Chapter 7 REACTIONS IN AQUEOUS SOLUTIONS Classification of

- Slides: 9

Chapter 7 REACTIONS IN AQUEOUS SOLUTIONS

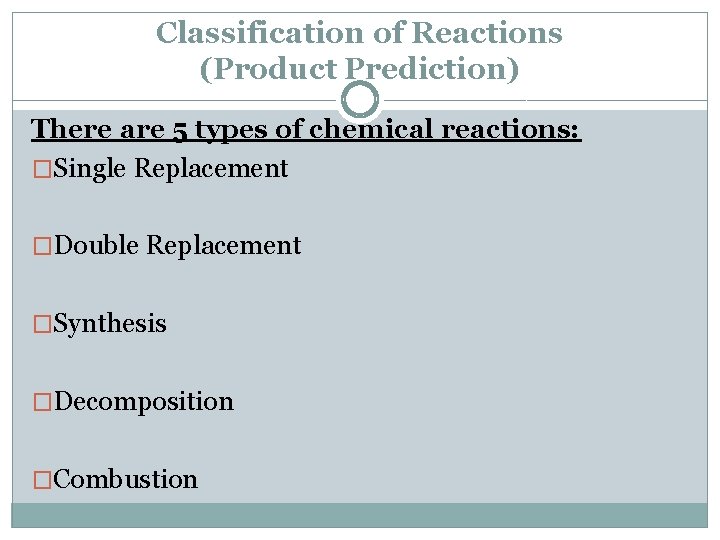
Classification of Reactions (Product Prediction) There are 5 types of chemical reactions: �Single Replacement �Double Replacement �Synthesis �Decomposition �Combustion

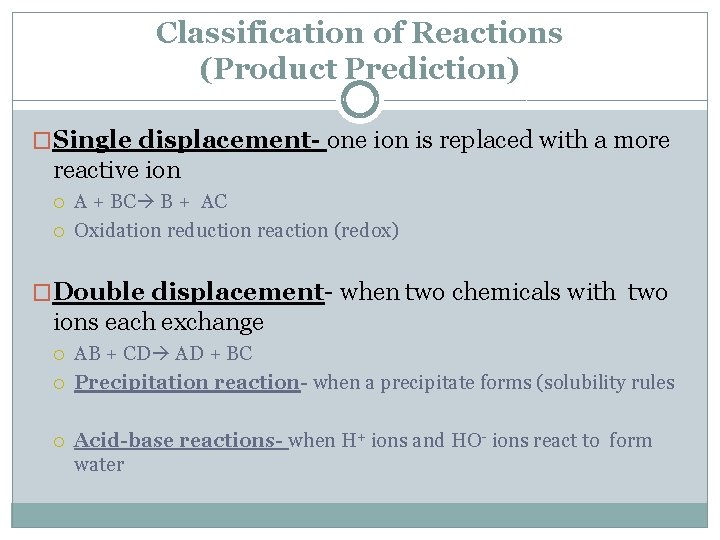
Classification of Reactions (Product Prediction) �Single displacement- one ion is replaced with a more reactive ion A + BC B + AC Oxidation reduction reaction (redox) �Double displacement- when two chemicals with two ions each exchange AB + CD AD + BC Precipitation reaction- when a precipitate forms (solubility rules Acid-base reactions- when H+ ions and HO- ions react to form water

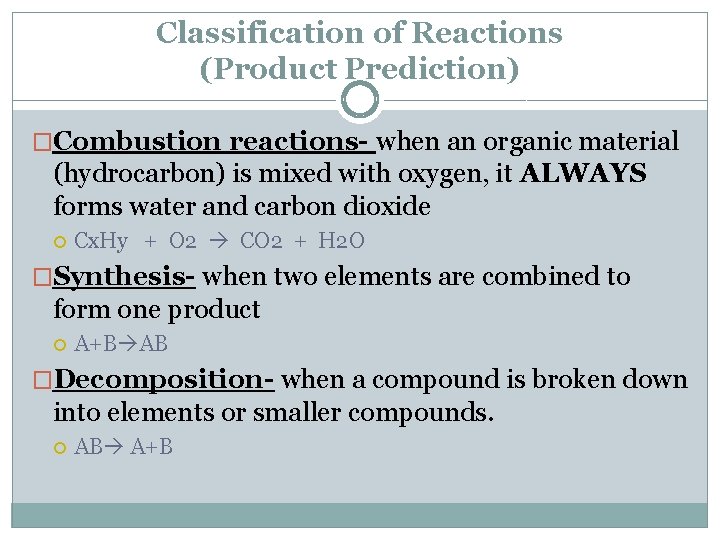
Classification of Reactions (Product Prediction) �Combustion reactions- when an organic material (hydrocarbon) is mixed with oxygen, it ALWAYS forms water and carbon dioxide Cx. Hy + O 2 CO 2 + H 2 O �Synthesis- when two elements are combined to form one product A+B AB �Decomposition- when a compound is broken down into elements or smaller compounds. AB A+B

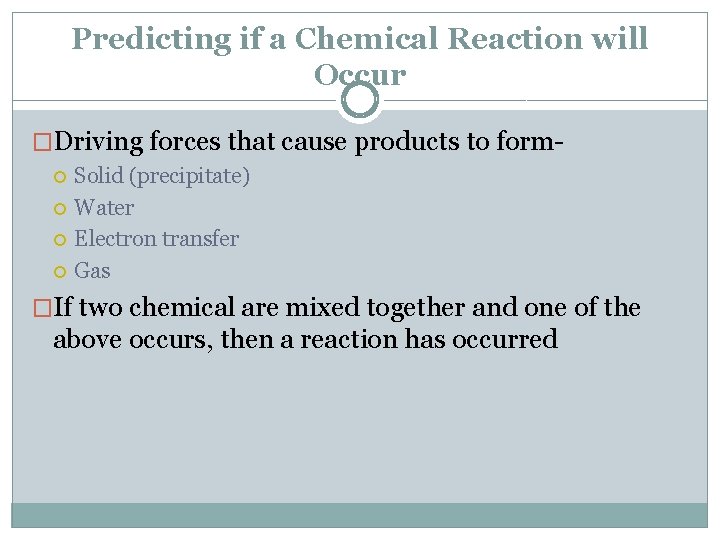
Predicting if a Chemical Reaction will Occur �Driving forces that cause products to form Solid (precipitate) Water Electron transfer Gas �If two chemical are mixed together and one of the above occurs, then a reaction has occurred

Reaction in which a solid forms �Precipitate (ppt) - This when a solid forms after two chemicals have been mixed �Determine this by: Swap the cations and anions (double replacement reaction) follow solubility rules to determine if any of the products are insoluble in water (ppt) Any solutions that are soluble just dissolve (stay ions – spectator ions)

Describing Reactions in Aqueous Solutions �Molecular equation- the two reactants and the product with their state �Complete ionic equation- each reactant and product is broken apart and given an individual charge and state �Spectator ions- ions that do not change throughout the reaction. Usually aqueous solutions �Net ionic equation- contains only the compounds that changed in the reaction

Acid and Base Reactions �Acids produce H+ ions �Base produces OH- ions �Strong bases and acids have strong electrolytes that either contain H+ or OH- ions �Acid/base reactions always produce water as a product

Reactions of Metals and Nonmetals �Oxidation-Reduction reaction- involves transfer of electrons �OIL RIG- oxidation is a loss: Reduction is a gain �Usually it is the metal that has an electron change (Gain or loss)