Chapter 7 Seawater Chemistry AcidBase Balance and Density
Chapter 7 Seawater Chemistry Acid/Base Balance and Density © 2002 Brooks/Cole, a division of Thomson Learning, Inc.
Acid-Base Balance What are acids and bases? An acid is a substance that releases a hydrogen ion in solution. A base is a substance that combines with a hydrogen ion in solution. A solution containing an acid is called an acidic solution. A solution containing a base is called an alkaline solution. Acidity or alkalinity is measured on the p. H scale. © 2002 Brooks/Cole, a division of Thomson Learning, Inc.
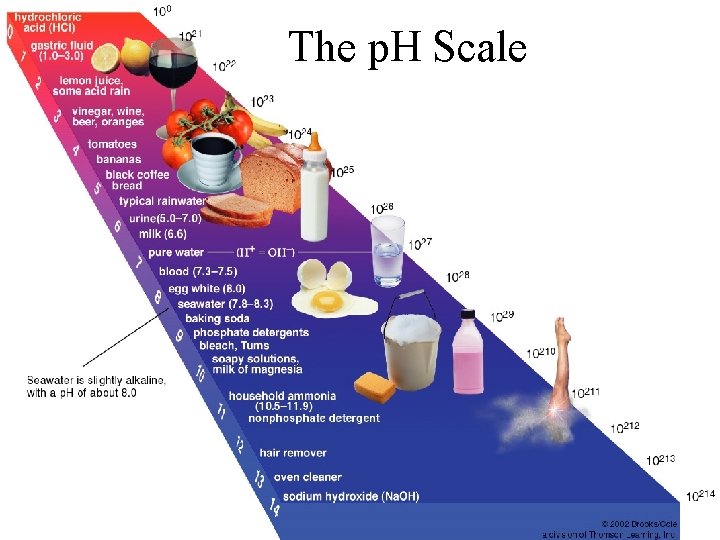
The p. H Scale
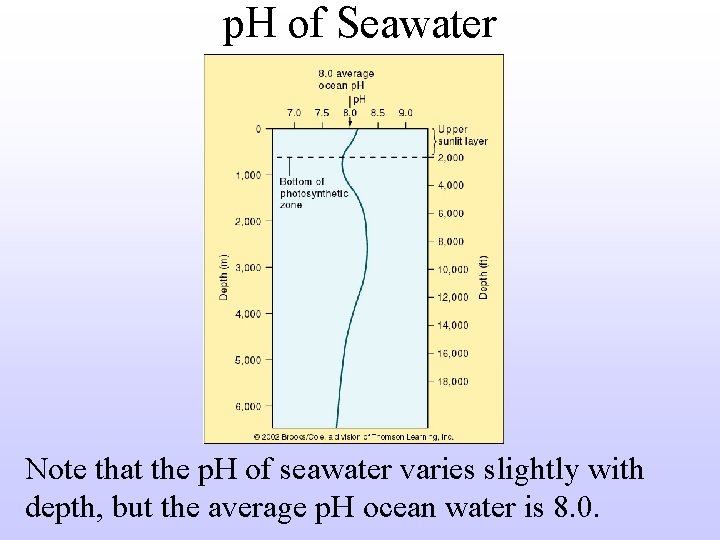
p. H of Seawater Note that the p. H of seawater varies slightly with depth, but the average p. H ocean water is 8. 0.

Carbonate buffering • Keeps ocean p. H about same (8. 1) – If p. H gets too high (water is too basic), carbonic acid (H 2 CO 3) releases H+ to become bicarbonate (HCO 3) – If p. H gets too low (water is too acidic), bicarbonate (HCO 3) combines with H+ to become carbonic acid (H 2 CO 3) • Precipitation/dissolution of calcium carbonate Ca. CO 3 buffers ocean p. H • Oceans can absorb CO 2 from atmosphere without much change in p. H

Carbonate buffering

Density of seawater • 1. 022 to 1. 030 g/cm 3 surface seawater • Ocean layered according to density • Density seawater controlled by temperature, salinity, and pressure – Most important influence is temperature – Density increases with decreasing temperature (cold water is denser than warm water)

Density of seawater • Polar ocean is isothermal • Salinity greatest influence on density in polar oceans – Salty water is denser than fresh water
Density versus depth • Density differences cause a layered ocean • Pycnocline, abrupt change of density with depth • Thermocline, abrupt change of temperature with depth
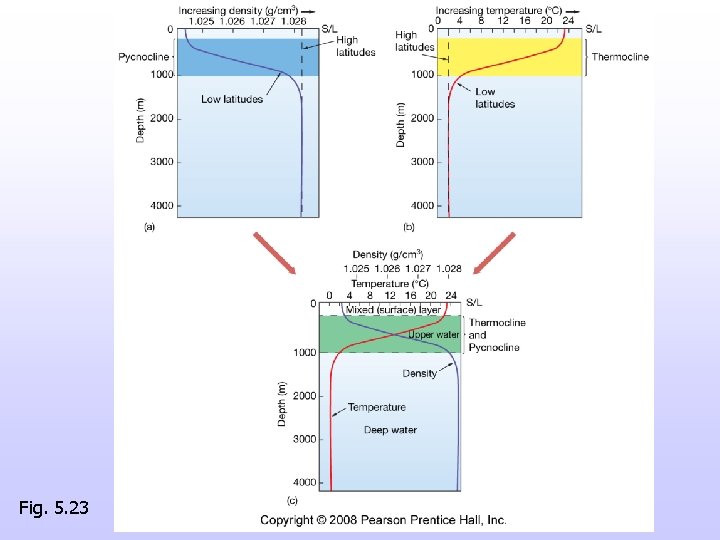
Fig. 5. 23

Layered ocean – Mixed surface water – Pycnocline and thermocline – Deep water • High latitude oceans (poles) are not as layered – Isothermal – Isopycnal
- Slides: 11