Chapter 7 Solutions 7 2 Electrolytes and Nonelectrolytes
- Slides: 11

Chapter 7 Solutions 7. 2 Electrolytes and Nonelectrolytes Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

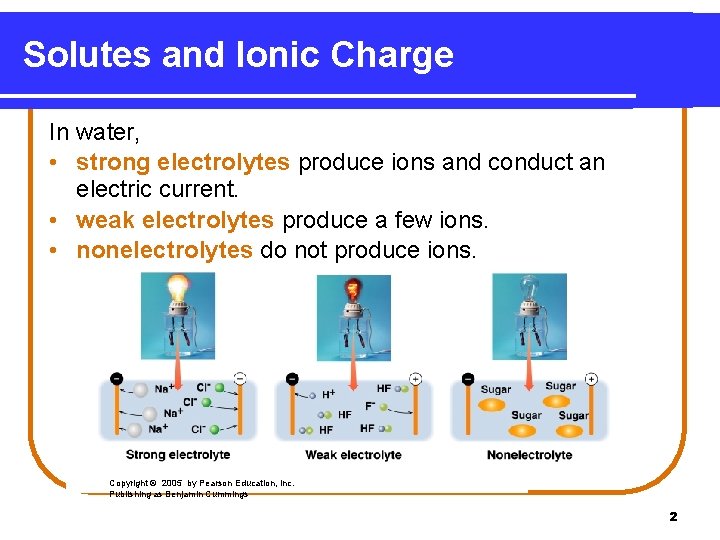
Solutes and Ionic Charge In water, • strong electrolytes produce ions and conduct an electric current. • weak electrolytes produce a few ions. • nonelectrolytes do not produce ions. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 2

Strong Electrolytes Strong electrolytes • dissociate in water producing positive and negative ions. • conduct an electric current in water. • in equations show the formation of ions in aqueous (aq) solutions. H 2 O 100% ions Na. Cl(s) Na+(aq) + Cl− (aq) H 2 O Ca. Br 2(s) Ca 2+(aq) + 2 Br− (aq) 3

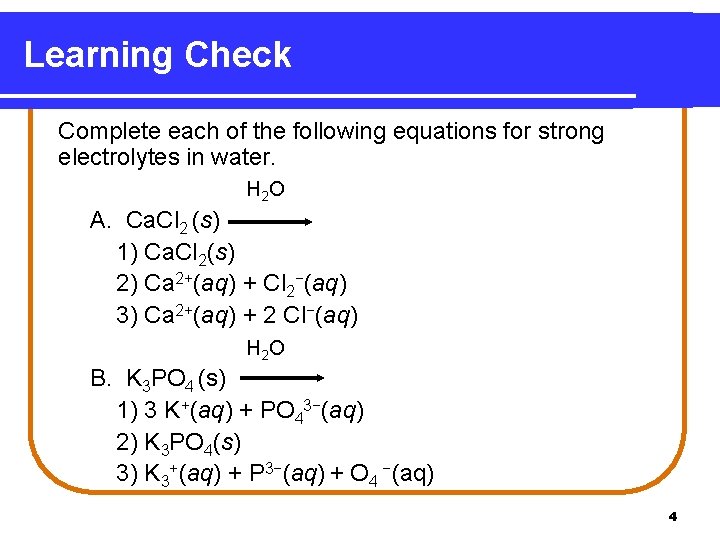
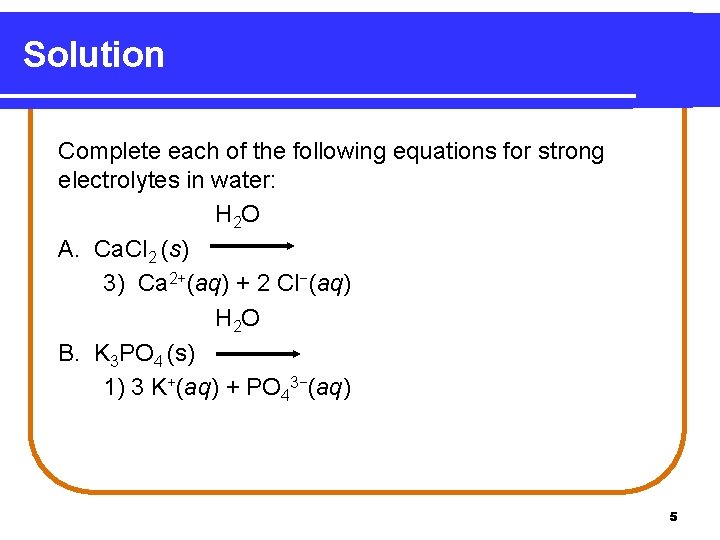
Learning Check Complete each of the following equations for strong electrolytes in water. H 2 O A. Ca. Cl 2 (s) 1) Ca. Cl 2(s) 2) Ca 2+(aq) + Cl 2−(aq) 3) Ca 2+(aq) + 2 Cl−(aq) H 2 O B. K 3 PO 4 (s) 1) 3 K+(aq) + PO 43−(aq) 2) K 3 PO 4(s) 3) K 3+(aq) + P 3−(aq) + O 4 −(aq) 4

Solution Complete each of the following equations for strong electrolytes in water: H 2 O A. Ca. Cl 2 (s) 3) Ca 2+(aq) + 2 Cl−(aq) H 2 O B. K 3 PO 4 (s) 1) 3 K+(aq) + PO 43−(aq) 5

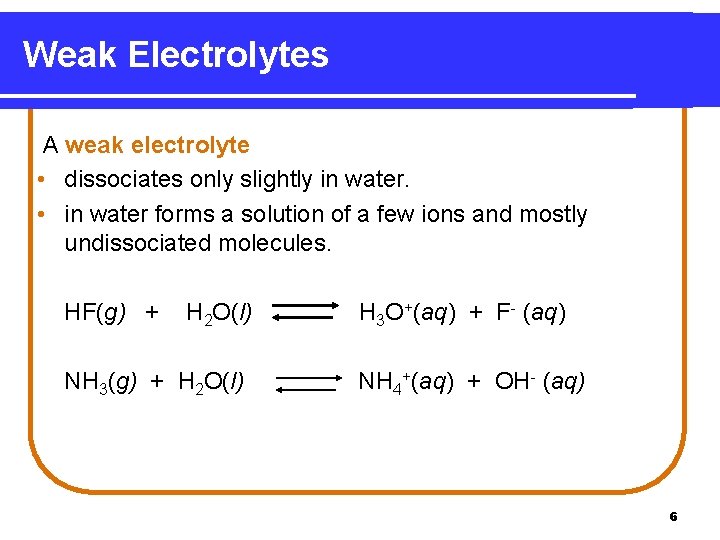
Weak Electrolytes A weak electrolyte • dissociates only slightly in water. • in water forms a solution of a few ions and mostly undissociated molecules. HF(g) + H 2 O(l) NH 3(g) + H 2 O(l) H 3 O+(aq) + F- (aq) NH 4+(aq) + OH- (aq) 6

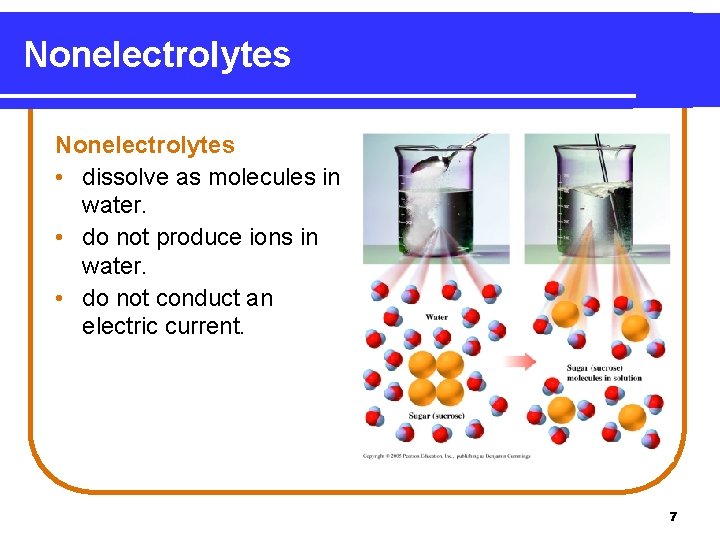
Nonelectrolytes • dissolve as molecules in water. • do not produce ions in water. • do not conduct an electric current. 7

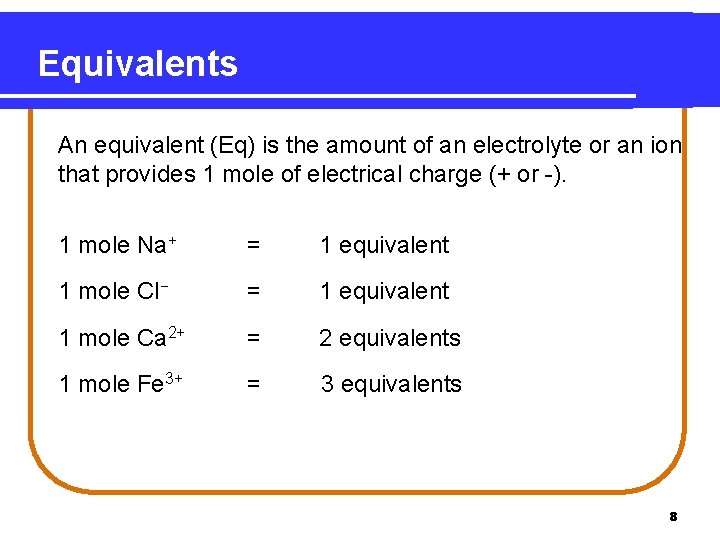
Equivalents An equivalent (Eq) is the amount of an electrolyte or an ion that provides 1 mole of electrical charge (+ or -). 1 mole Na+ = 1 equivalent 1 mole Cl− = 1 equivalent 1 mole Ca 2+ = 2 equivalents 1 mole Fe 3+ = 3 equivalents 8

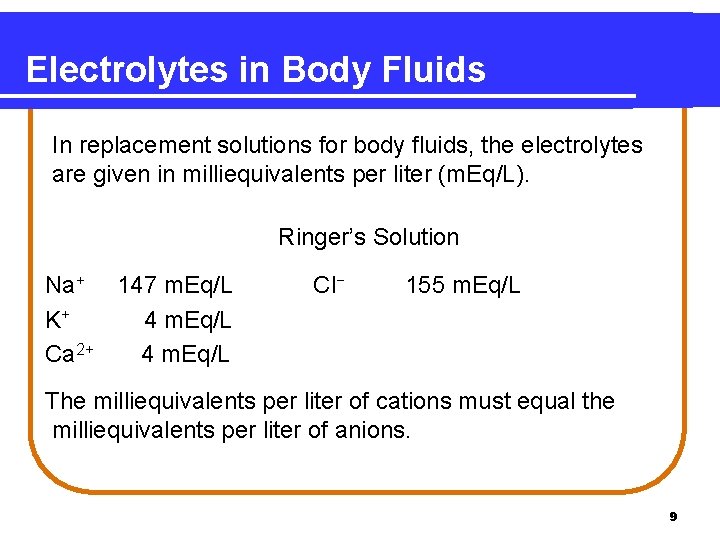
Electrolytes in Body Fluids In replacement solutions for body fluids, the electrolytes are given in milliequivalents per liter (m. Eq/L). Ringer’s Solution Na+ 147 m. Eq/L K+ 4 m. Eq/L Ca 2+ 4 m. Eq/L Cl− 155 m. Eq/L The milliequivalents per liter of cations must equal the milliequivalents per liter of anions. 9

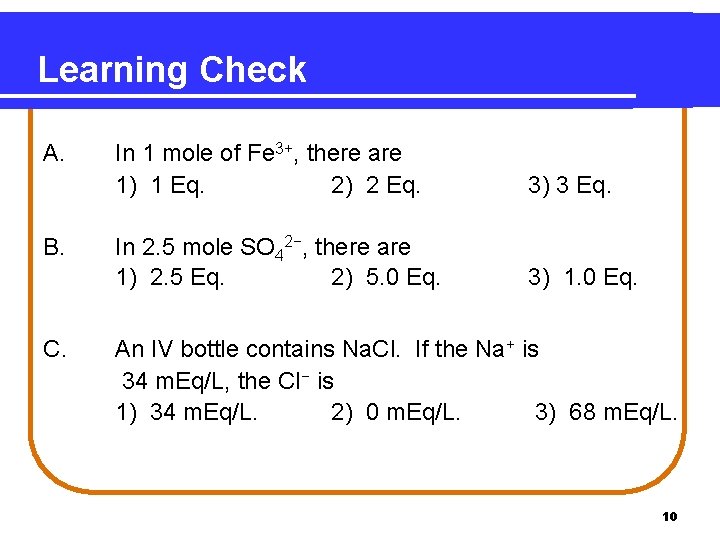
Learning Check A. B. C. In 1 mole of Fe 3+, there are 1) 1 Eq. 2) 2 Eq. 3) 3 Eq. In 2. 5 mole SO 42−, there are 1) 2. 5 Eq. 2) 5. 0 Eq. 3) 1. 0 Eq. An IV bottle contains Na. Cl. If the Na+ is 34 m. Eq/L, the Cl− is 1) 34 m. Eq/L. 2) 0 m. Eq/L. 3) 68 m. Eq/L. 10

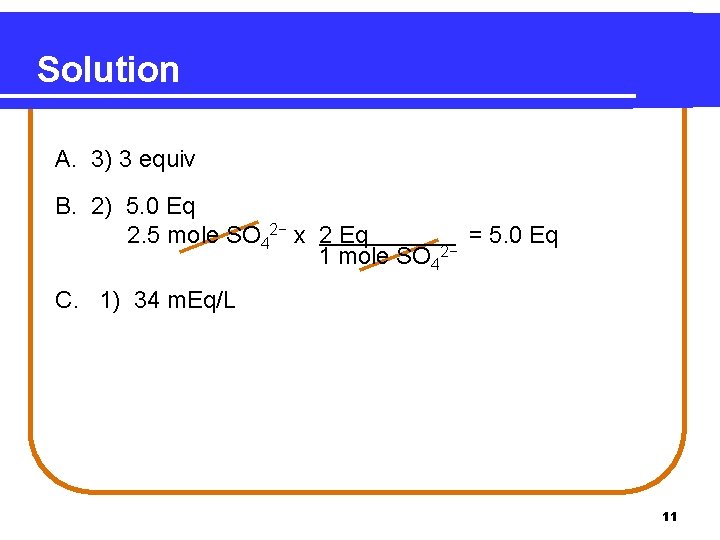
Solution A. 3) 3 equiv B. 2) 5. 0 Eq 2. 5 mole SO 42− x 2 Eq = 5. 0 Eq 1 mole SO 42− C. 1) 34 m. Eq/L 11