Chapter 7 Solutions 7 5 Molarity and Dilution
- Slides: 25

Chapter 7 Solutions 7. 5 Molarity and Dilution 1

Molarity (M) is • a concentration term for solutions. • gives the moles of solute in 1 L solution. • moles of solute liter of solution 2

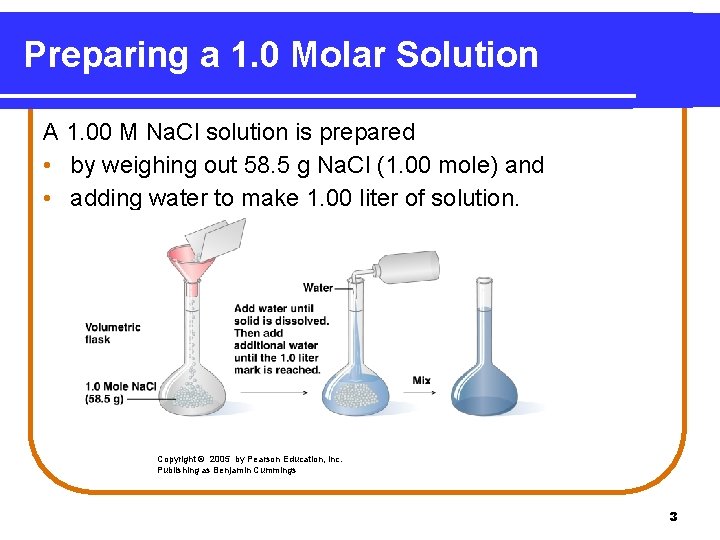
Preparing a 1. 0 Molar Solution A 1. 00 M Na. Cl solution is prepared • by weighing out 58. 5 g Na. Cl (1. 00 mole) and • adding water to make 1. 00 liter of solution. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3

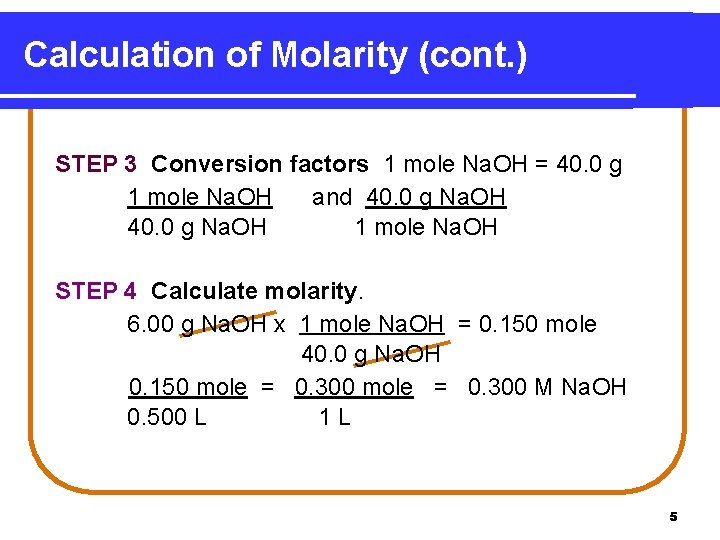
Calculation of Molarity What is the molarity of 0. 500 L Na. OH solution if it contains 6. 00 g Na. OH? STEP 1 Given 6. 00 g Na. OH in 0. 500 L solution Need molarity (mole/L) STEP 2 Plan g Na. OH mole Na. OH molarity 4

Calculation of Molarity (cont. ) STEP 3 Conversion factors 1 mole Na. OH = 40. 0 g 1 mole Na. OH and 40. 0 g Na. OH 1 mole Na. OH STEP 4 Calculate molarity. 6. 00 g Na. OH x 1 mole Na. OH = 0. 150 mole 40. 0 g Na. OH 0. 150 mole = 0. 300 M Na. OH 0. 500 L 1 L 5

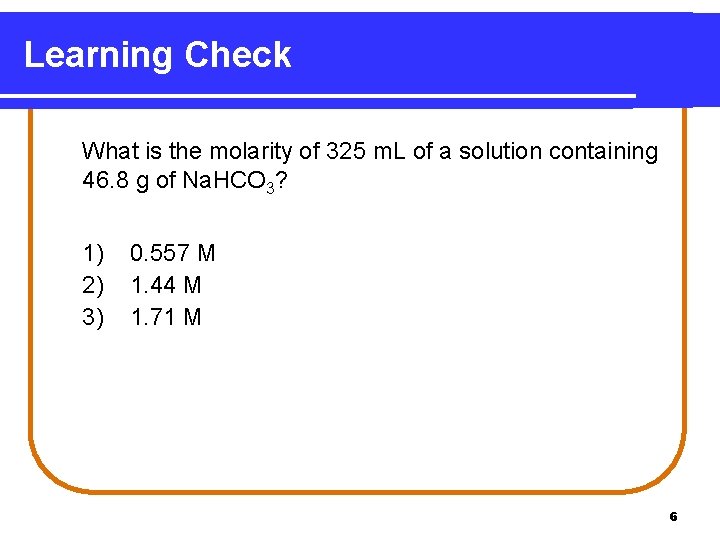
Learning Check What is the molarity of 325 m. L of a solution containing 46. 8 g of Na. HCO 3? 1) 2) 3) 0. 557 M 1. 44 M 1. 71 M 6

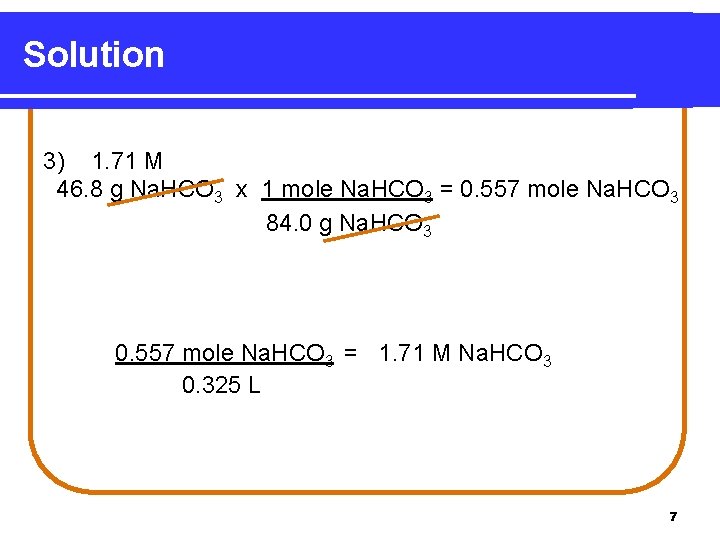
Solution 3) 1. 71 M 46. 8 g Na. HCO 3 x 1 mole Na. HCO 3 = 0. 557 mole Na. HCO 3 84. 0 g Na. HCO 3 0. 557 mole Na. HCO 3 = 1. 71 M Na. HCO 3 0. 325 L 7

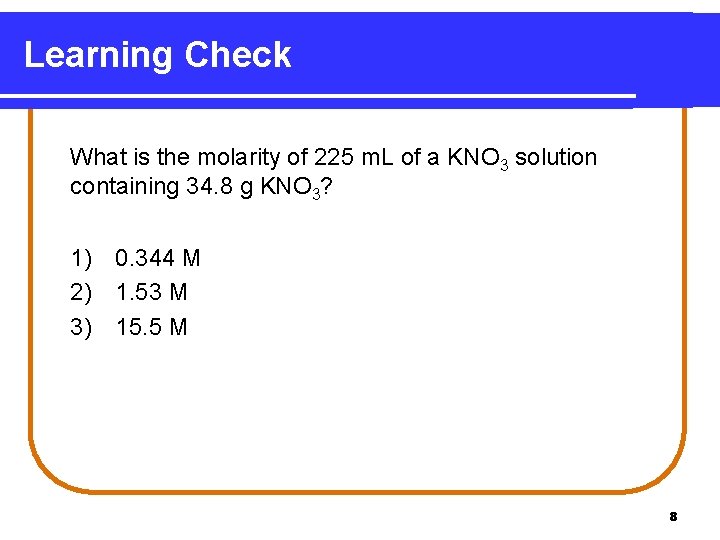
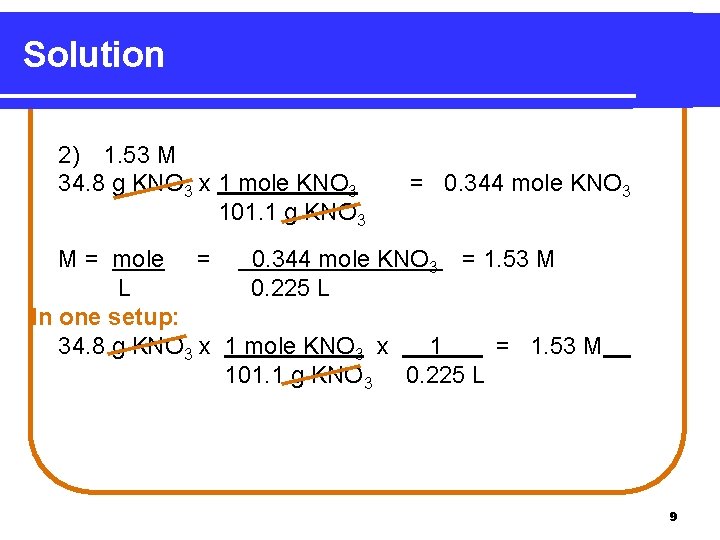
Learning Check What is the molarity of 225 m. L of a KNO 3 solution containing 34. 8 g KNO 3? 1) 0. 344 M 2) 1. 53 M 3) 15. 5 M 8

Solution 2) 1. 53 M 34. 8 g KNO 3 x 1 mole KNO 3 101. 1 g KNO 3 = 0. 344 mole KNO 3 M = mole = 0. 344 mole KNO 3 = 1. 53 M L 0. 225 L In one setup: 34. 8 g KNO 3 x 1 mole KNO 3 x 1 = 1. 53 M 101. 1 g KNO 3 0. 225 L 9

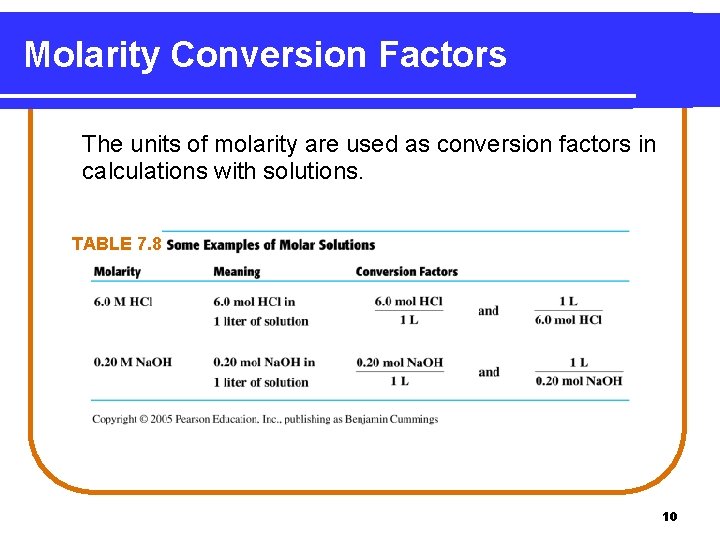
Molarity Conversion Factors The units of molarity are used as conversion factors in calculations with solutions. TABLE 7. 8 10

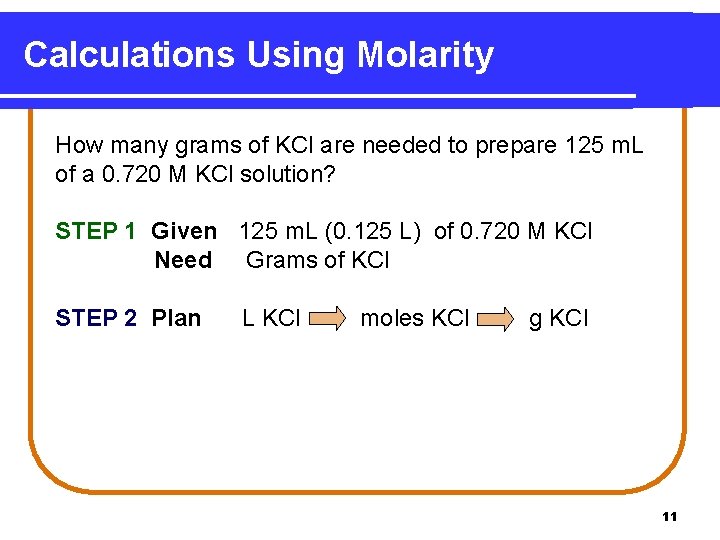
Calculations Using Molarity How many grams of KCl are needed to prepare 125 m. L of a 0. 720 M KCl solution? STEP 1 Given 125 m. L (0. 125 L) of 0. 720 M KCl Need Grams of KCl STEP 2 Plan L KCl moles KCl g KCl 11

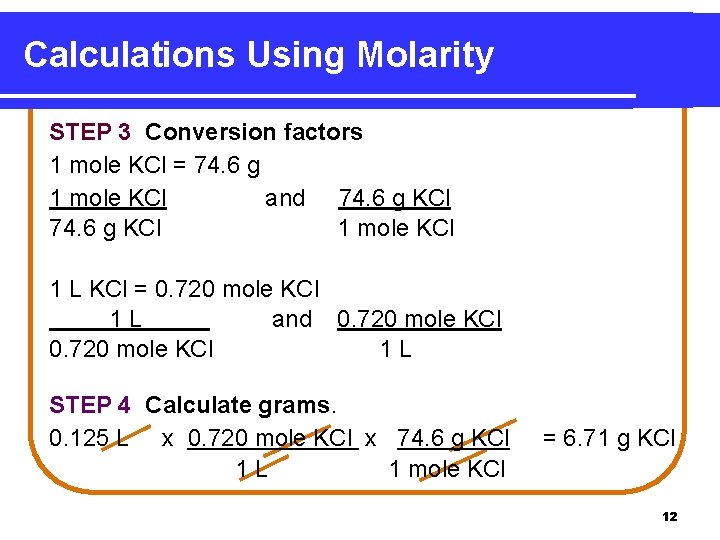
Calculations Using Molarity STEP 3 Conversion factors 1 mole KCl = 74. 6 g 1 mole KCl and 74. 6 g KCl 1 mole KCl 1 L KCl = 0. 720 mole KCl 1 L and 0. 720 mole KCl 1 L STEP 4 Calculate grams. 0. 125 L x 0. 720 mole KCl x 74. 6 g KCl 1 L 1 mole KCl = 6. 71 g KCl 12

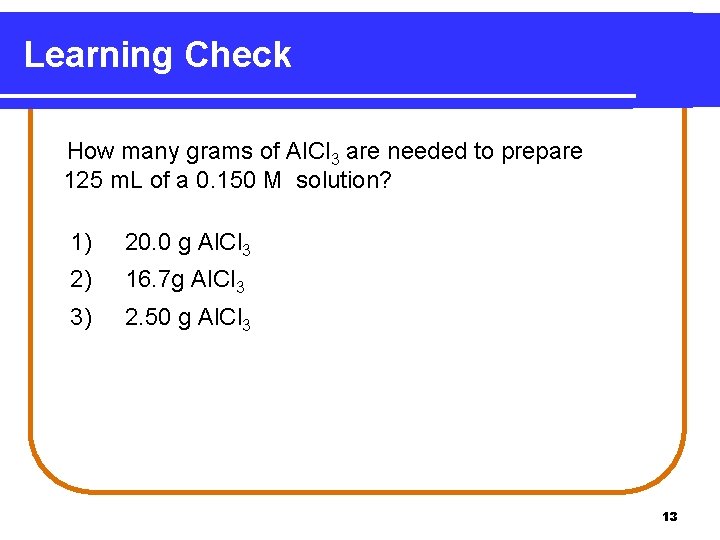
Learning Check How many grams of Al. Cl 3 are needed to prepare 125 m. L of a 0. 150 M solution? 1) 20. 0 g Al. Cl 3 2) 16. 7 g Al. Cl 3 3) 2. 50 g Al. Cl 3 13

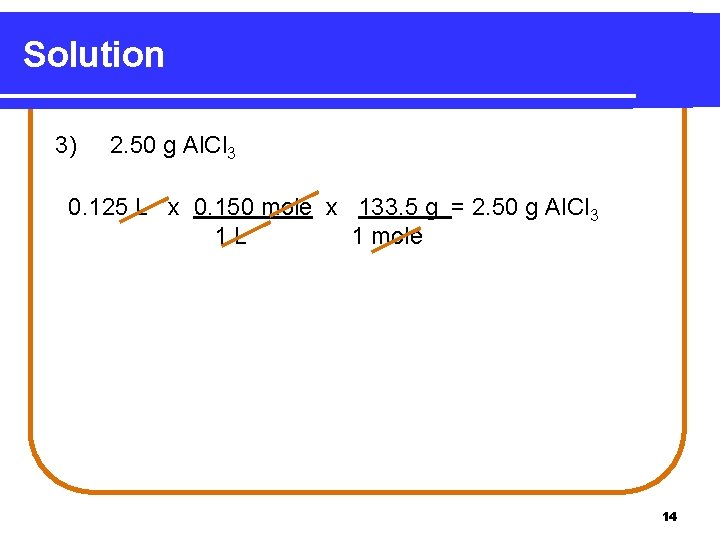
Solution 3) 2. 50 g Al. Cl 3 0. 125 L x 0. 150 mole x 133. 5 g = 2. 50 g Al. Cl 3 1 L 1 mole 14

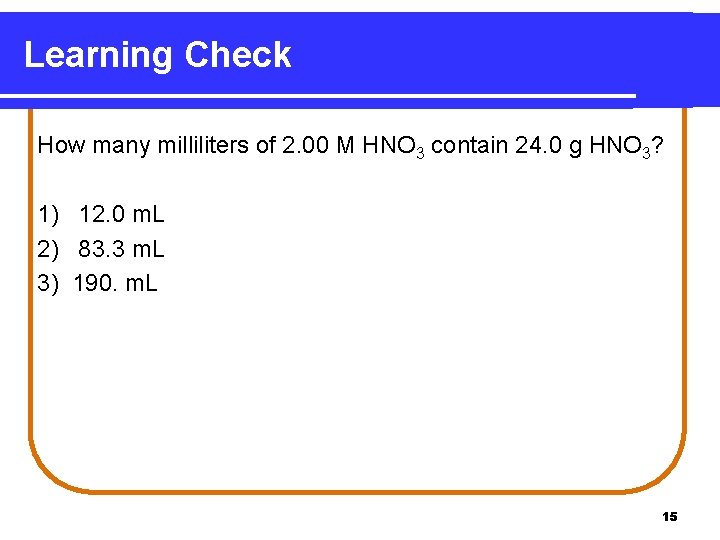
Learning Check How many milliliters of 2. 00 M HNO 3 contain 24. 0 g HNO 3? 1) 12. 0 m. L 2) 83. 3 m. L 3) 190. m. L 15

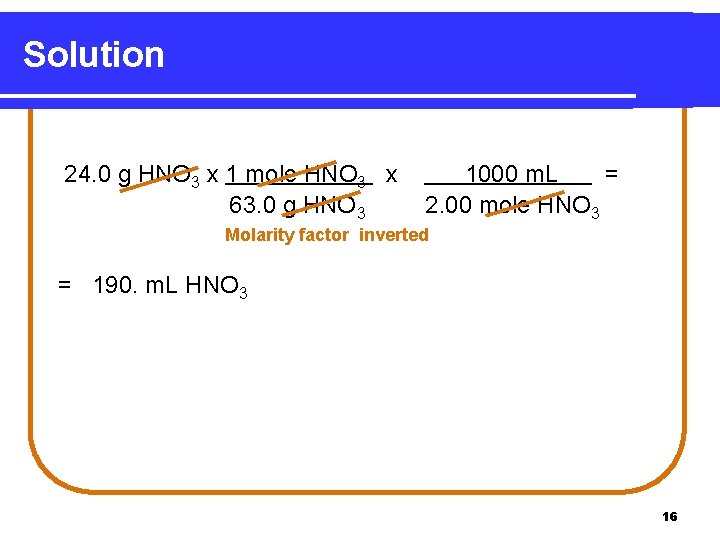
Solution 24. 0 g HNO 3 x 1 mole HNO 3 x 63. 0 g HNO 3 1000 m. L = 2. 00 mole HNO 3 Molarity factor inverted = 190. m. L HNO 3 16

Dilution In a dilution • water is added. • volume increases. • concentration decreases. 17

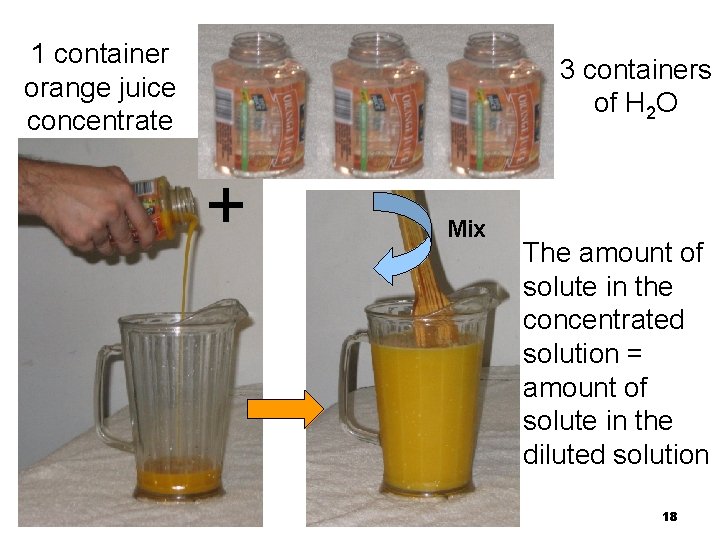
1 container orange juice concentrate 3 containers of H 2 O + Mix The amount of solute in the concentrated solution = amount of solute in the diluted solution 18

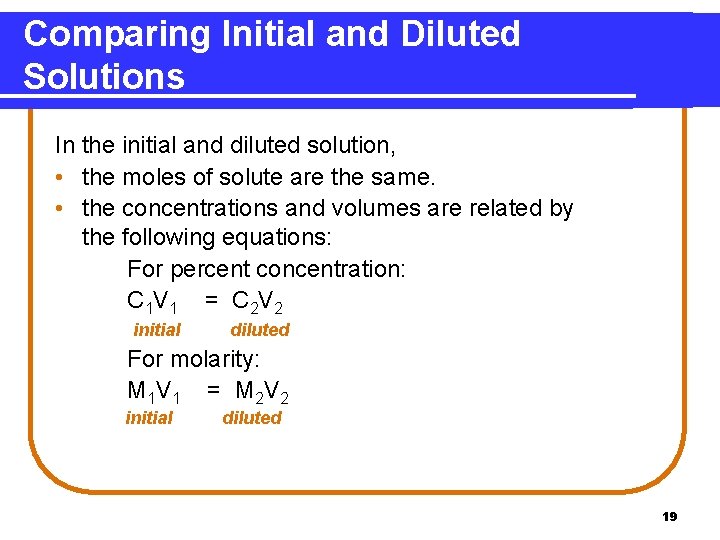
Comparing Initial and Diluted Solutions In the initial and diluted solution, • the moles of solute are the same. • the concentrations and volumes are related by the following equations: For percent concentration: C 1 V 1 = C 2 V 2 initial diluted For molarity: M 1 V 1 = M 2 V 2 initial diluted 19

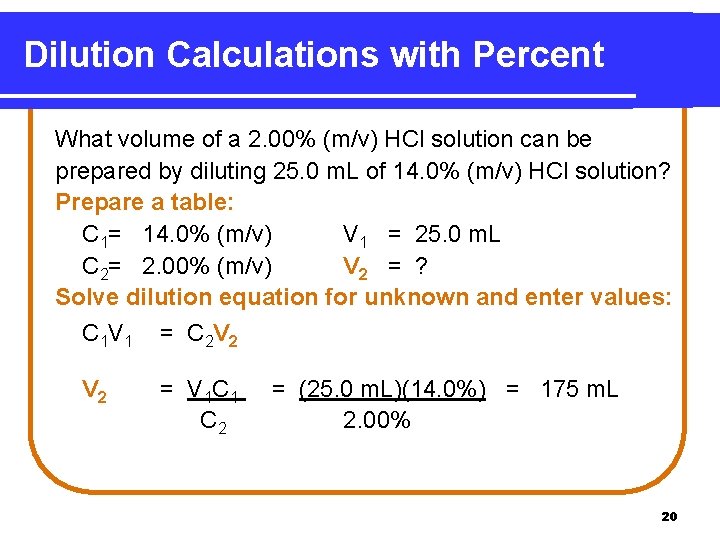
Dilution Calculations with Percent What volume of a 2. 00% (m/v) HCl solution can be prepared by diluting 25. 0 m. L of 14. 0% (m/v) HCl solution? Prepare a table: C 1= 14. 0% (m/v) V 1 = 25. 0 m. L C 2= 2. 00% (m/v) V 2 = ? Solve dilution equation for unknown and enter values: C 1 V 1 = C 2 V 2 = V 1 C 1 C 2 = (25. 0 m. L)(14. 0%) = 175 m. L 2. 00% 20

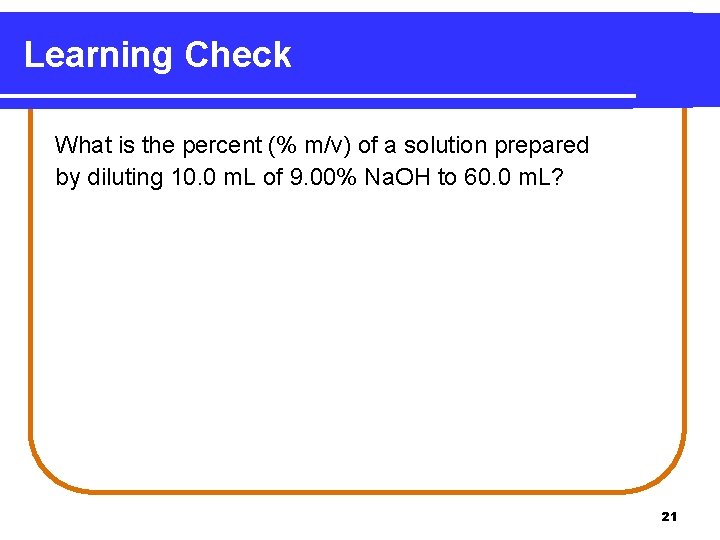
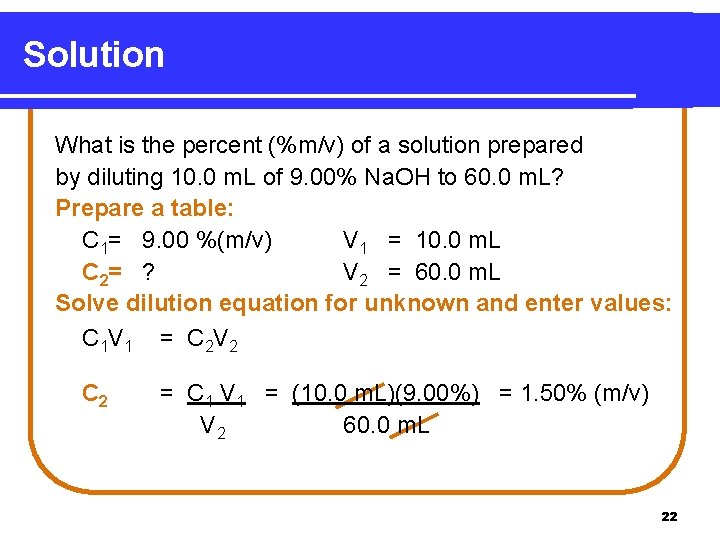
Learning Check What is the percent (% m/v) of a solution prepared by diluting 10. 0 m. L of 9. 00% Na. OH to 60. 0 m. L? 21

Solution What is the percent (%m/v) of a solution prepared by diluting 10. 0 m. L of 9. 00% Na. OH to 60. 0 m. L? Prepare a table: C 1= 9. 00 %(m/v) V 1 = 10. 0 m. L C 2= ? V 2 = 60. 0 m. L Solve dilution equation for unknown and enter values: C 1 V 1 = C 2 V 2 C 2 = C 1 V 1 = (10. 0 m. L)(9. 00%) = 1. 50% (m/v) V 2 60. 0 m. L 22

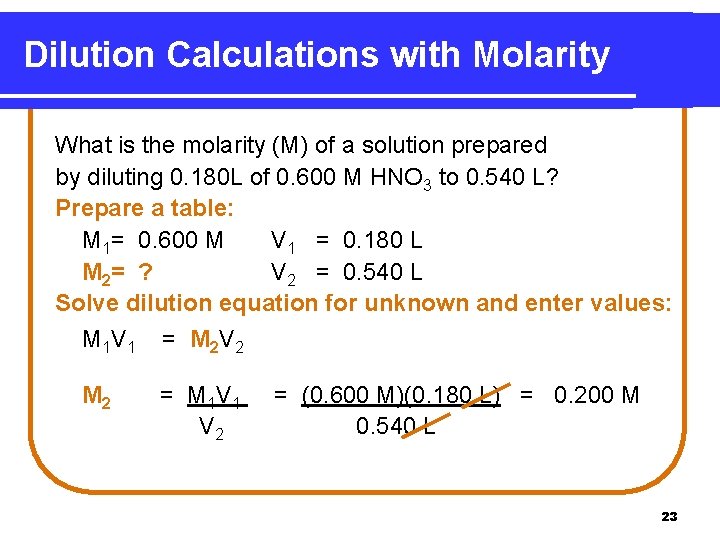
Dilution Calculations with Molarity What is the molarity (M) of a solution prepared by diluting 0. 180 L of 0. 600 M HNO 3 to 0. 540 L? Prepare a table: M 1= 0. 600 M V 1 = 0. 180 L M 2 = ? V 2 = 0. 540 L Solve dilution equation for unknown and enter values: M 1 V 1 = M 2 V 2 M 2 = M 1 V 2 = (0. 600 M)(0. 180 L) = 0. 200 M 0. 540 L 23

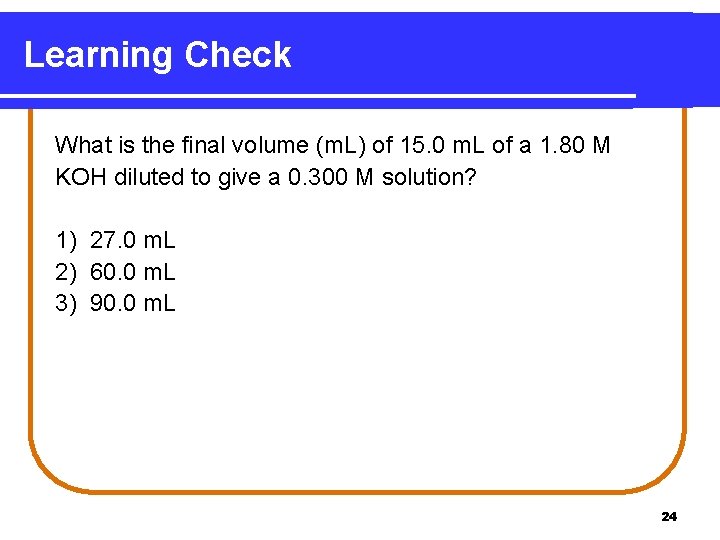
Learning Check What is the final volume (m. L) of 15. 0 m. L of a 1. 80 M KOH diluted to give a 0. 300 M solution? 1) 27. 0 m. L 2) 60. 0 m. L 3) 90. 0 m. L 24

Solution What is the final volume (m. L) of 15. 0 m. L of a 1. 80 M KOH diluted to give a 0. 300 M solution? Prepare a table: M 1= 1. 80 M V 1 = 15. 0 m. L M 2= 0. 300 M V 2 = ? Solve dilution equation for V 2 and enter values: M 1 V 1 = M 2 V 2 = M 1 V 1 M 2 = (1. 80 M)(15. 0 m. L) = 90. 0 m. L 0. 300 M 25