes such as sugar and ionic solutes such as Na. Cl. • when a nonpolar solvent such as hexane (C 6 H 14) dissolves nonpolar solutes such as oil or grease. 12
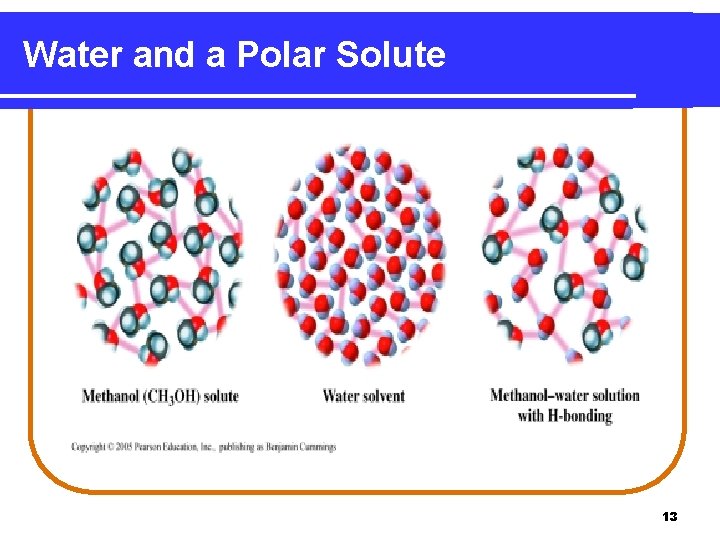
Water and a Polar Solute 13
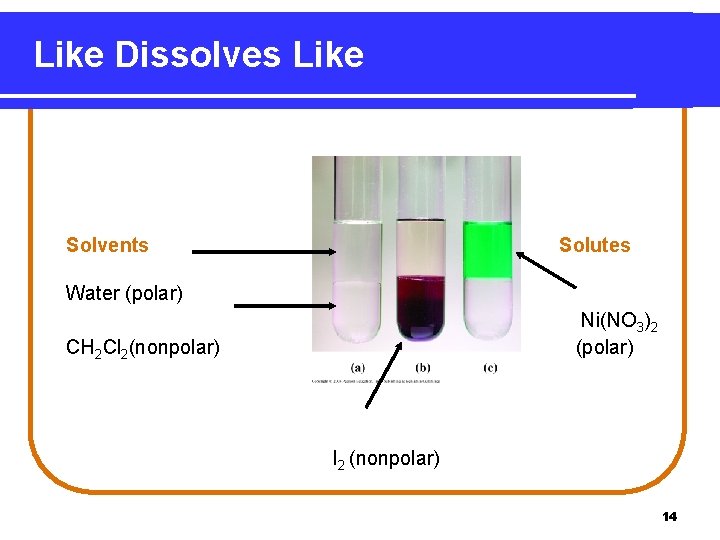
Like Dissolves Like Solvents Solutes Water (polar) Ni(NO 3)2 (polar) CH 2 Cl 2(nonpolar) I 2 (nonpolar) 14
Learning Check Which of the following solutes will dissolve in water? Why? 1) Na 2 SO 4 2) gasoline (nonpolar) 3) I 2 4) HCl 15

Solution Which of the following solutes will dissolve in water? Why? 1) Na 2 SO 4 Yes, ionic 2) gasoline No, nonpolar 3) I 2 No, nonpolar 4) HCl Yes, polar Most polar and ionic solutes dissolve in water because water is a polar solvent. 16
- Slides: 16