Chapter 7 Solutions and Colloids 7 2 Solubility

Chapter 7 Solutions and Colloids 7. 2 Solubility Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Solubility is • the maximum amount of solute that dissolves in a specific amount of solvent. • expressed as grams of solute in 100 grams of solvent water. g of solute 100 g water 2

Unsaturated Solutions Unsaturated solutions • contain less than the maximum amount of solute. • can dissolve more solute. Dissolved solute Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3
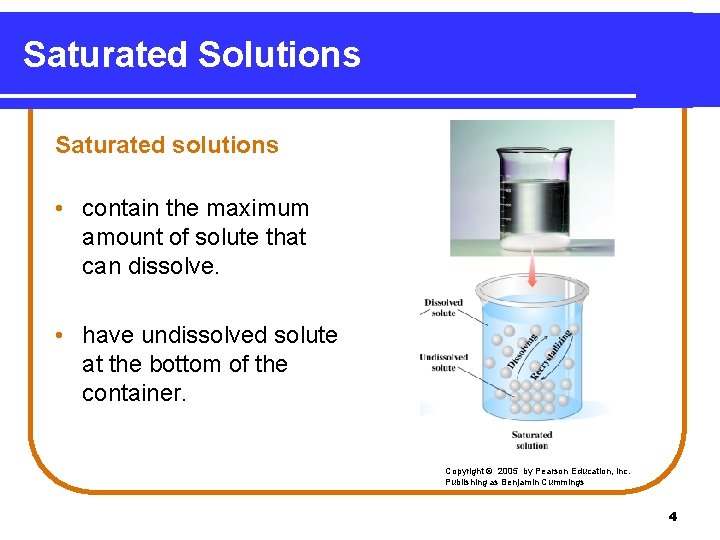
Saturated Solutions Saturated solutions • contain the maximum amount of solute that can dissolve. • have undissolved solute at the bottom of the container. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 4

Supersaturated Solutions Supersaturated solutions • An unstable solution that contains an amount of solute greater than the solute solubility. • Also has undissolved solute at the bottom of the container. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 5
Learning Check At 40 C, the solubility of KBr is 80 g/100 g H 2 O. Identify the following solutions as either 1) saturated or (2) unsaturated. Explain. A. 60 g KBr added to 100 g of water at 40 C. B. 200 g KBr added to 200 g of water at 40 C. C. 25 g KBr added to 50 g of water at 40 C. 6
Solution A. 2 Amount of 60 g KBr/100 g water is less than the solubility of 80 g KBr/100 g water. B. 1 In 100 g of water, 100 g KBr exceeds the solubility of 80 g KBr water at 40 C. 2 This is the same as 50 g KBr in 100 g of water, which is less than the solubility of 80 g KBr/100 g water at 40 C. 7
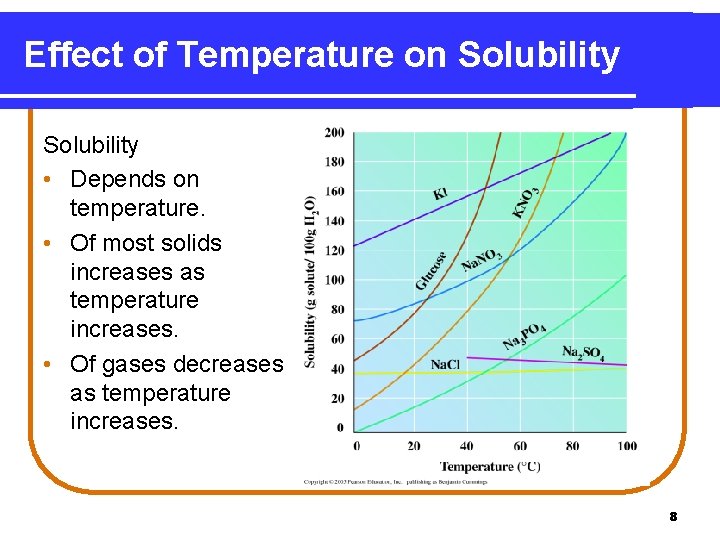
Effect of Temperature on Solubility • Depends on temperature. • Of most solids increases as temperature increases. • Of gases decreases as temperature increases. 8

Learning Check A. Why could a bottle of carbonated drink possibly burst (explode) when it is left out in the hot sun ? B. Why do fish die in water that is too warm? 9

Solution A. The pressure in a bottle increases as the gas leaves solution as it becomes less soluble at high temperatures. As pressure increases, the bottle could burst. B. Because O 2 gas is less soluble in warm water, fish cannot obtain the amount of O 2 required for their survival. 10
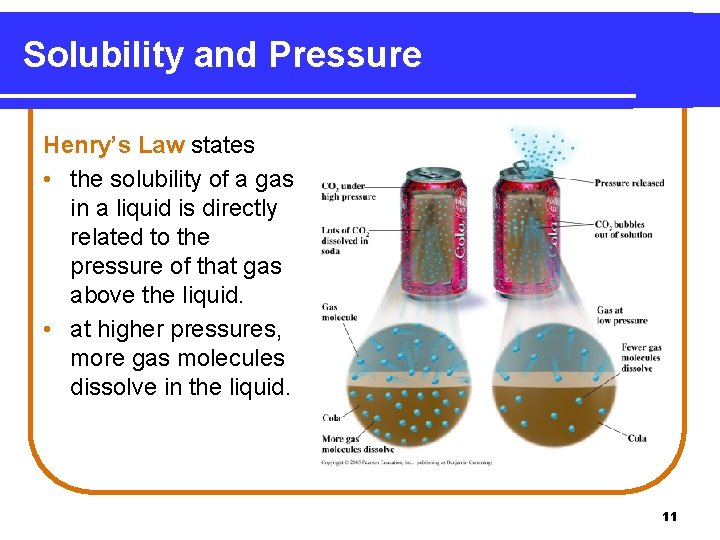
Solubility and Pressure Henry’s Law states • the solubility of a gas in a liquid is directly related to the pressure of that gas above the liquid. • at higher pressures, more gas molecules dissolve in the liquid. 11
- Slides: 11