Chapter 7 Reactions of Alkenes and Alkynes 2006








- Slides: 70

Chapter 7 Reactions of Alkenes and Alkynes © 2006 Thomson Higher Education

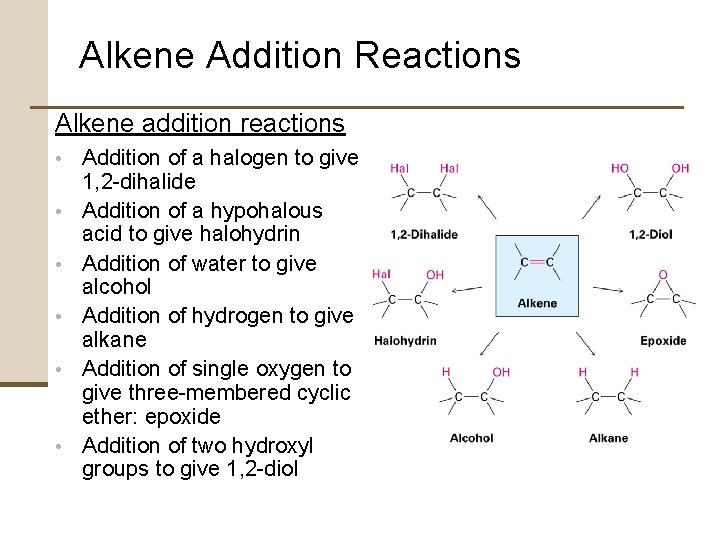
Alkene Addition Reactions Alkene addition reactions • Addition of a halogen to give • • • 1, 2 -dihalide Addition of a hypohalous acid to give halohydrin Addition of water to give alcohol Addition of hydrogen to give alkane Addition of single oxygen to give three-membered cyclic ether: epoxide Addition of two hydroxyl groups to give 1, 2 -diol

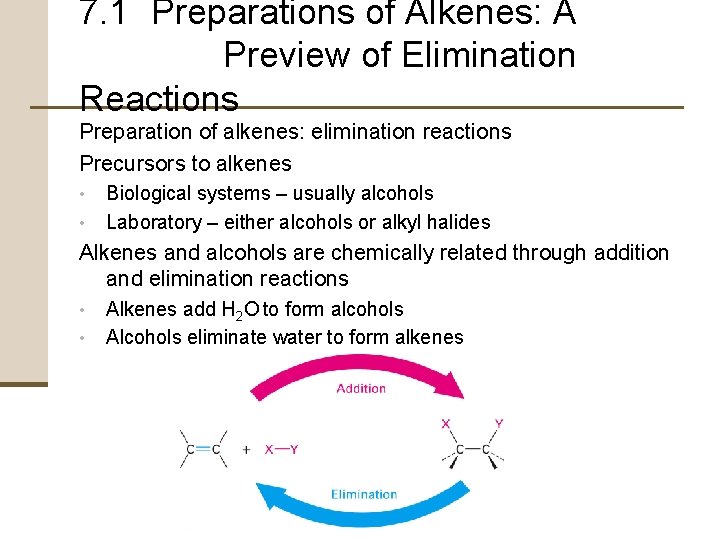
7. 1 Preparations of Alkenes: A Preview of Elimination Reactions Preparation of alkenes: elimination reactions Precursors to alkenes • • Biological systems – usually alcohols Laboratory – either alcohols or alkyl halides Alkenes and alcohols are chemically related through addition and elimination reactions • • Alkenes add H 2 O to form alcohols Alcohols eliminate water to form alkenes

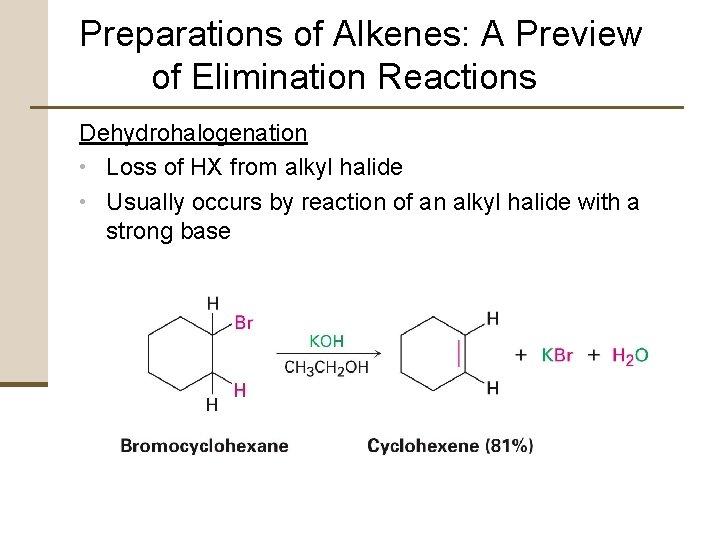
Preparations of Alkenes: A Preview of Elimination Reactions Dehydrohalogenation • Loss of HX from alkyl halide • Usually occurs by reaction of an alkyl halide with a strong base

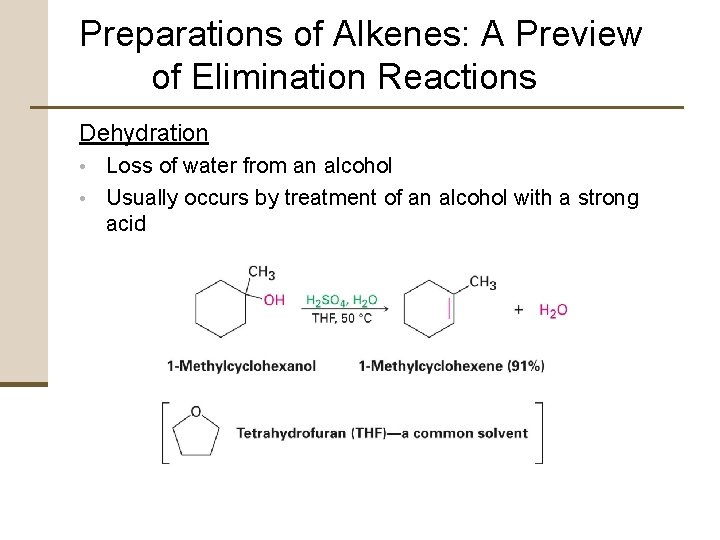
Preparations of Alkenes: A Preview of Elimination Reactions Dehydration • Loss of water from an alcohol • Usually occurs by treatment of an alcohol with a strong acid

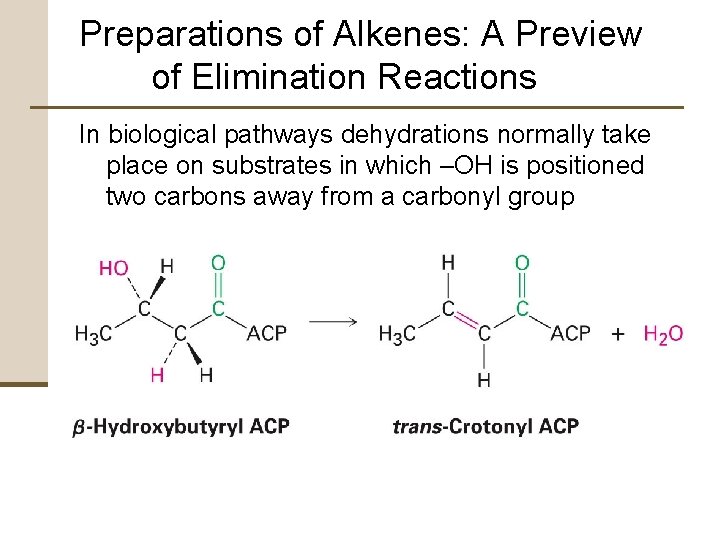
Preparations of Alkenes: A Preview of Elimination Reactions In biological pathways dehydrations normally take place on substrates in which –OH is positioned two carbons away from a carbonyl group

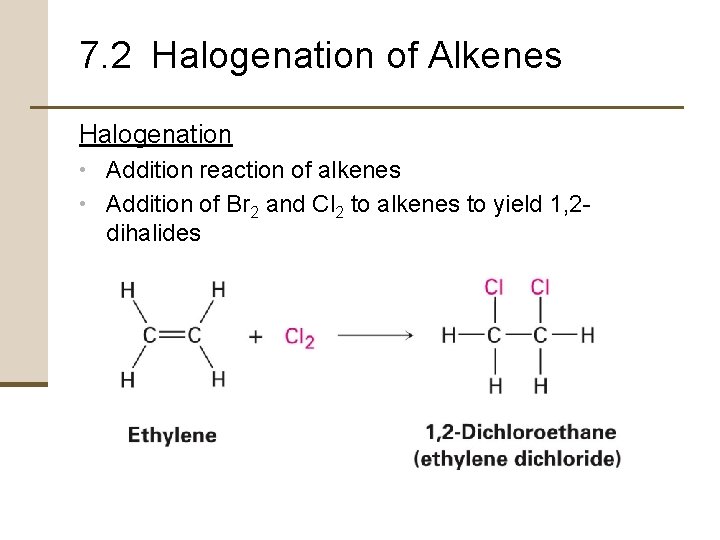
7. 2 Halogenation of Alkenes Halogenation • Addition reaction of alkenes • Addition of Br 2 and Cl 2 to alkenes to yield 1, 2 - dihalides

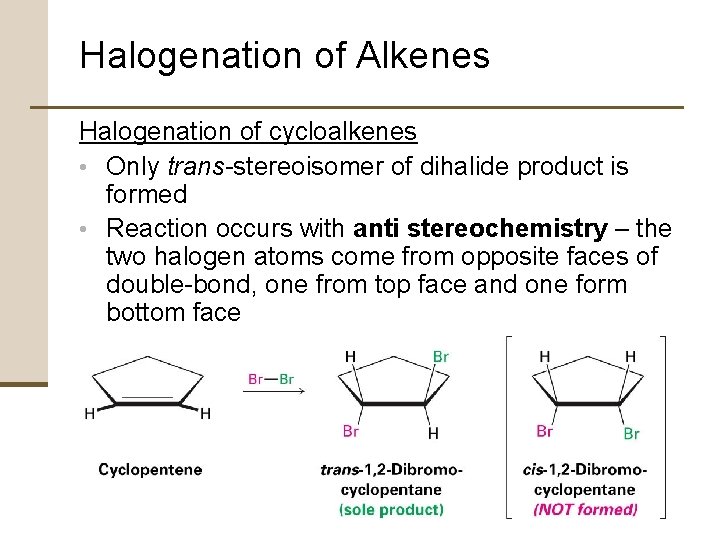
Halogenation of Alkenes Halogenation of cycloalkenes • Only trans-stereoisomer of dihalide product is formed • Reaction occurs with anti stereochemistry – the two halogen atoms come from opposite faces of double-bond, one from top face and one form bottom face

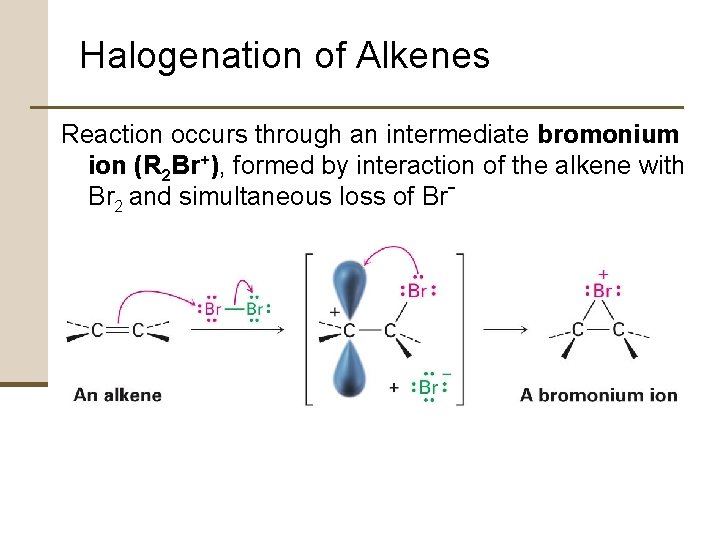
Halogenation of Alkenes Reaction occurs through an intermediate bromonium ion (R 2 Br+), formed by interaction of the alkene with Br 2 and simultaneous loss of Br-

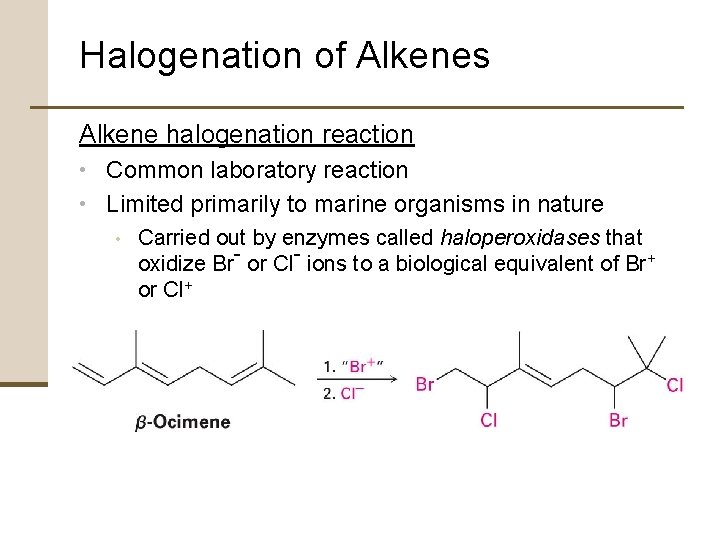
Halogenation of Alkenes Alkene halogenation reaction • Common laboratory reaction • Limited primarily to marine organisms in nature • Carried out by enzymes called haloperoxidases that oxidize Br- or Cl- ions to a biological equivalent of Br+ or Cl+

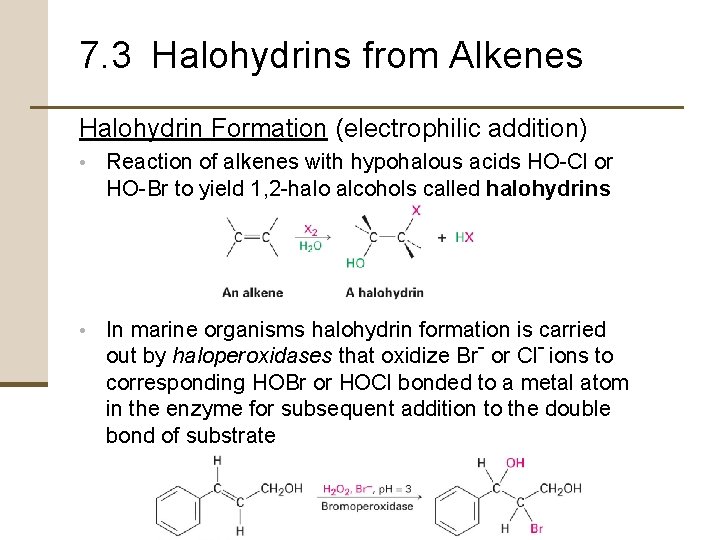
7. 3 Halohydrins from Alkenes Halohydrin Formation (electrophilic addition) • Reaction of alkenes with hypohalous acids HO-Cl or HO-Br to yield 1, 2 -halo alcohols called halohydrins • In marine organisms halohydrin formation is carried out by haloperoxidases that oxidize Br- or Cl- ions to corresponding HOBr or HOCl bonded to a metal atom in the enzyme for subsequent addition to the double bond of substrate

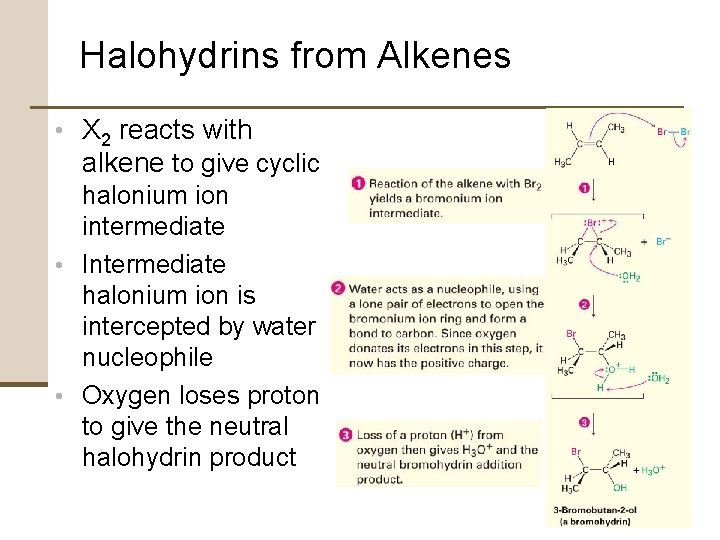
Halohydrins from Alkenes • X 2 reacts with alkene to give cyclic halonium ion intermediate • Intermediate halonium ion is intercepted by water nucleophile • Oxygen loses proton to give the neutral halohydrin product

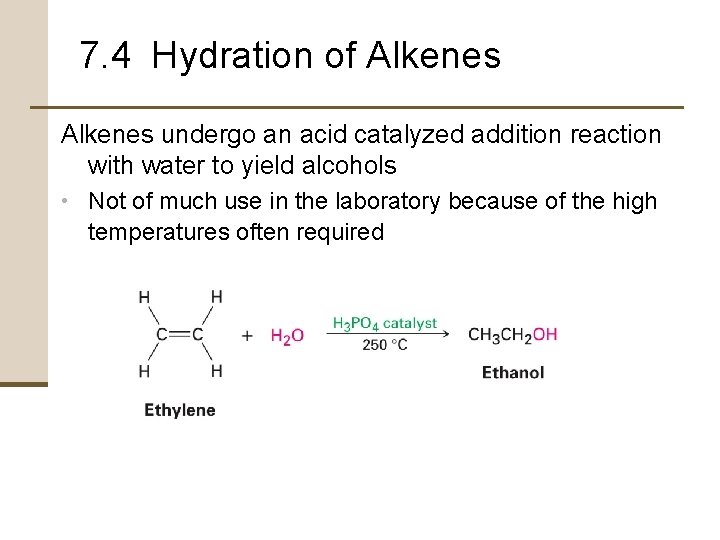
7. 4 Hydration of Alkenes undergo an acid catalyzed addition reaction with water to yield alcohols • Not of much use in the laboratory because of the high temperatures often required

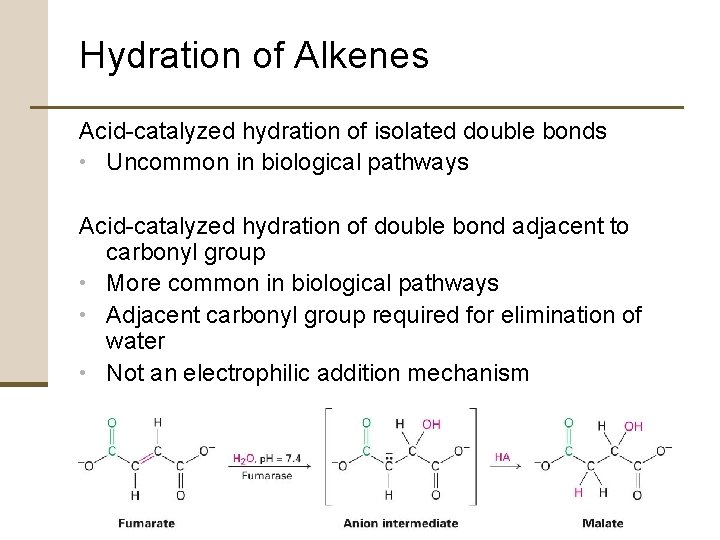
Hydration of Alkenes Acid-catalyzed hydration of isolated double bonds • Uncommon in biological pathways Acid-catalyzed hydration of double bond adjacent to carbonyl group • More common in biological pathways • Adjacent carbonyl group required for elimination of water • Not an electrophilic addition mechanism

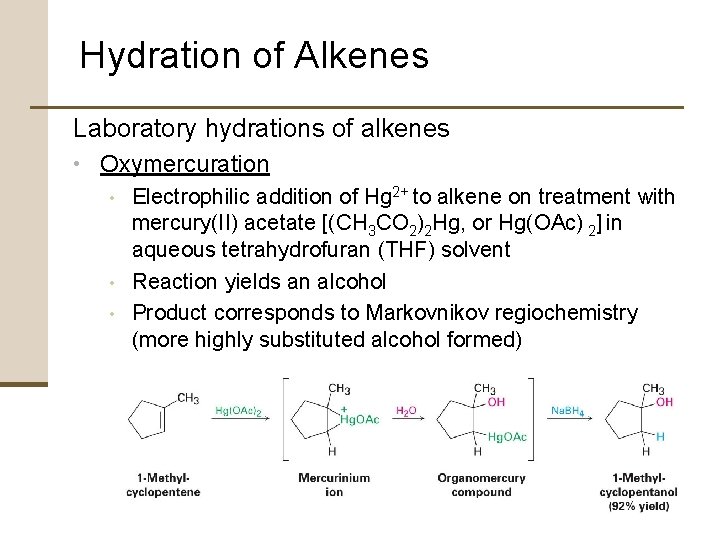
Hydration of Alkenes Laboratory hydrations of alkenes • Oxymercuration • Electrophilic addition of Hg 2+ to alkene on treatment with mercury(II) acetate [(CH 3 CO 2)2 Hg, or Hg(OAc) 2] in aqueous tetrahydrofuran (THF) solvent • Reaction yields an alcohol • Product corresponds to Markovnikov regiochemistry (more highly substituted alcohol formed)

Hydration of Alkenes • Hydroboration/oxidation • Addition of a B-H bond of borane, BH 3, to an alkene • Occurs in single step • No carbocation intermediate • Reaction yields an alcohol • Syn stereochemistry • Both C-H and C-B bonds form at the same time and from the same face of the double-bond • Product has non-Markovnikov regiochemistry

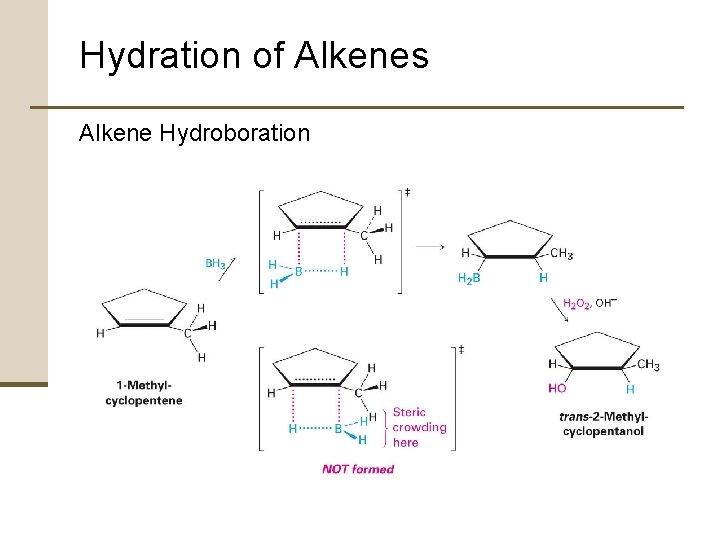
Hydration of Alkenes Alkene Hydroboration

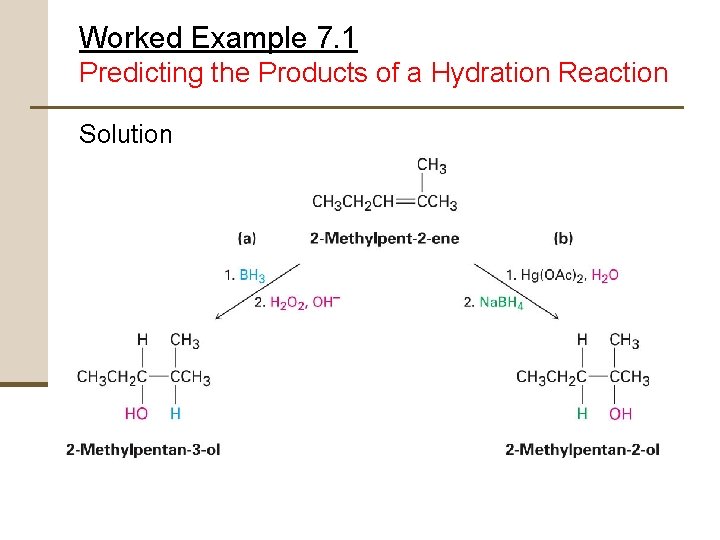
Worked Example 7. 1 Predicting the Products of a Hydration Reaction What products would you obtain from reaction of 2 methylpent-2 -ene with: (a) BH 3, followed by H 2 O 2, OH(b) Hg(OAc)2, followed by Na. BH 4

Worked Example 7. 1 Predicting the Products of a Hydration Reaction Strategy • Determine type of reaction being carried out • Two methods of hydration • • Hydroboration/oxidation • Occurs with syn stereochemistry • Gives non-Markovnikov alcohol Oxymercuration • Gives the Markovnikov alcohol

Worked Example 7. 1 Predicting the Products of a Hydration Reaction Solution

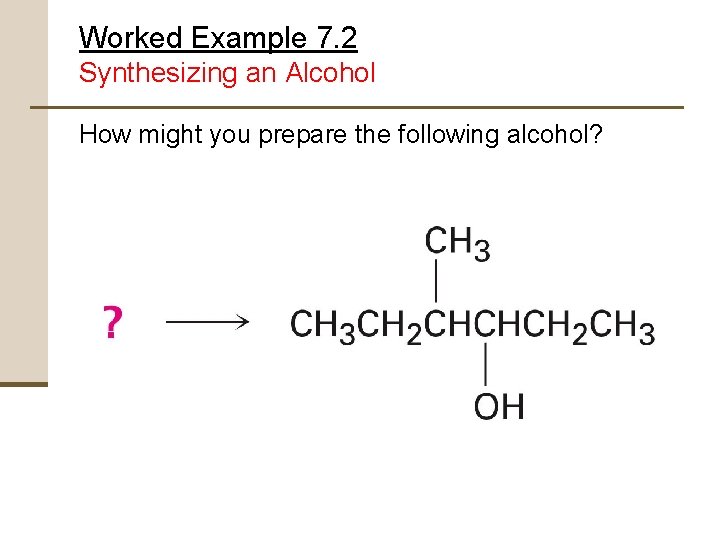
Worked Example 7. 2 Synthesizing an Alcohol How might you prepare the following alcohol?

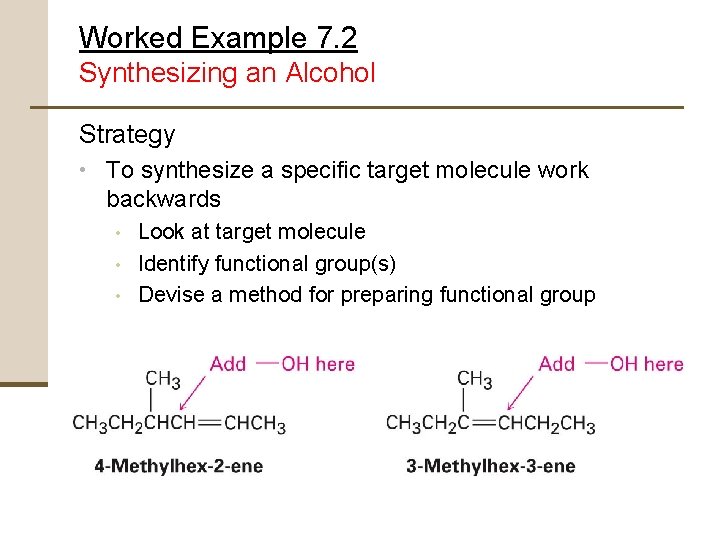
Worked Example 7. 2 Synthesizing an Alcohol Strategy • To synthesize a specific target molecule work backwards • • • Look at target molecule Identify functional group(s) Devise a method for preparing functional group

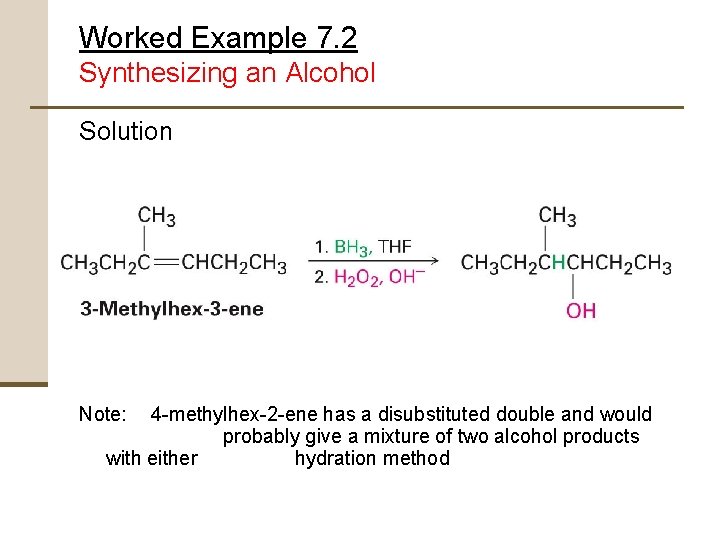
Worked Example 7. 2 Synthesizing an Alcohol Solution Note: 4 -methylhex-2 -ene has a disubstituted double and would probably give a mixture of two alcohol products with either hydration method

7. 5 Reduction of Alkenes Hydrogenation • Addition reaction process by which alkenes are reduced to alkanes Reduction • Increases electron density on carbon by • • Forming C-H Breaking C-O, C-N, or C-X bond Common catalysts for alkene hydrogenation: • Platinum – Pt. O 2 (Adams’ Catalyst) • Palladium – very fine powder supported on inert material such as charcoal (Pd/C)

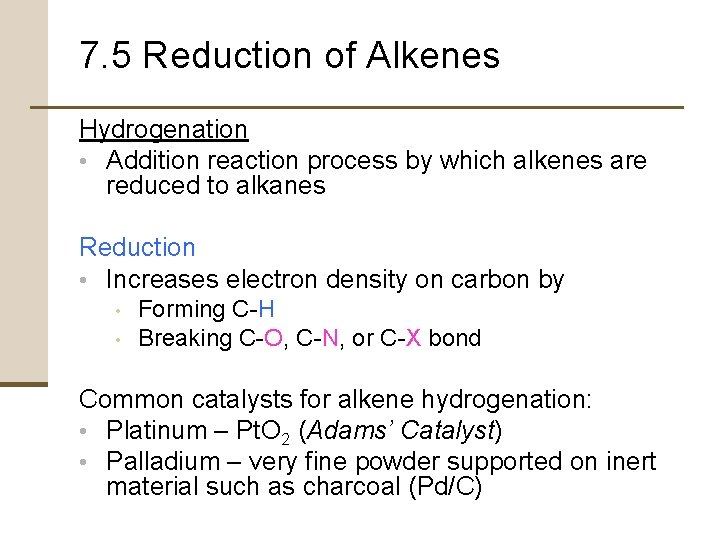
Reduction of Alkenes Catalytic hydrogenation • A heterogeneous process that takes place on the surface of insoluble catalyst particles • Occurs with syn stereochemistry • Both hydrogens add to the double bond from the same side

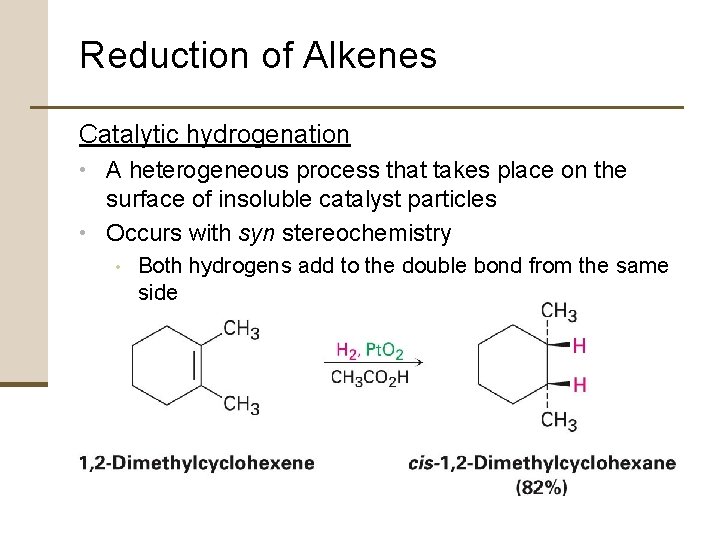
Reduction of Alkenes Steps of Catalytic hydrogenation: 1. 2. 3. 4. Adsorption of H 2 onto catalyst surface Complexation between catalyst and alkene occurs as a vacant orbital on metal interacts with filled p orbitals Hydrogen added to double bond Saturated product diffuses away from catalyst

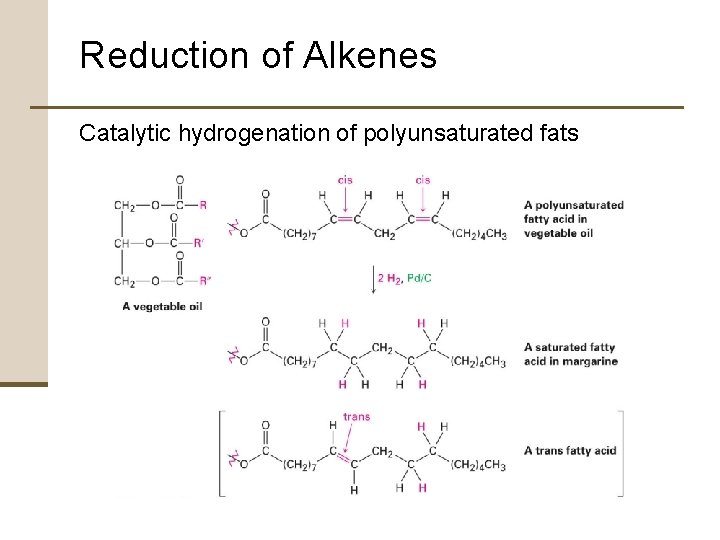
Reduction of Alkenes Hydrogenation • Unsaturated vegetable oils reduced to produce saturated fats used in margarine and cooking products • Vegetable oils • Triesters of glycerol, HOCH 2 CH(OH)CH 2 OH, with three long-chain carboxylic acids called fatty acids • Fatty acids • Polyunsaturated carboxylic acids containing long hydrocarbon chains • Double bonds have cis stereochemistry

Reduction of Alkenes Catalytic hydrogenation of polyunsaturated fats

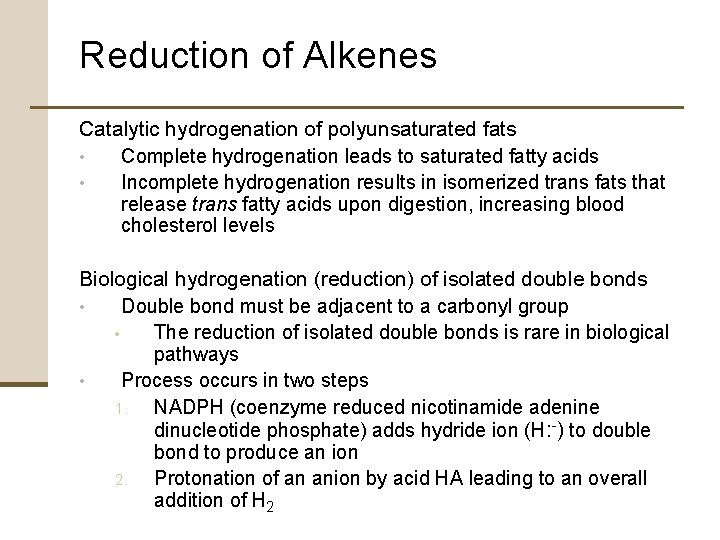
Reduction of Alkenes Catalytic hydrogenation of polyunsaturated fats • Complete hydrogenation leads to saturated fatty acids • Incomplete hydrogenation results in isomerized trans fats that release trans fatty acids upon digestion, increasing blood cholesterol levels Biological hydrogenation (reduction) of isolated double bonds • Double bond must be adjacent to a carbonyl group • The reduction of isolated double bonds is rare in biological pathways • Process occurs in two steps 1. NADPH (coenzyme reduced nicotinamide adenine dinucleotide phosphate) adds hydride ion (H: -) to double bond to produce an ion 2. Protonation of an anion by acid HA leading to an overall addition of H 2

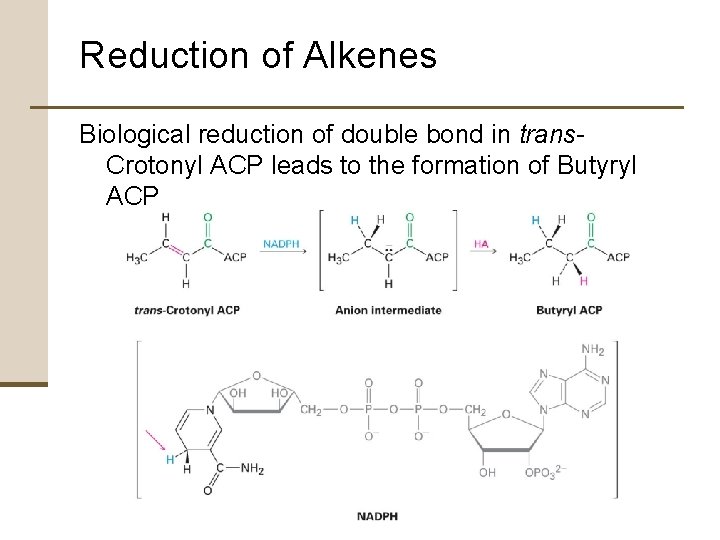
Reduction of Alkenes Biological reduction of double bond in trans. Crotonyl ACP leads to the formation of Butyryl ACP

7. 6 Oxidation of Alkenes: Epoxidation Oxidation • A reaction that results in a loss of electron density by carbon Oxidation • Decreases electron density on carbon by • • Breaking C-H bond Forming C-O, C-N, or C-X bond Note: oxidation often adds oxygen; reduction often adds hydrogen

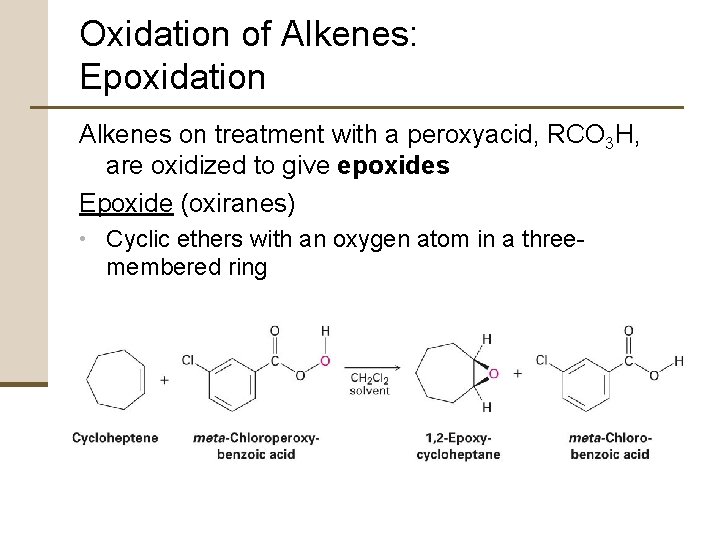
Oxidation of Alkenes: Epoxidation Alkenes on treatment with a peroxyacid, RCO 3 H, are oxidized to give epoxides Epoxide (oxiranes) • Cyclic ethers with an oxygen atom in a three- membered ring

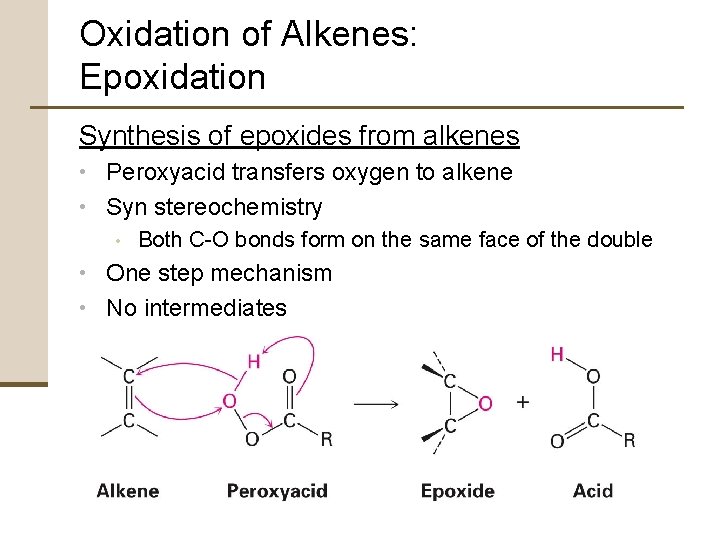
Oxidation of Alkenes: Epoxidation Synthesis of epoxides from alkenes • Peroxyacid transfers oxygen to alkene • Syn stereochemistry • Both C-O bonds form on the same face of the double • One step mechanism • No intermediates

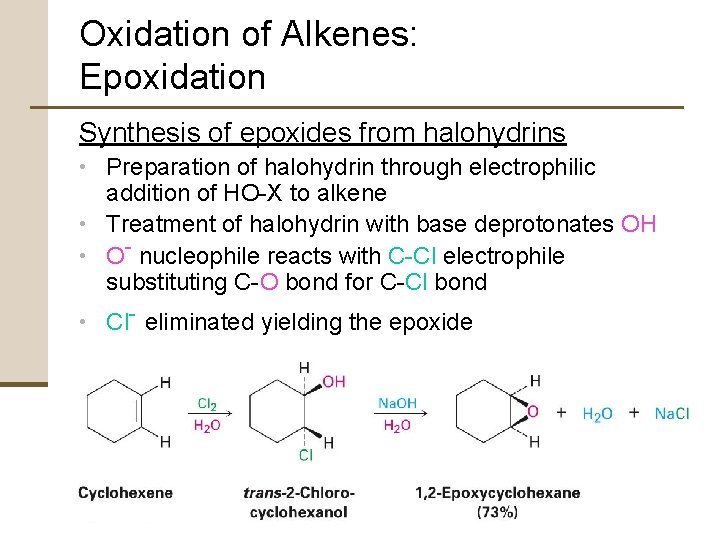
Oxidation of Alkenes: Epoxidation Synthesis of epoxides from halohydrins • Preparation of halohydrin through electrophilic addition of HO-X to alkene • Treatment of halohydrin with base deprotonates OH • O- nucleophile reacts with C-Cl electrophile substituting C-O bond for C-Cl bond • Cl- eliminated yielding the epoxide

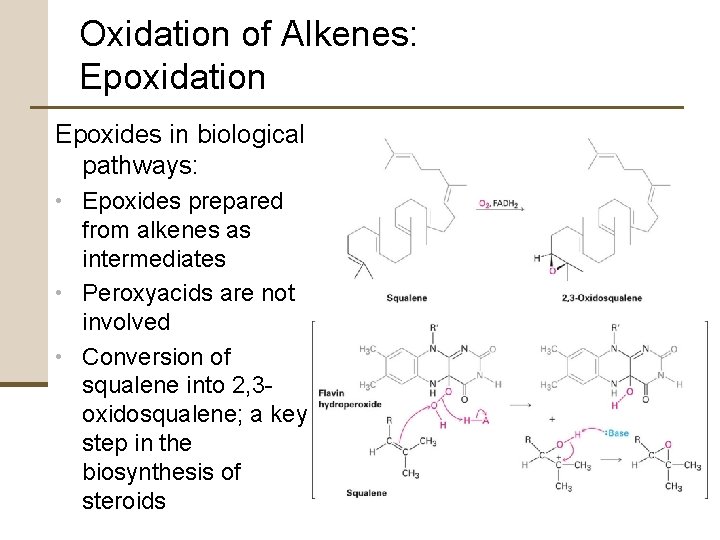
Oxidation of Alkenes: Epoxidation Epoxides in biological pathways: • Epoxides prepared from alkenes as intermediates • Peroxyacids are not involved • Conversion of squalene into 2, 3 oxidosqualene; a key step in the biosynthesis of steroids

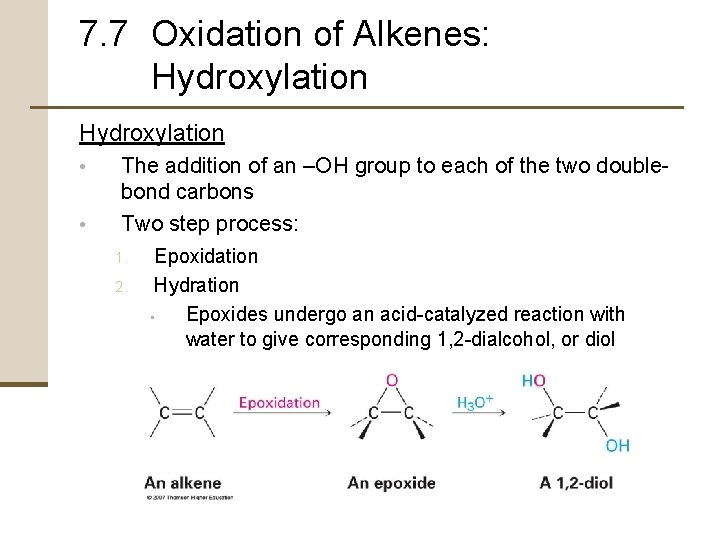
7. 7 Oxidation of Alkenes: Hydroxylation • • The addition of an –OH group to each of the two doublebond carbons Two step process: 1. 2. Epoxidation Hydration • Epoxides undergo an acid-catalyzed reaction with water to give corresponding 1, 2 -dialcohol, or diol

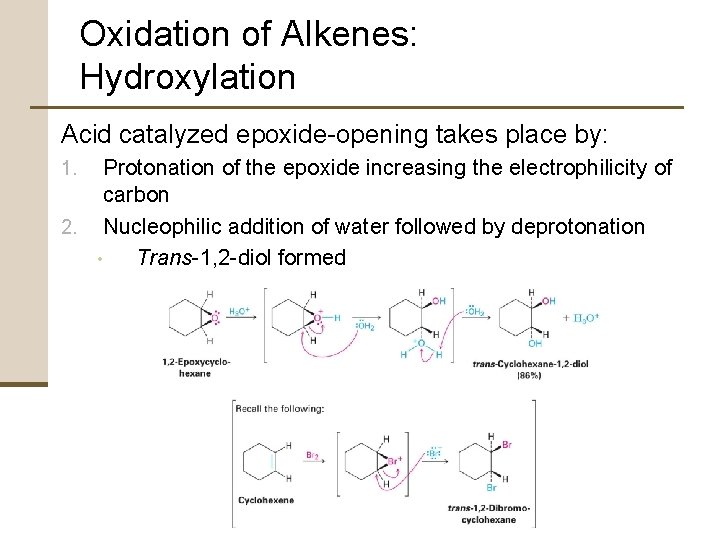
Oxidation of Alkenes: Hydroxylation Acid catalyzed epoxide-opening takes place by: Protonation of the epoxide increasing the electrophilicity of carbon 2. Nucleophilic addition of water followed by deprotonation • Trans-1, 2 -diol formed 1.

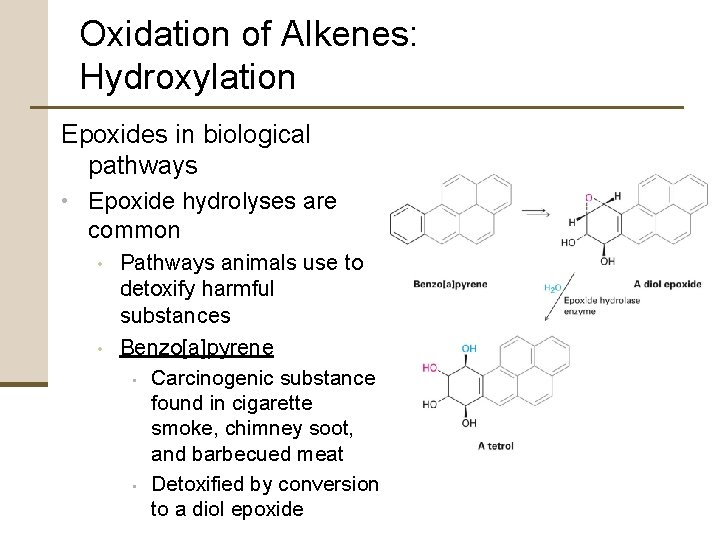
Oxidation of Alkenes: Hydroxylation Epoxides in biological pathways • Epoxide hydrolyses are common • • Pathways animals use to detoxify harmful substances Benzo[a]pyrene • Carcinogenic substance found in cigarette smoke, chimney soot, and barbecued meat • Detoxified by conversion to a diol epoxide

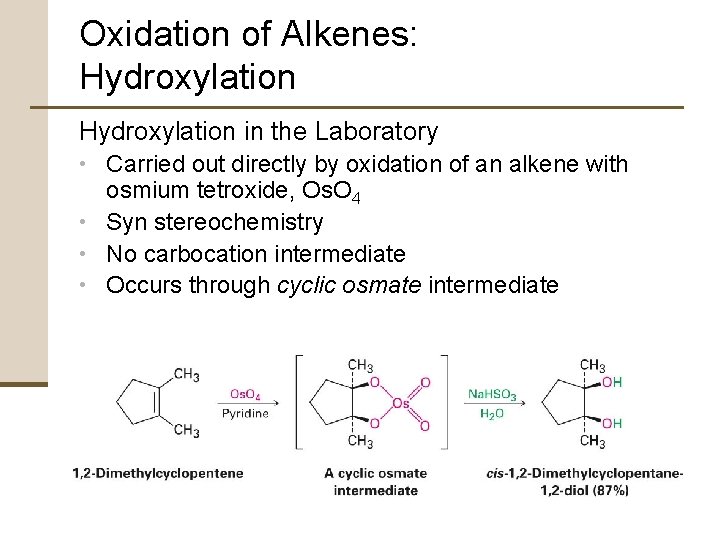
Oxidation of Alkenes: Hydroxylation in the Laboratory • Carried out directly by oxidation of an alkene with osmium tetroxide, Os. O 4 • Syn stereochemistry • No carbocation intermediate • Occurs through cyclic osmate intermediate

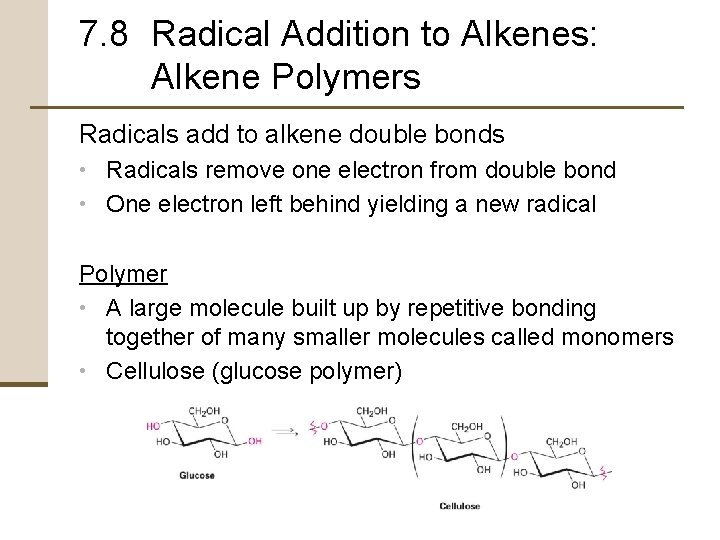
7. 8 Radical Addition to Alkenes: Alkene Polymers Radicals add to alkene double bonds • Radicals remove one electron from double bond • One electron left behind yielding a new radical Polymer • A large molecule built up by repetitive bonding together of many smaller molecules called monomers • Cellulose (glucose polymer)

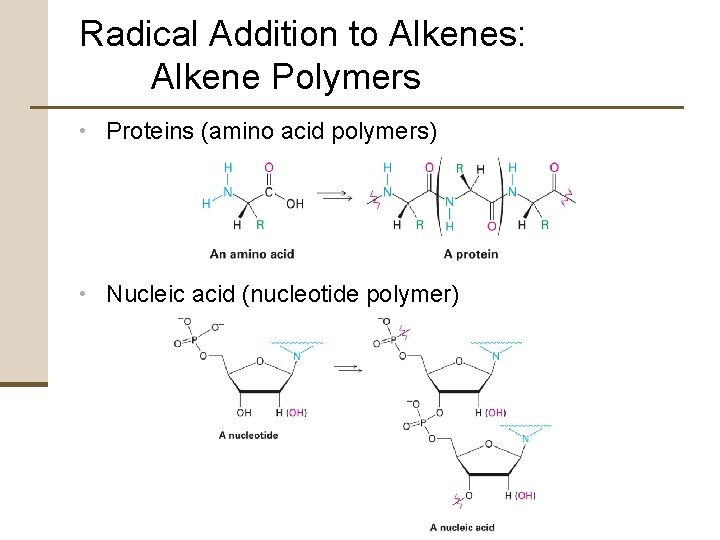
Radical Addition to Alkenes: Alkene Polymers • Proteins (amino acid polymers) • Nucleic acid (nucleotide polymer)

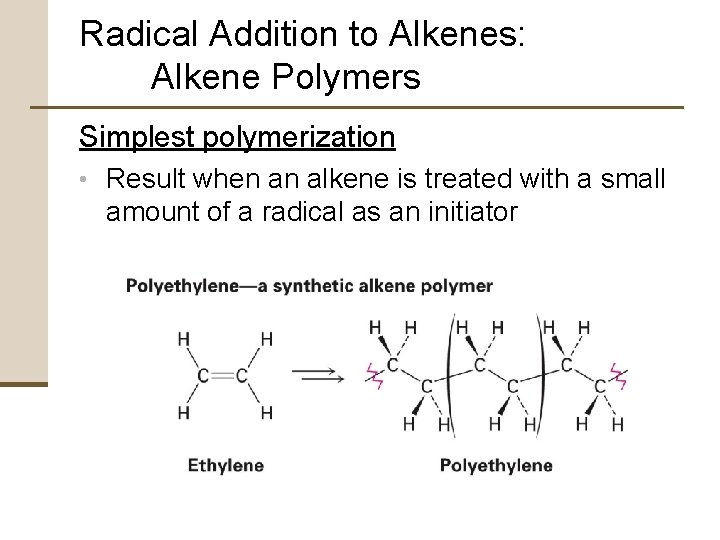
Radical Addition to Alkenes: Alkene Polymers Simplest polymerization • Result when an alkene is treated with a small amount of a radical as an initiator

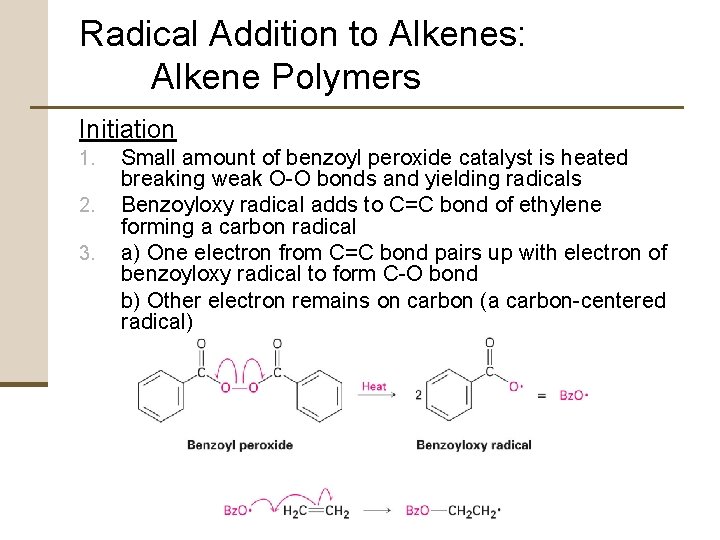
Radical Addition to Alkenes: Alkene Polymers Initiation 1. 2. 3. Small amount of benzoyl peroxide catalyst is heated breaking weak O-O bonds and yielding radicals Benzoyloxy radical adds to C=C bond of ethylene forming a carbon radical a) One electron from C=C bond pairs up with electron of benzoyloxy radical to form C-O bond b) Other electron remains on carbon (a carbon-centered radical)

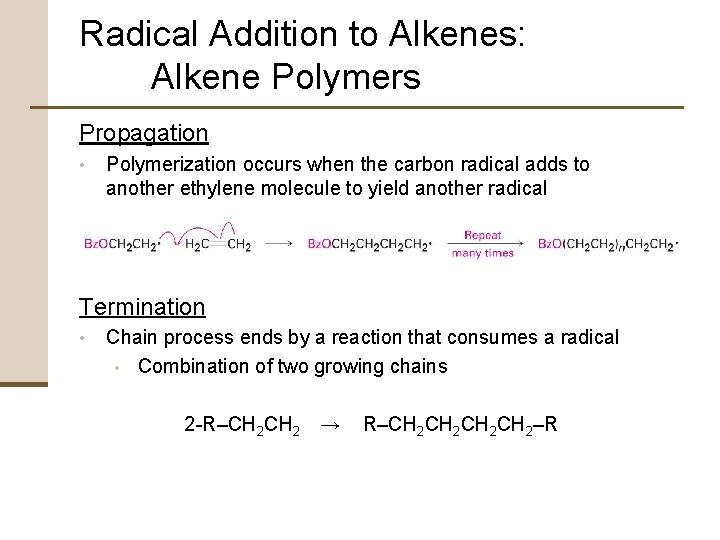
Radical Addition to Alkenes: Alkene Polymers Propagation • Polymerization occurs when the carbon radical adds to another ethylene molecule to yield another radical Termination • Chain process ends by a reaction that consumes a radical • Combination of two growing chains 2 -R–CH 2 → R–CH 2 CH 2–R

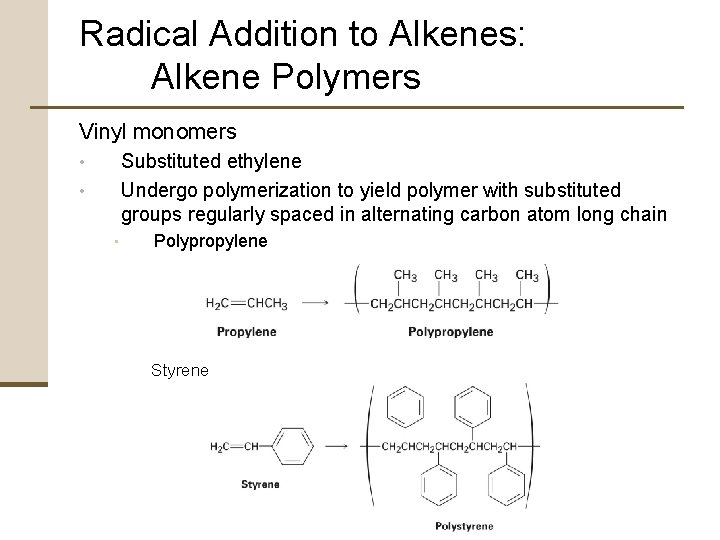
Radical Addition to Alkenes: Alkene Polymers Vinyl monomers Substituted ethylene Undergo polymerization to yield polymer with substituted groups regularly spaced in alternating carbon atom long chain • • • Polypropylene Styrene

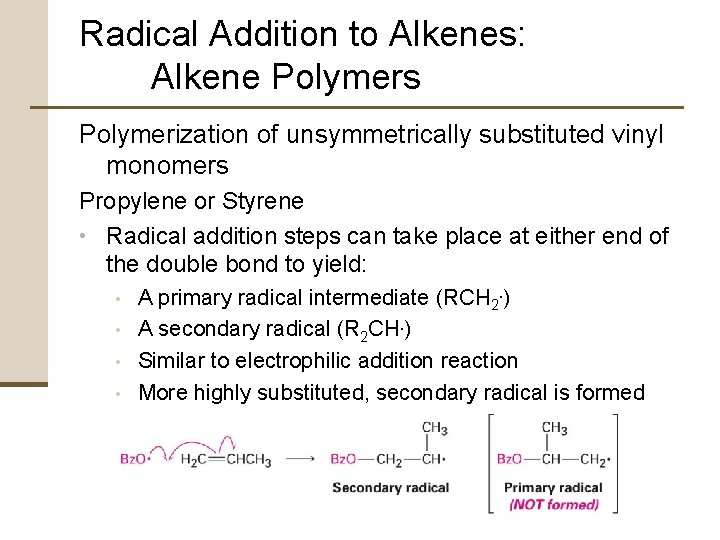
Radical Addition to Alkenes: Alkene Polymers Polymerization of unsymmetrically substituted vinyl monomers Propylene or Styrene • Radical addition steps can take place at either end of the double bond to yield: • • A primary radical intermediate (RCH 2. ) A secondary radical (R 2 CH. ) Similar to electrophilic addition reaction More highly substituted, secondary radical is formed

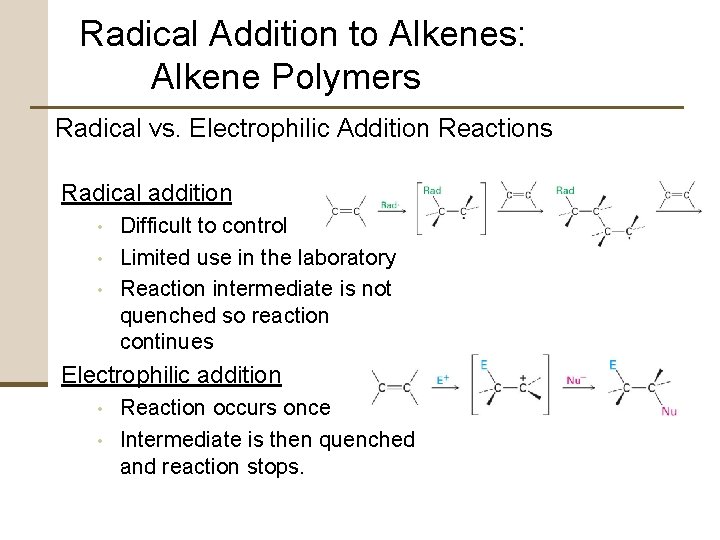
Radical Addition to Alkenes: Alkene Polymers Radical vs. Electrophilic Addition Reactions Radical addition • • • Difficult to control Limited use in the laboratory Reaction intermediate is not quenched so reaction continues Electrophilic addition • • Reaction occurs once Intermediate is then quenched and reaction stops.

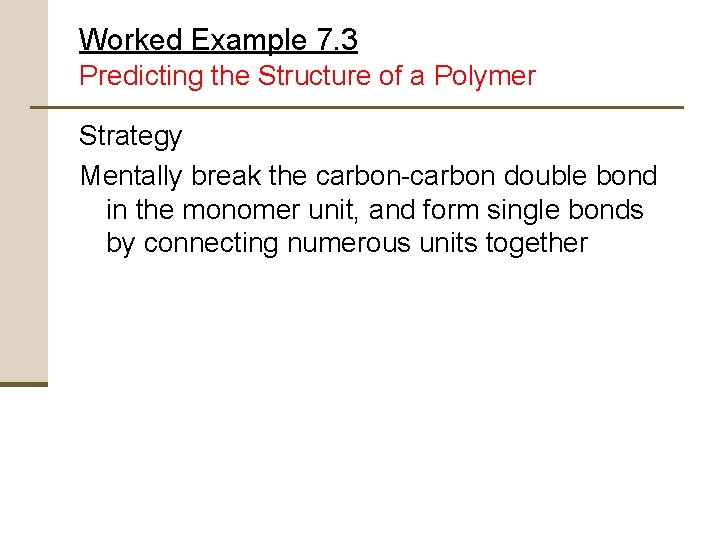
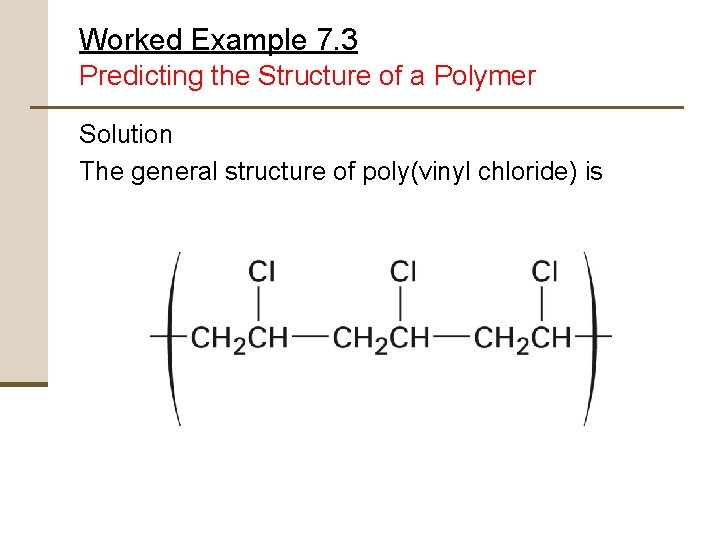
Worked Example 7. 3 Predicting the Structure of a Polymer Show the structure of poly(vinyl chloride), a polymer made from H 2 C=CHCl, by drawing several repeating units

Worked Example 7. 3 Predicting the Structure of a Polymer Strategy Mentally break the carbon-carbon double bond in the monomer unit, and form single bonds by connecting numerous units together

Worked Example 7. 3 Predicting the Structure of a Polymer Solution The general structure of poly(vinyl chloride) is

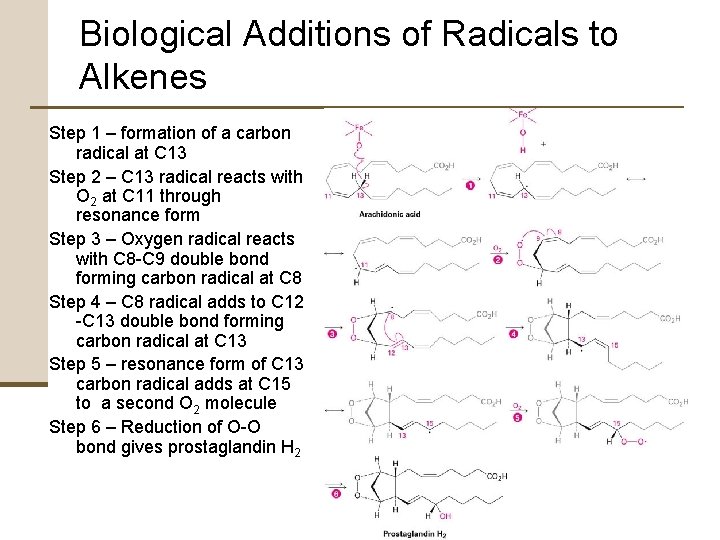
7. 9 Biological Additions of Radicals to Alkenes Biological Reactions • Only one substrate molecule at a time is present in the active site of the enzyme where the reaction occurs (necessary reactant groups nearby) • More controlled • More common than laboratory radical reactions

Biological Additions of Radicals to Alkenes Step 1 – formation of a carbon radical at C 13 Step 2 – C 13 radical reacts with O 2 at C 11 through resonance form Step 3 – Oxygen radical reacts with C 8 -C 9 double bond forming carbon radical at C 8 Step 4 – C 8 radical adds to C 12 -C 13 double bond forming carbon radical at C 13 Step 5 – resonance form of C 13 carbon radical adds at C 15 to a second O 2 molecule Step 6 – Reduction of O-O bond gives prostaglandin H 2

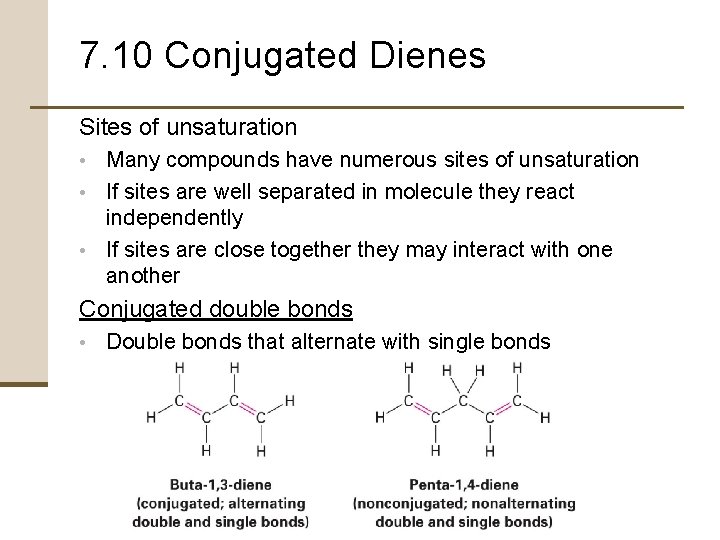
7. 10 Conjugated Dienes Sites of unsaturation • Many compounds have numerous sites of unsaturation • If sites are well separated in molecule they react independently • If sites are close together they may interact with one another Conjugated double bonds • Double bonds that alternate with single bonds

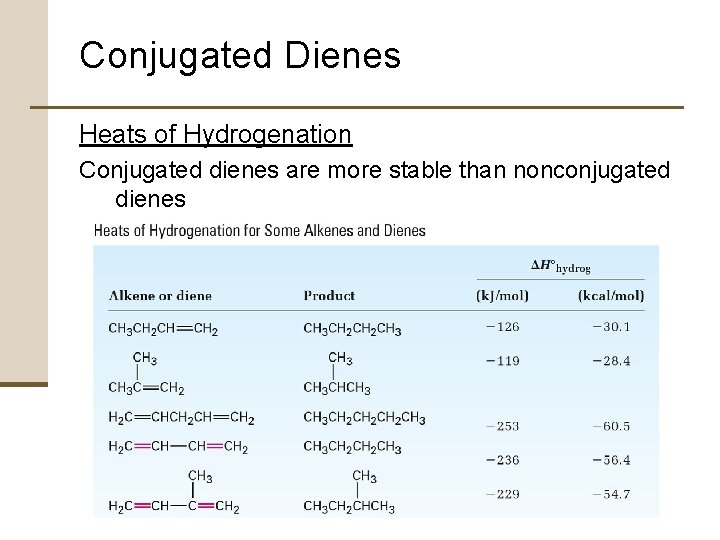
Conjugated Dienes Heats of Hydrogenation Conjugated dienes are more stable than nonconjugated dienes

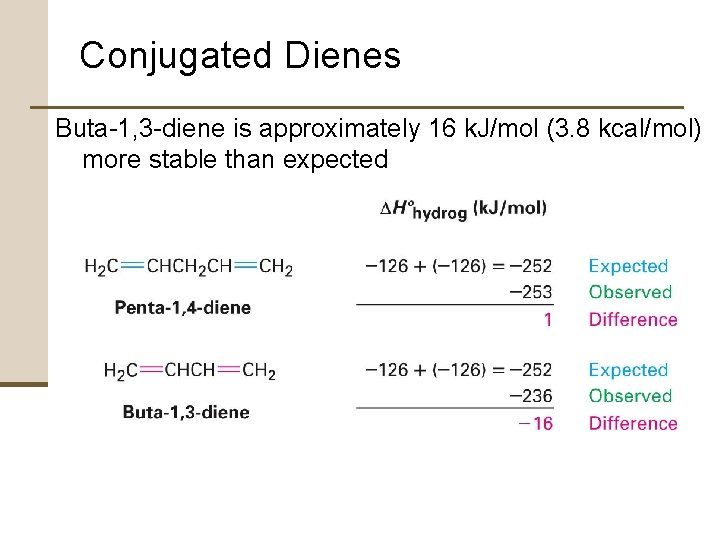
Conjugated Dienes Buta-1, 3 -diene is approximately 16 k. J/mol (3. 8 kcal/mol) more stable than expected

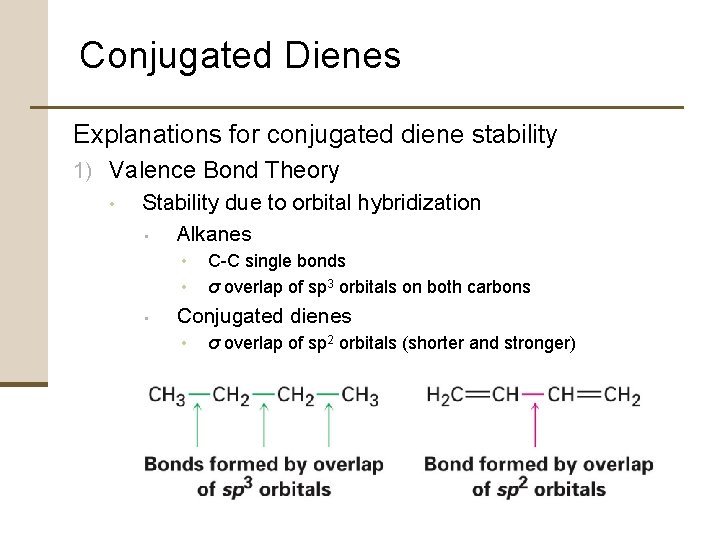
Conjugated Dienes Explanations for conjugated diene stability 1) Valence Bond Theory • Stability due to orbital hybridization • Alkanes • • • C-C single bonds σ overlap of sp 3 orbitals on both carbons Conjugated dienes • σ overlap of sp 2 orbitals (shorter and stronger)

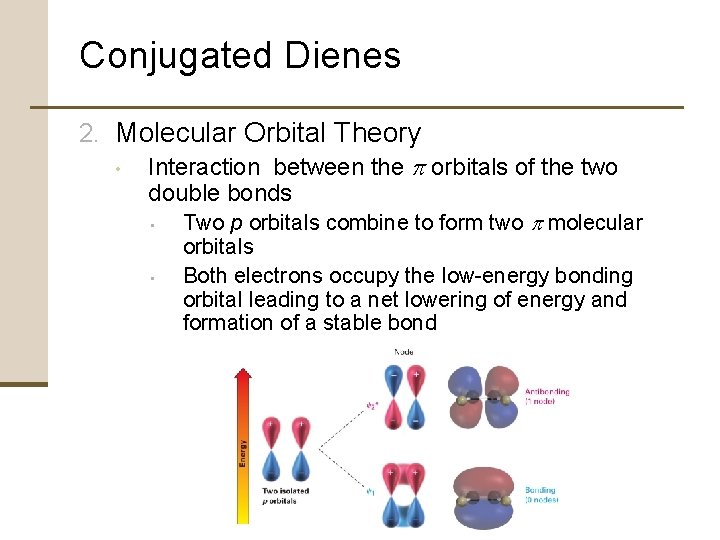
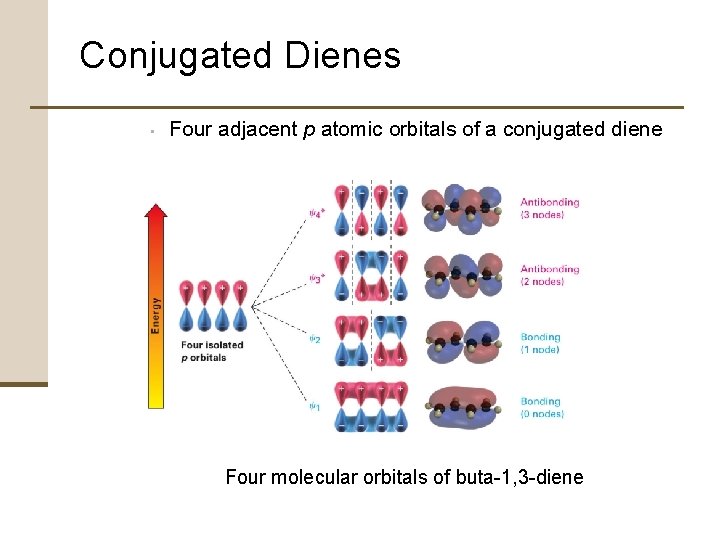
Conjugated Dienes 2. Molecular Orbital Theory • Interaction between the p orbitals of the two double bonds • • Two p orbitals combine to form two p molecular orbitals Both electrons occupy the low-energy bonding orbital leading to a net lowering of energy and formation of a stable bond

Conjugated Dienes • Four adjacent p atomic orbitals of a conjugated diene Four molecular orbitals of buta-1, 3 -diene

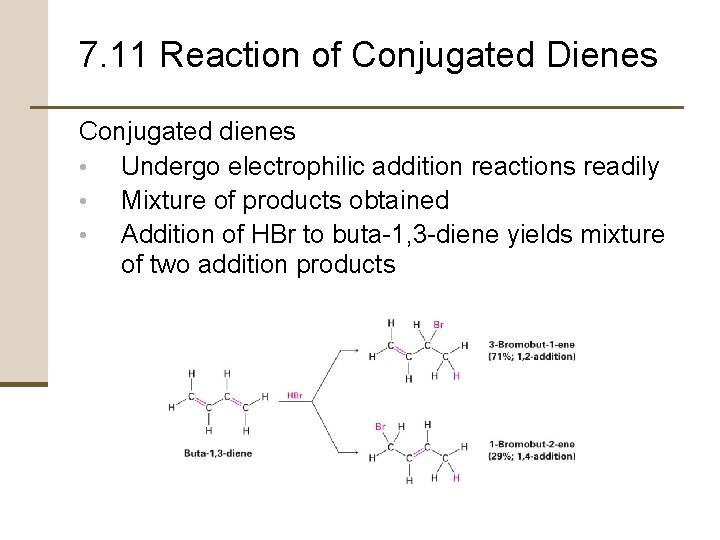
7. 11 Reaction of Conjugated Dienes Conjugated dienes • Undergo electrophilic addition reactions readily • Mixture of products obtained • Addition of HBr to buta-1, 3 -diene yields mixture of two addition products

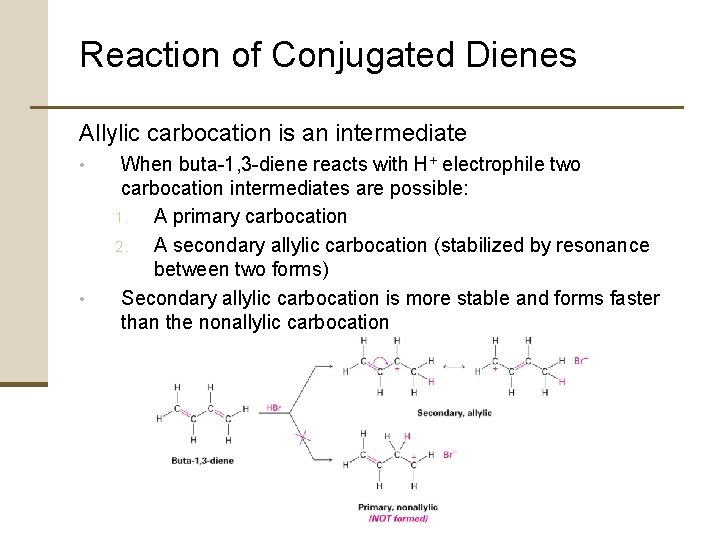
Reaction of Conjugated Dienes Allylic carbocation is an intermediate • • When buta-1, 3 -diene reacts with H+ electrophile two carbocation intermediates are possible: 1. A primary carbocation 2. A secondary allylic carbocation (stabilized by resonance between two forms) Secondary allylic carbocation is more stable and forms faster than the nonallylic carbocation

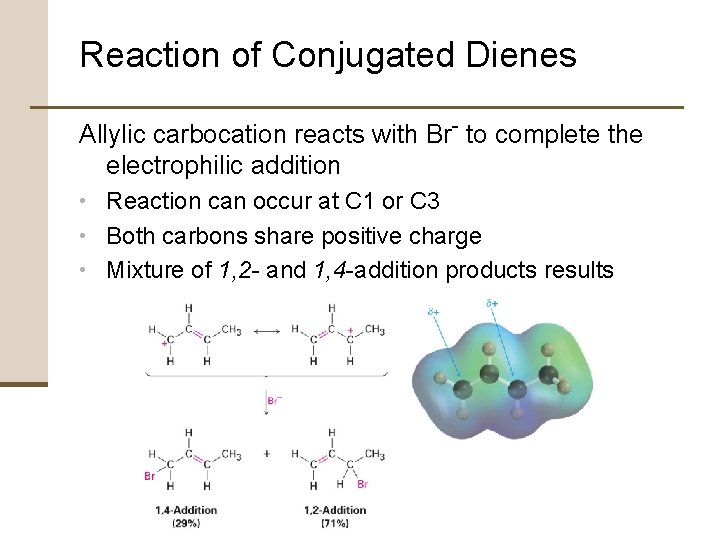
Reaction of Conjugated Dienes Allylic carbocation reacts with Br- to complete the electrophilic addition • Reaction can occur at C 1 or C 3 • Both carbons share positive charge • Mixture of 1, 2 - and 1, 4 -addition products results

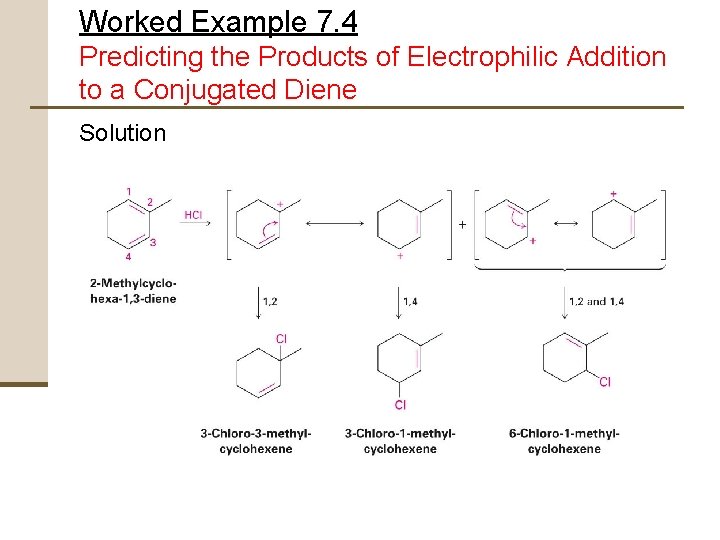
Worked Example 7. 4 Predicting the Products of Electrophilic Addition to a Conjugated Diene Give the structures of the likely products from reaction of 1 equivalent of HCl with 2 methylcyclohexa-1, 3 -diene. Show both 1, 2 and 1, 4 - adducts.

Worked Example 7. 4 Predicting the Products of Electrophilic Addition to a Conjugated Diene Strategy Electrophilic addition of HCl to a conjugated diene involves the formation of allylic carbocation intermediates First – • Protonate the two ends of the diene • Draw resonance forms of the two allylic carbocations that result Second – • Allow each resonance form to react with Cl- to generate four possible products

Worked Example 7. 4 Predicting the Products of Electrophilic Addition to a Conjugated Diene Solution

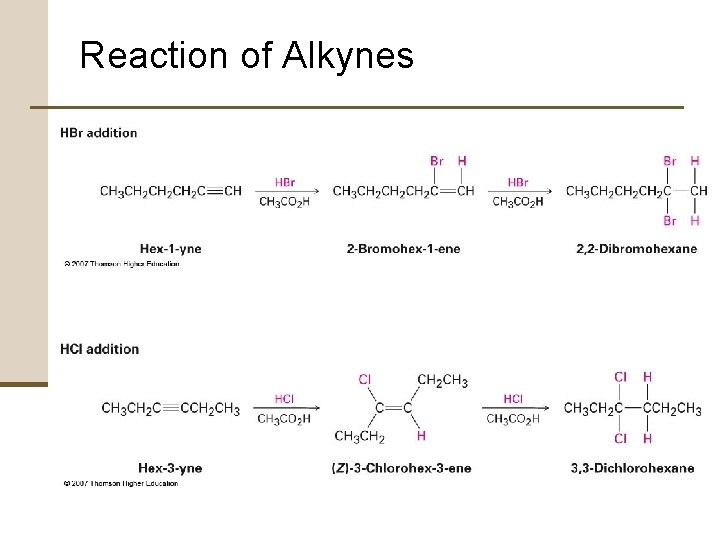
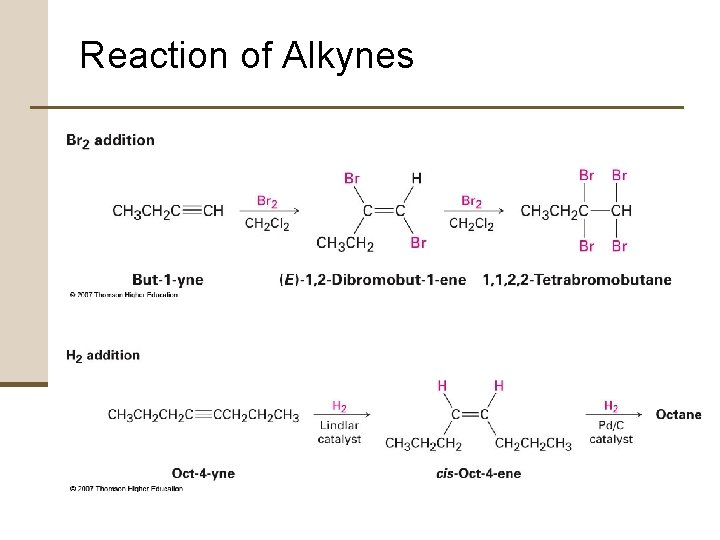
7. 12 Reaction of Alkynes Alkyne Addition Reactions • Alkynes behave similarly to alkenes • Alkynes are less reactive than alkenes • Various reactions can often be stopped at the monoaddition stage if one molar equivalent of reagent is used

Reaction of Alkynes

Reaction of Alkynes

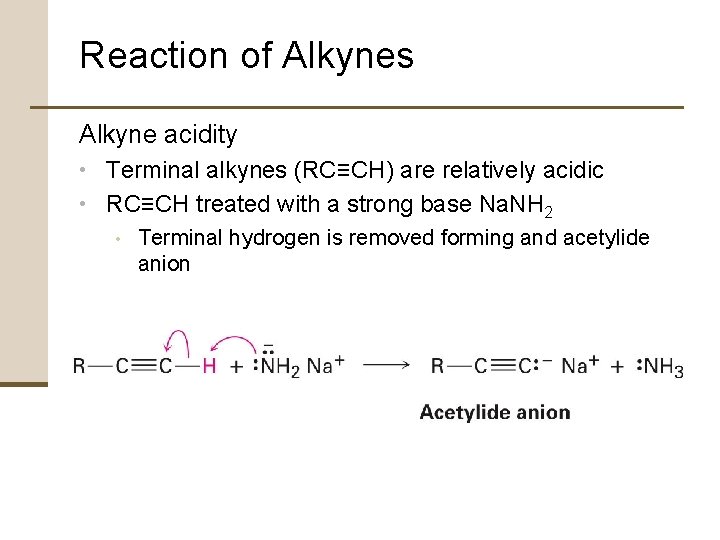
Reaction of Alkynes Alkyne acidity • Terminal alkynes (RC≡CH) are relatively acidic • RC≡CH treated with a strong base Na. NH 2 • Terminal hydrogen is removed forming and acetylide anion

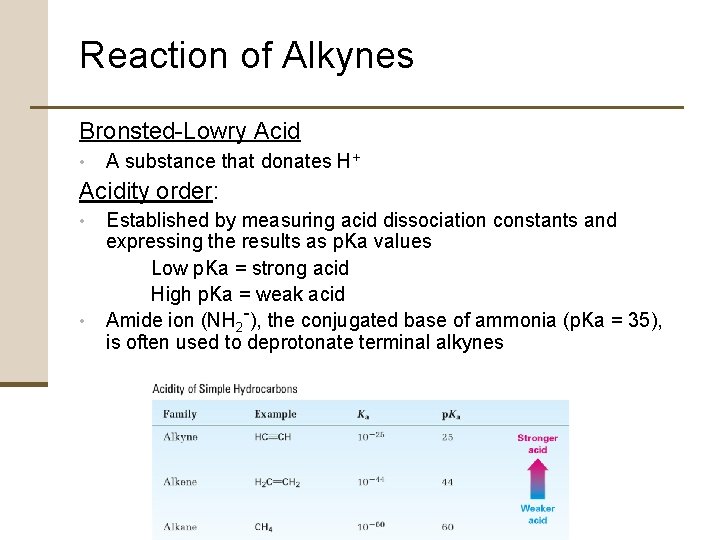
Reaction of Alkynes Bronsted-Lowry Acid • A substance that donates H+ Acidity order: • • Established by measuring acid dissociation constants and expressing the results as p. Ka values Low p. Ka = strong acid High p. Ka = weak acid Amide ion (NH 2 -), the conjugated base of ammonia (p. Ka = 35), is often used to deprotonate terminal alkynes

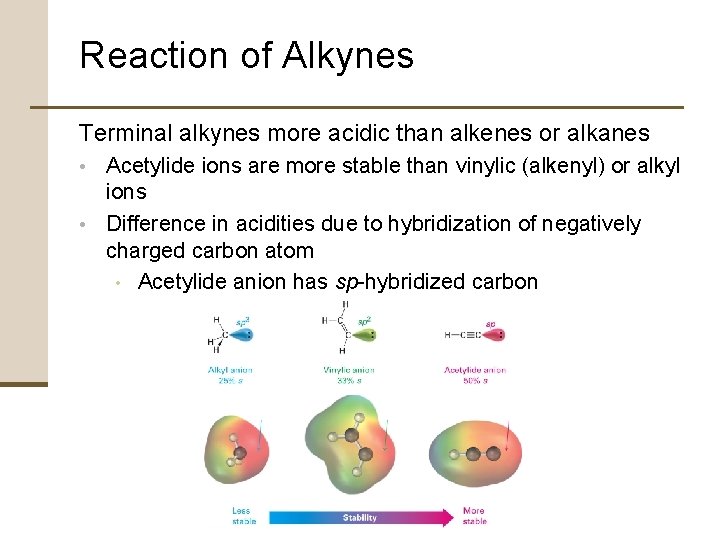
Reaction of Alkynes Terminal alkynes more acidic than alkenes or alkanes • Acetylide ions are more stable than vinylic (alkenyl) or alkyl ions • Difference in acidities due to hybridization of negatively charged carbon atom • Acetylide anion has sp-hybridized carbon