Chapter 7 Solutions 1 7 3 Solubility Chemistry

Chapter 7 Solutions 1 7. 3 Solubility Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
Solubility 2 Solubility is • the maximum amount of solute that dissolves in a specific amount of solvent • • temperature sensitive for solutes expressed as grams of solute in 100 grams of solvent, usually water g of solute 100 g water Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Unsaturated Solution 3 Unsaturated solutions • contain less than the maximum amount of solute • can dissolve more solute Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Saturated Solution 4 Saturated solutions • contain the maximum amount of solute that can dissolve • have undissolved solute at the bottom of the container • contain solute that dissolves as well as solute that recrystallizes in an equilibrium process Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Learning Check 5 Label each of the following solutions as saturated or unsaturated. A. Salt disappears when put in water. B. Sugar added to a cup of water does not disappear, but sits at the bottom of the cup. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Solution 6 Label each of the following solutions as saturated or unsaturated. A. Unsaturated: Salt disappears when put in water. B. Saturated: Sugar added to a cup of water does not disappear, but sits at the bottom of the cup. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
Learning Check 7 At 40 C, the solubility of KBr is 80 g/100 g of H 2 O. Identify the following solutions as either saturated or unsaturated. Explain. A. 60 g KBr added to 100 g of water at 40 C B. 200 g KBr added to 200 g of water at 40 C C. 25 g KBr added to 50 g of water at 40 C Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
Solution 8 At 40 C, the solubility of KBr is 80 g/100 g of H 2 O. Identify the following solutions as either saturated or unsaturated. Explain. A. Unsaturated: 60 g KBr/100 g of water at 40 C is less than the solubility of KBr in water (80 g KBr/ 100 g water) B. Saturated: 200 g KBr/200 g of water at 40 C is greater than the solubility of KBr in water (80 g KBr/ 100 g water) C. Unsaturated: 25 g KBr/50 g of water at 40 C is less than the solubility of KBr in water (80 g KBr/ 100 g water) Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
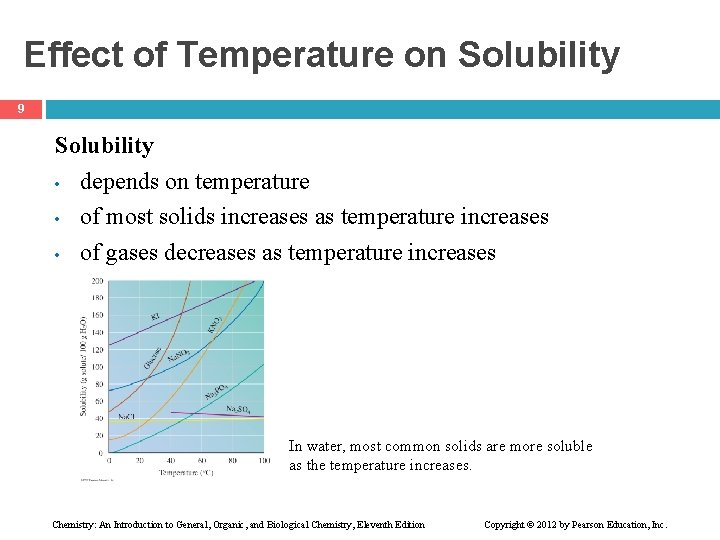
Effect of Temperature on Solubility 9 Solubility • depends on temperature • of most solids increases as temperature increases • of gases decreases as temperature increases In water, most common solids are more soluble as the temperature increases. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Learning Check 10 1. Why could a bottle of carbonated drink possibly burst (explode) when it is left out in the hot sun? 2. Why do fish die in water that is too warm? Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
Solution 11 1. The pressure in a bottle increases as the gas leaves solution when it becomes less soluble at higher temperatures. As pressure increases, the bottle could burst. 2. Because O 2 gas is less soluble in warm water, fish cannot obtain the amount of O 2 required for their survival. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
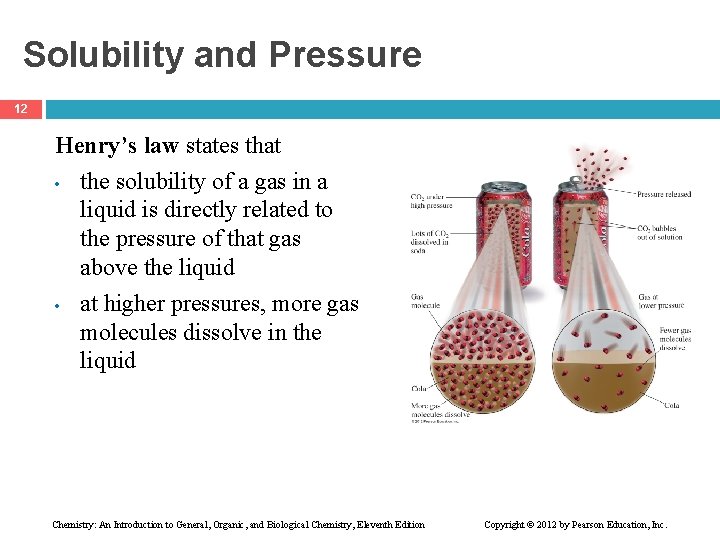
Solubility and Pressure 12 Henry’s law states that • the solubility of a gas in a liquid is directly related to the pressure of that gas above the liquid • at higher pressures, more gas molecules dissolve in the liquid Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
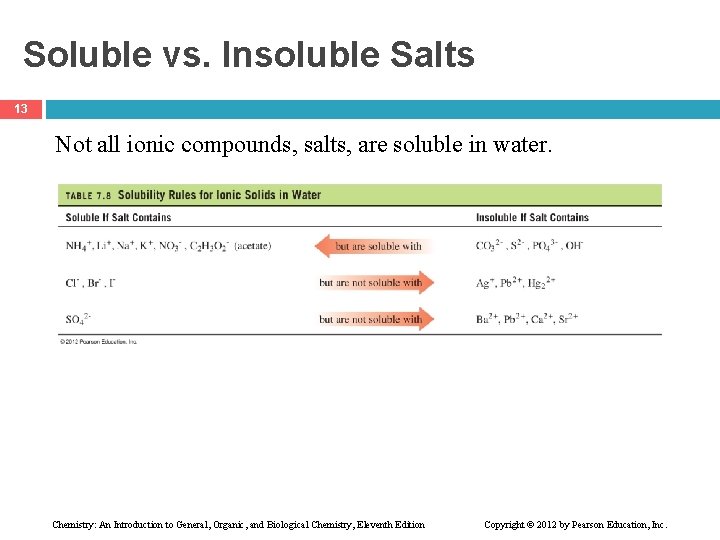
Soluble vs. Insoluble Salts 13 Not all ionic compounds, salts, are soluble in water. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
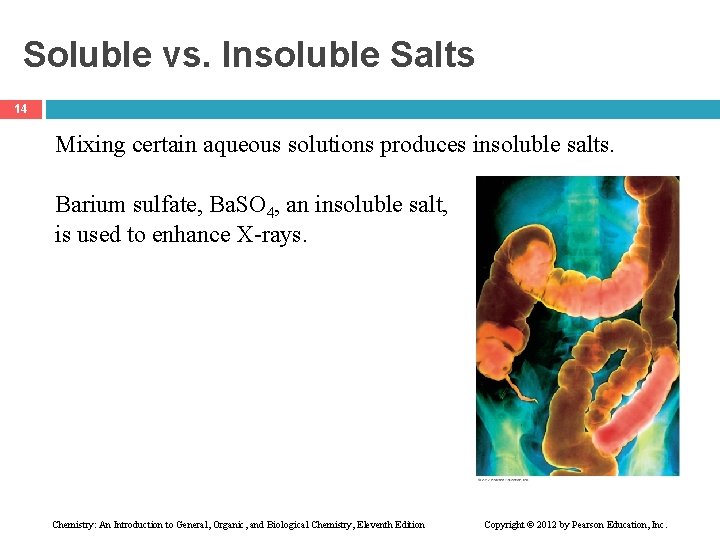
Soluble vs. Insoluble Salts 14 Mixing certain aqueous solutions produces insoluble salts. Barium sulfate, Ba. SO 4, an insoluble salt, is used to enhance X-rays. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
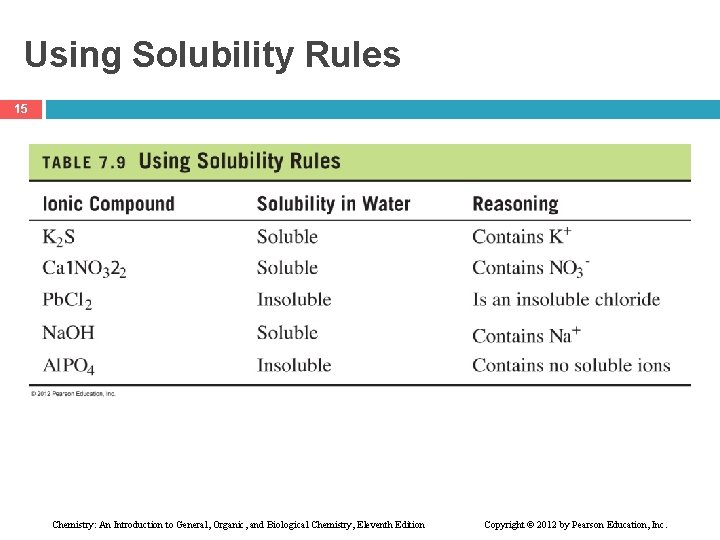
Using Solubility Rules 15 Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.

Learning Check 16 What ions make each of these compounds insoluble in water? Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
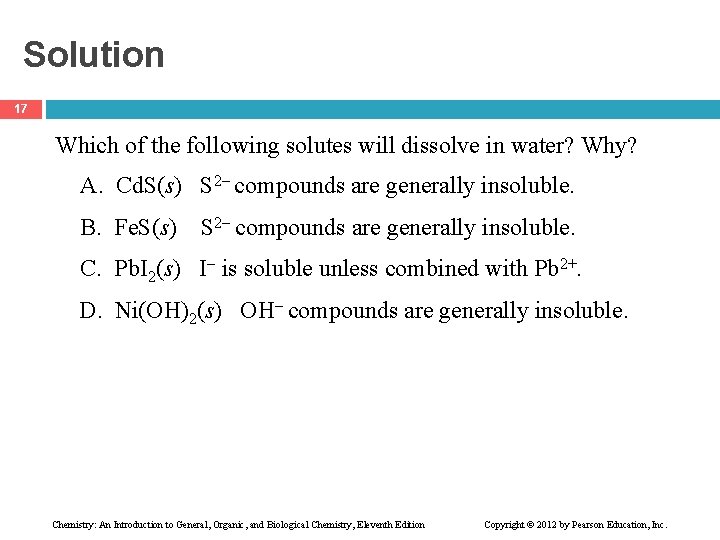
Solution 17 Which of the following solutes will dissolve in water? Why? A. Cd. S(s) S 2 compounds are generally insoluble. B. Fe. S(s) S 2 compounds are generally insoluble. C. Pb. I 2(s) I is soluble unless combined with Pb 2+. D. Ni(OH)2(s) OH compounds are generally insoluble. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright © 2012 by Pearson Education, Inc.
- Slides: 17