Chapter 7 Quantum Two Body Problem Hydrogen Atom

Chapter 7 Quantum Two Body Problem, Hydrogen Atom

The two-body problem 7. B. 2 • The two-body problem: two spinless point objects in 3 D interacting with each other (closed system) • Interaction between the objects depends only on the distance between them • The operators describing their positions and momenta satisfy these commutation relations:
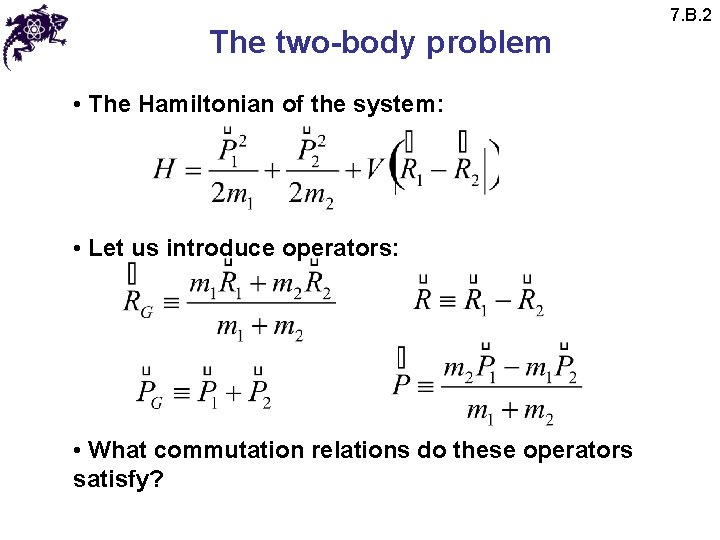
The two-body problem • The Hamiltonian of the system: • Let us introduce operators: • What commutation relations do these operators satisfy? 7. B. 2
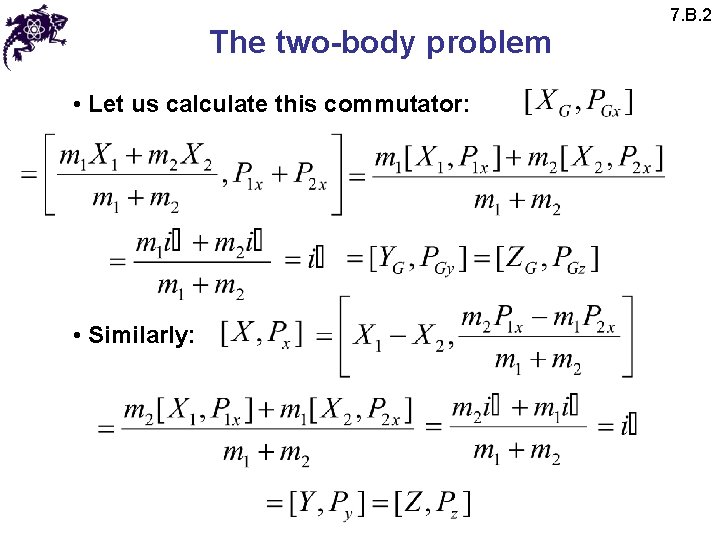
The two-body problem • Let us calculate this commutator: • Similarly: 7. B. 2
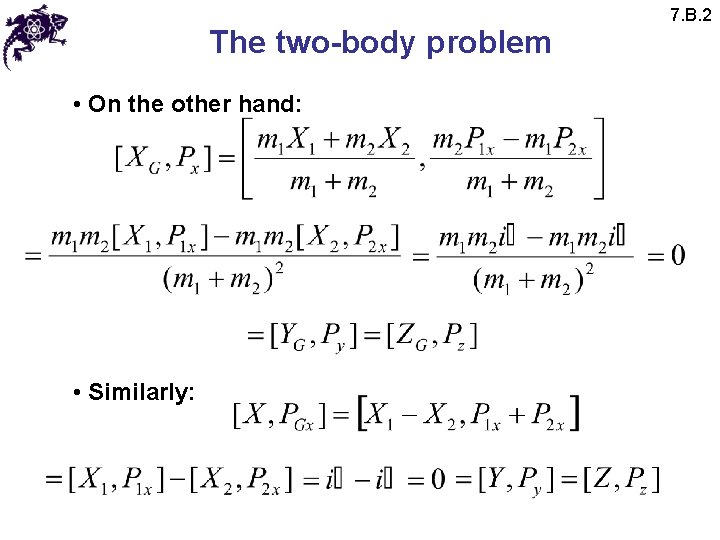
The two-body problem • On the other hand: • Similarly: 7. B. 2
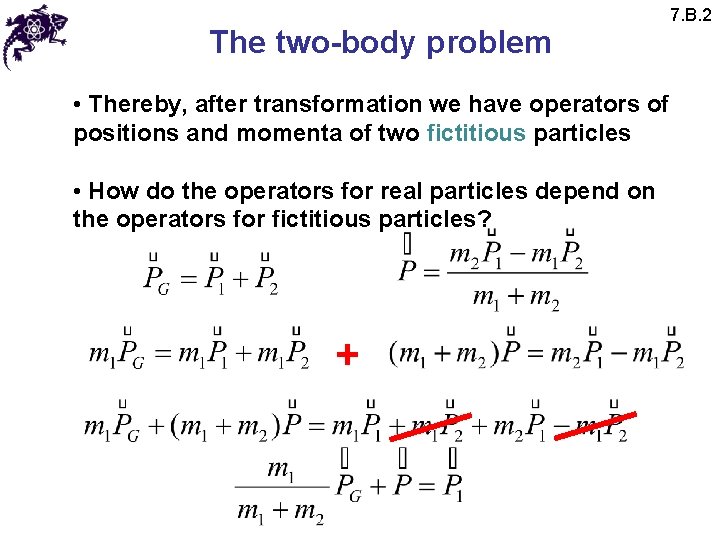
The two-body problem • Thereby, after transformation we have operators of positions and momenta of two fictitious particles • How do the operators for real particles depend on the operators for fictitious particles? + 7. B. 2
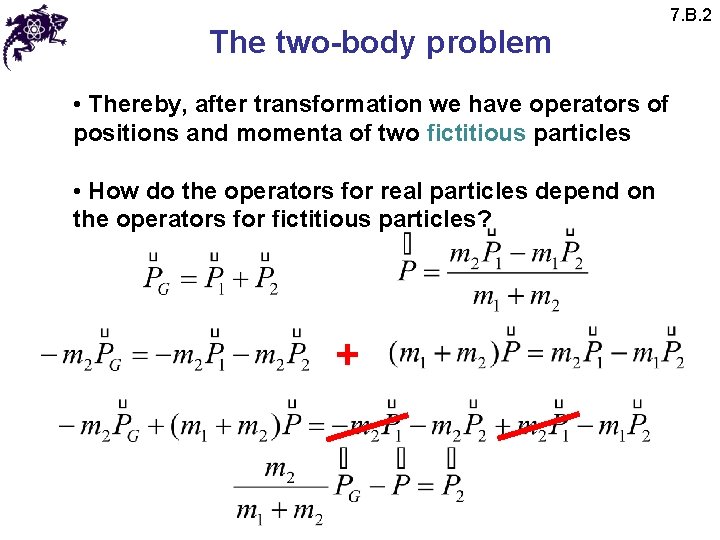
The two-body problem • Thereby, after transformation we have operators of positions and momenta of two fictitious particles • How do the operators for real particles depend on the operators for fictitious particles? + 7. B. 2
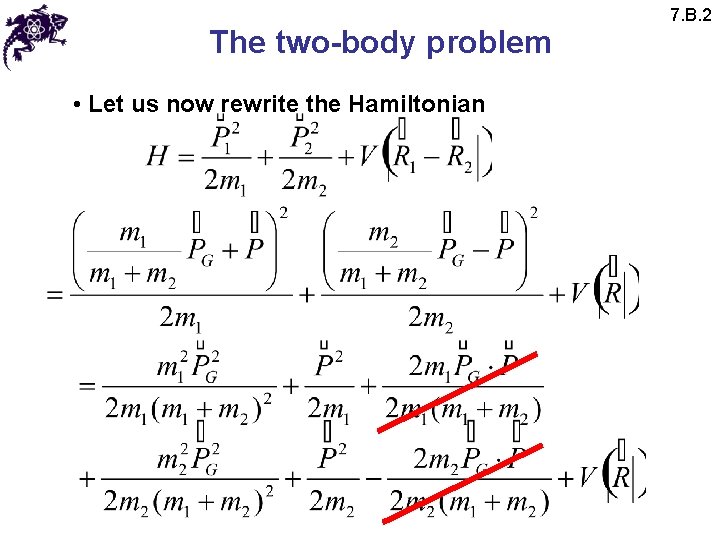
The two-body problem • Let us now rewrite the Hamiltonian 7. B. 2
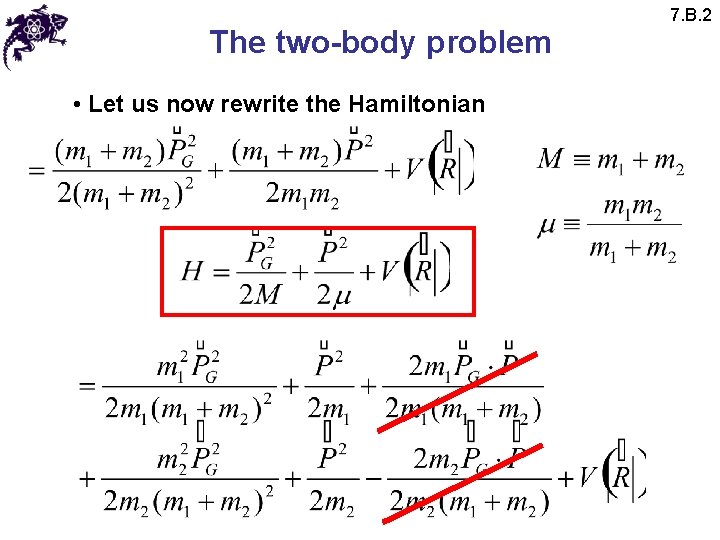
The two-body problem • Let us now rewrite the Hamiltonian 7. B. 2
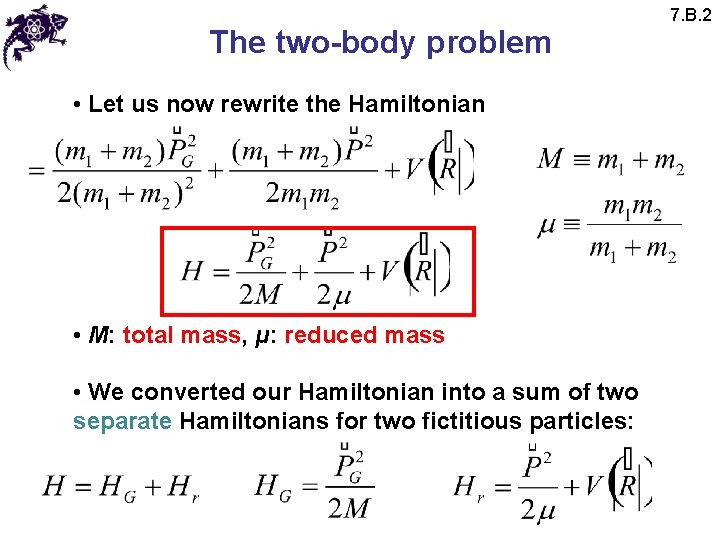
The two-body problem • Let us now rewrite the Hamiltonian • M: total mass, μ: reduced mass • We converted our Hamiltonian into a sum of two separate Hamiltonians for two fictitious particles: 7. B. 2

The two-body problem 7. B. 2 • The two parts of the Hamiltonian commute with each other: • Therefore they both commute with the full Hamiltonian • Thus there should be basis common for all three operators: • In this case
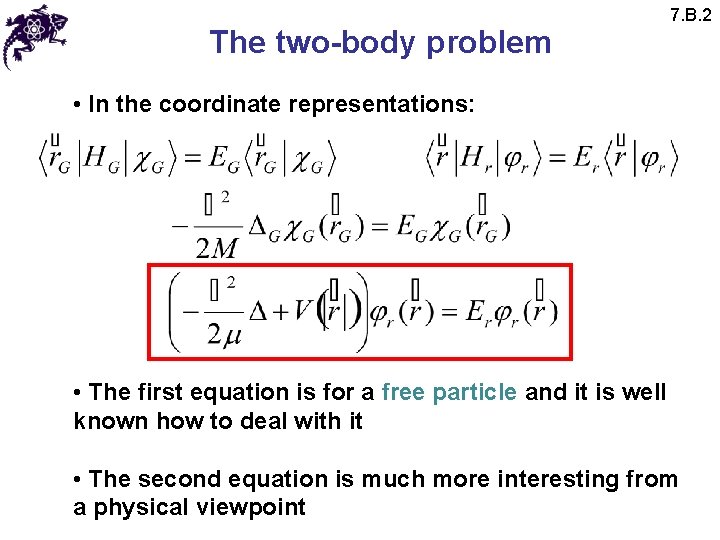
The two-body problem 7. B. 2 • In the coordinate representations: • The first equation is for a free particle and it is well known how to deal with it • The second equation is much more interesting from a physical viewpoint
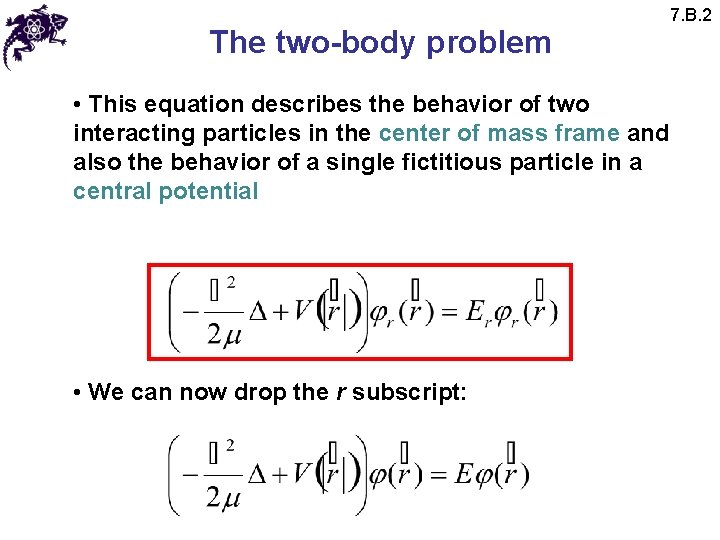
The two-body problem • This equation describes the behavior of two interacting particles in the center of mass frame and also the behavior of a single fictitious particle in a central potential • We can now drop the r subscript: 7. B. 2
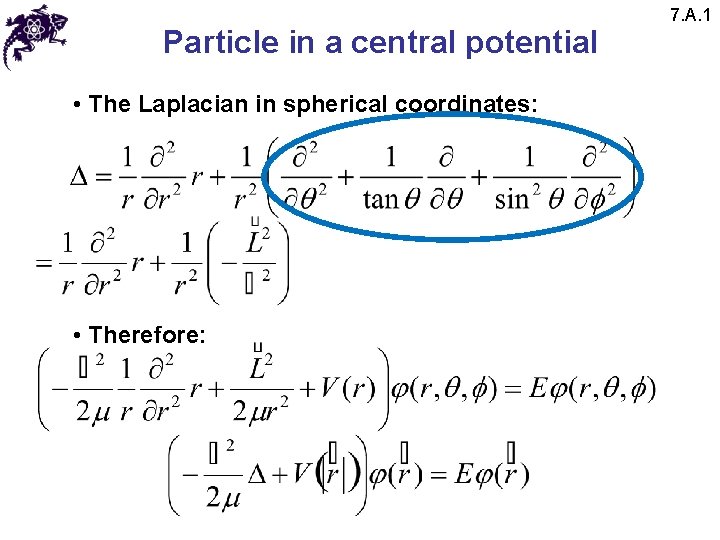
Particle in a central potential • The Laplacian in spherical coordinates: • Therefore: 7. A. 1
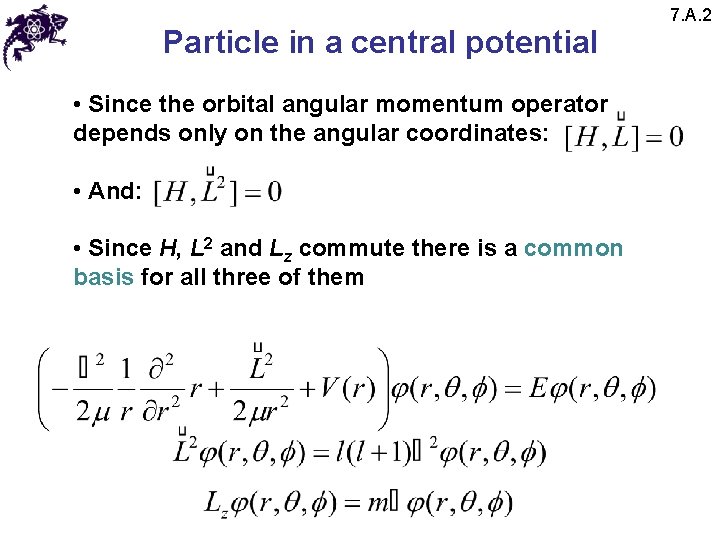
Particle in a central potential • Since the orbital angular momentum operator depends only on the angular coordinates: • And: • Since H, L 2 and Lz commute there is a common basis for all three of them 7. A. 2
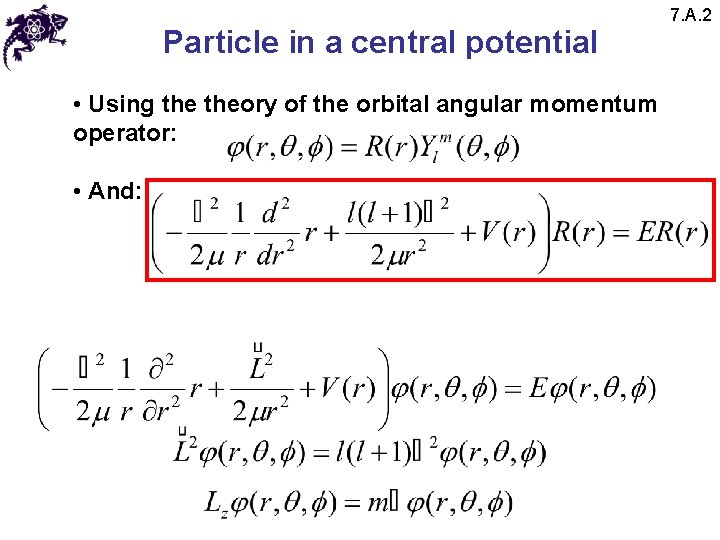
Particle in a central potential • Using theory of the orbital angular momentum operator: • And: 7. A. 2
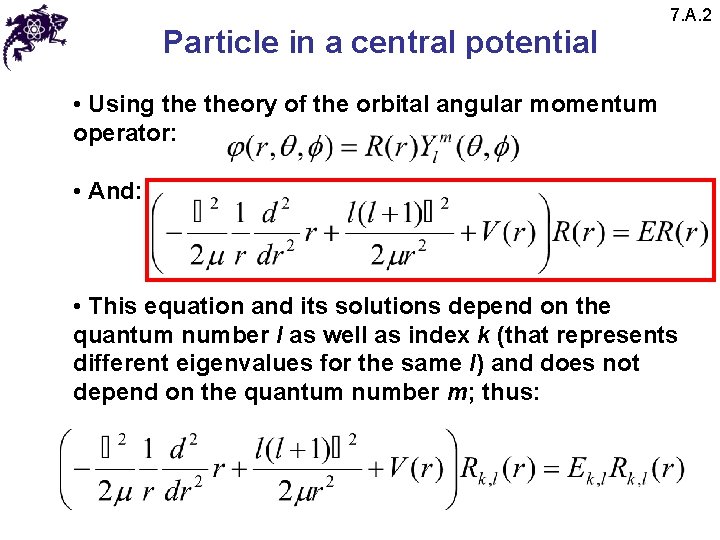
Particle in a central potential 7. A. 2 • Using theory of the orbital angular momentum operator: • And: • This equation and its solutions depend on the quantum number l as well as index k (that represents different eigenvalues for the same l) and does not depend on the quantum number m; thus:
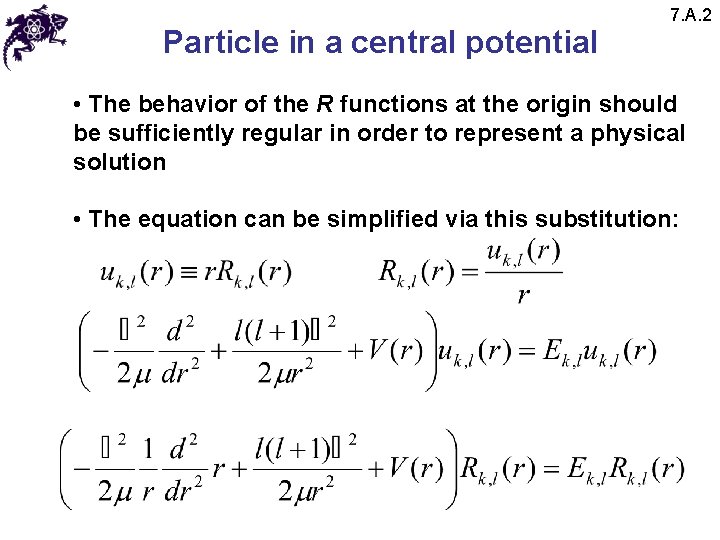
Particle in a central potential 7. A. 2 • The behavior of the R functions at the origin should be sufficiently regular in order to represent a physical solution • The equation can be simplified via this substitution:
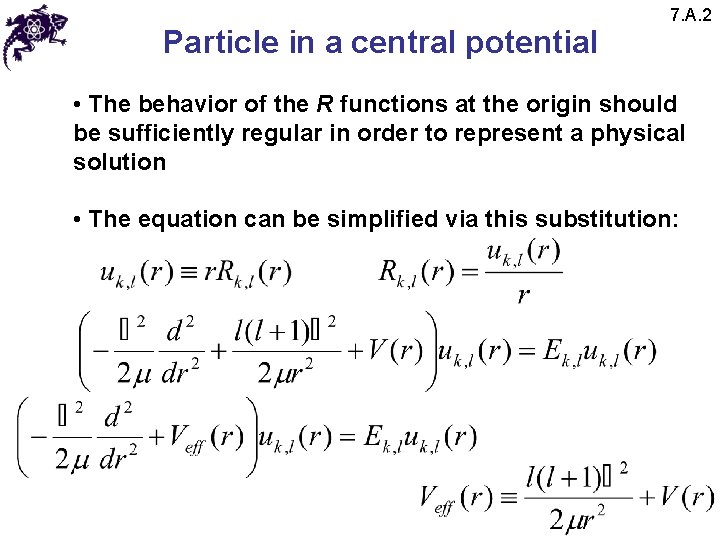
Particle in a central potential 7. A. 2 • The behavior of the R functions at the origin should be sufficiently regular in order to represent a physical solution • The equation can be simplified via this substitution:
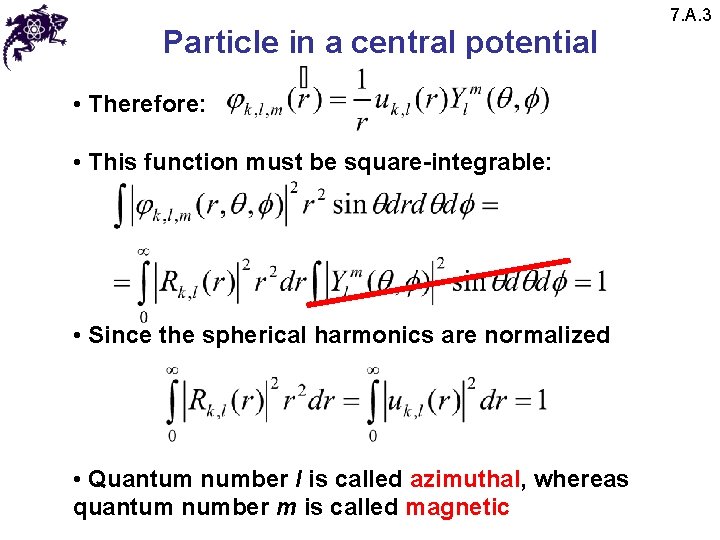
Particle in a central potential • Therefore: • This function must be square-integrable: • Since the spherical harmonics are normalized • Quantum number l is called azimuthal, whereas quantum number m is called magnetic 7. A. 3
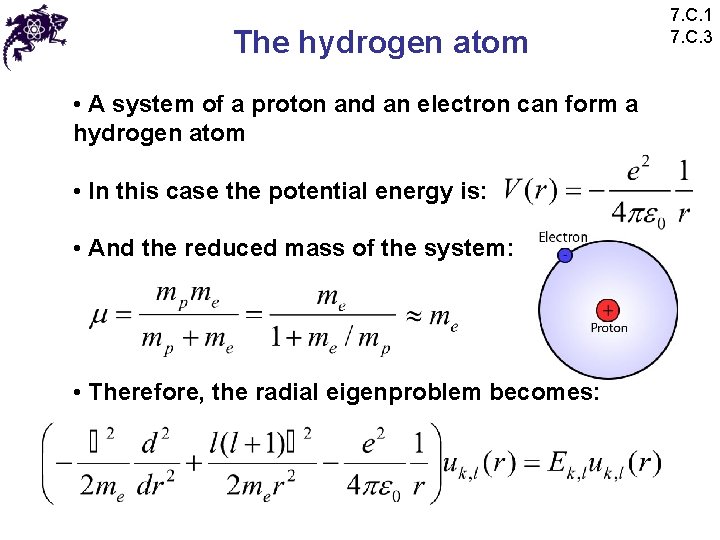
The hydrogen atom • A system of a proton and an electron can form a hydrogen atom • In this case the potential energy is: • And the reduced mass of the system: • Therefore, the radial eigenproblem becomes: 7. C. 1 7. C. 3
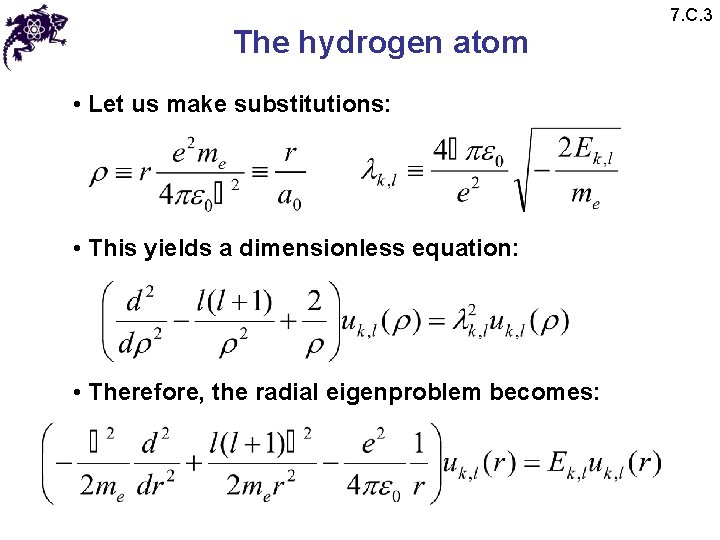
The hydrogen atom • Let us make substitutions: • This yields a dimensionless equation: • Therefore, the radial eigenproblem becomes: 7. C. 3
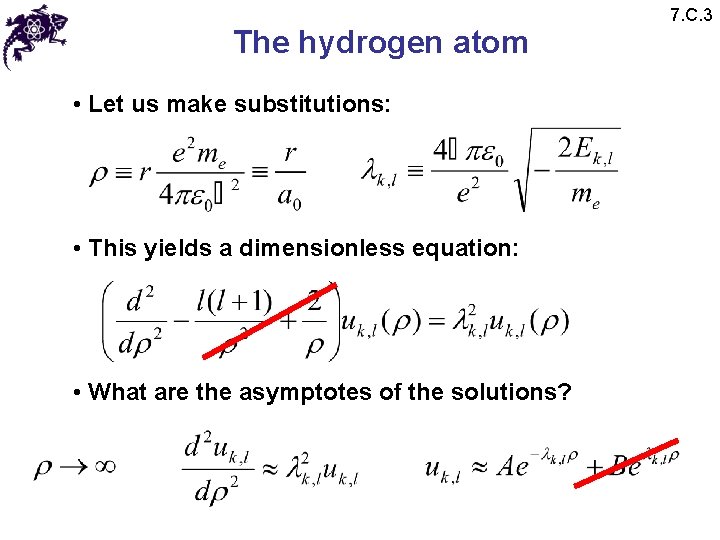
The hydrogen atom • Let us make substitutions: • This yields a dimensionless equation: • What are the asymptotes of the solutions? 7. C. 3
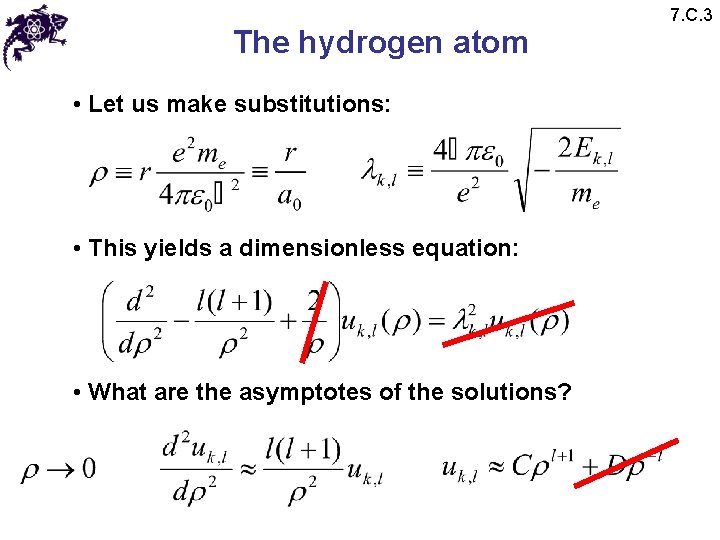
The hydrogen atom • Let us make substitutions: • This yields a dimensionless equation: • What are the asymptotes of the solutions? 7. C. 3
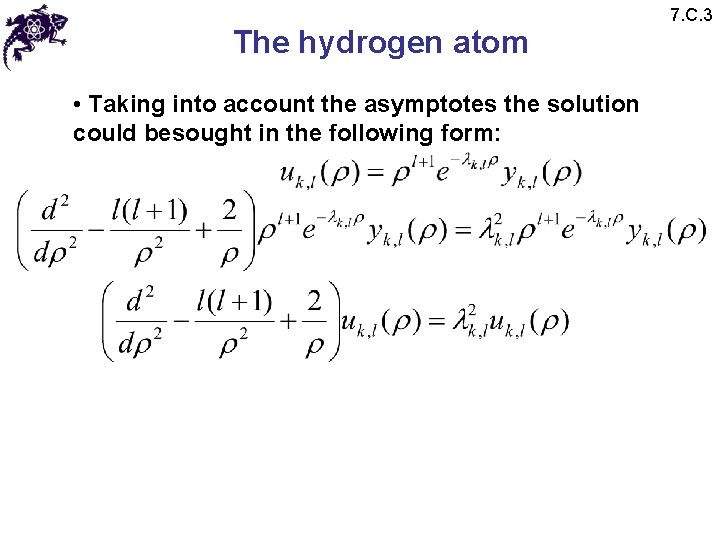
The hydrogen atom • Taking into account the asymptotes the solution could besought in the following form: 7. C. 3
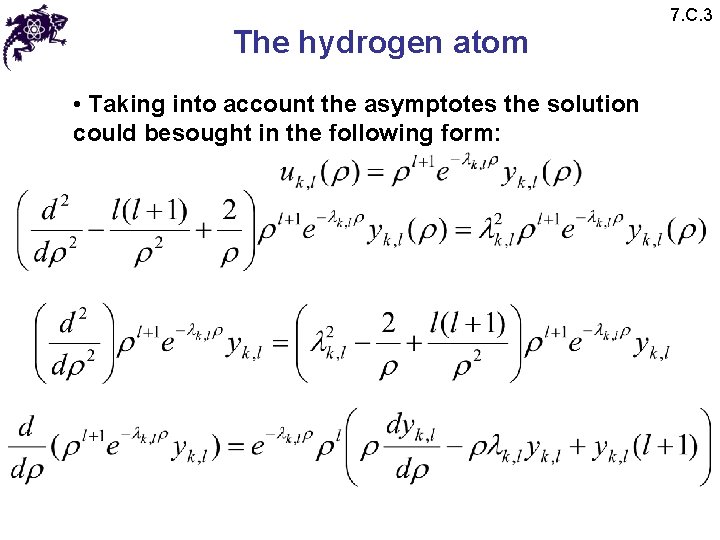
The hydrogen atom • Taking into account the asymptotes the solution could besought in the following form: 7. C. 3
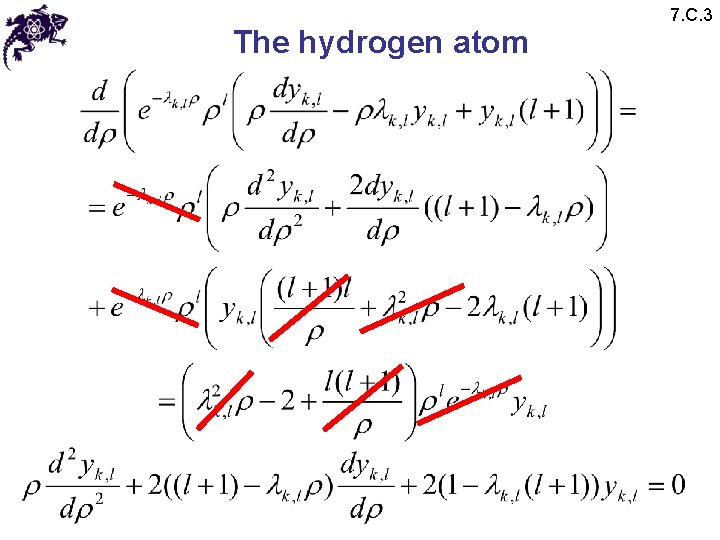
The hydrogen atom 7. C. 3
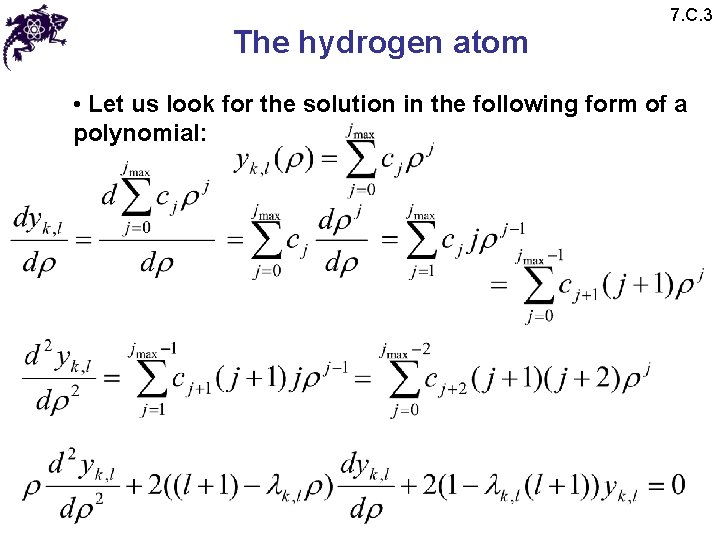
The hydrogen atom 7. C. 3 • Let us look for the solution in the following form of a polynomial:
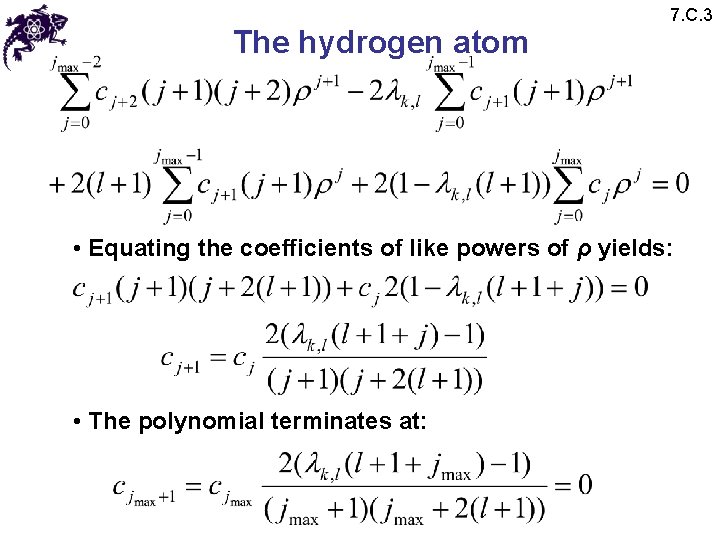
The hydrogen atom 7. C. 3 • Equating the coefficients of like powers of ρ yields: • The polynomial terminates at:
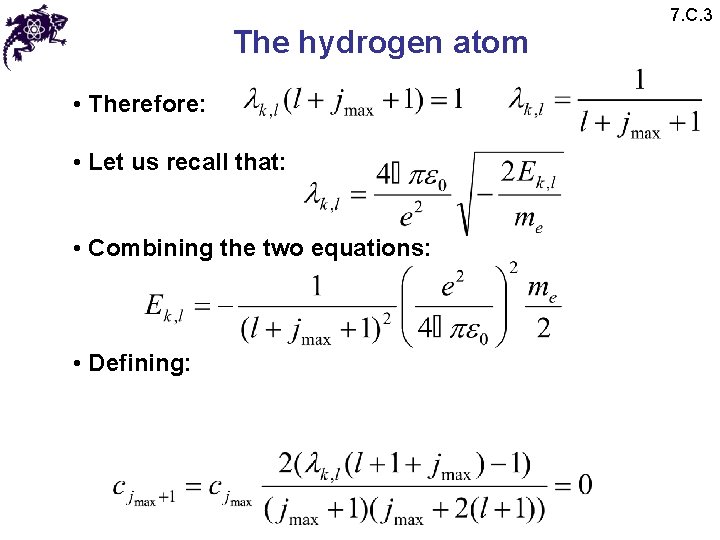
The hydrogen atom • Therefore: • Let us recall that: • Combining the two equations: • Defining: 7. C. 3
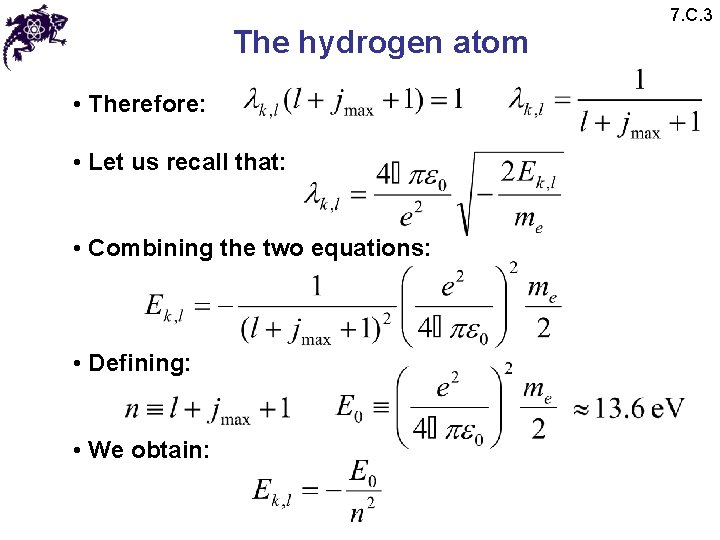
The hydrogen atom • Therefore: • Let us recall that: • Combining the two equations: • Defining: • We obtain: 7. C. 3
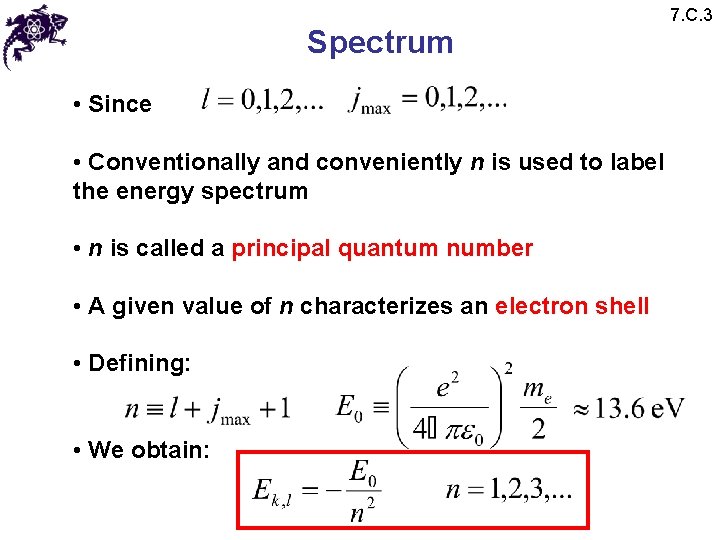
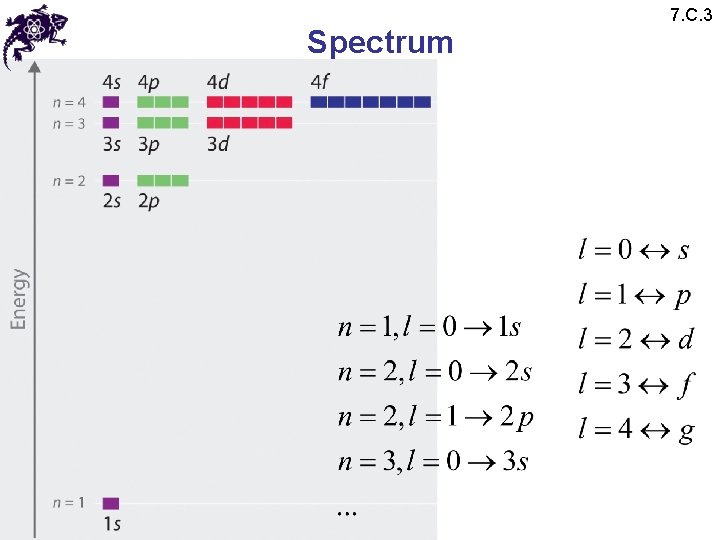
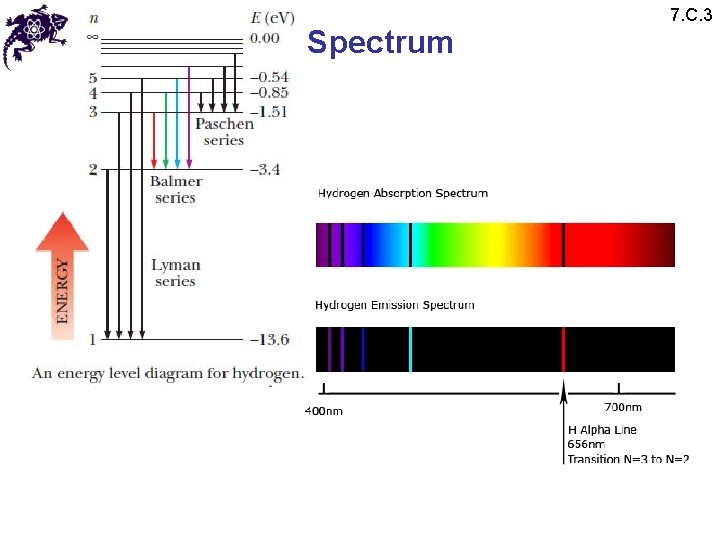
Spectrum • Since • Conventionally and conveniently n is used to label the energy spectrum • n is called a principal quantum number • A given value of n characterizes an electron shell • Defining: • We obtain: 7. C. 3
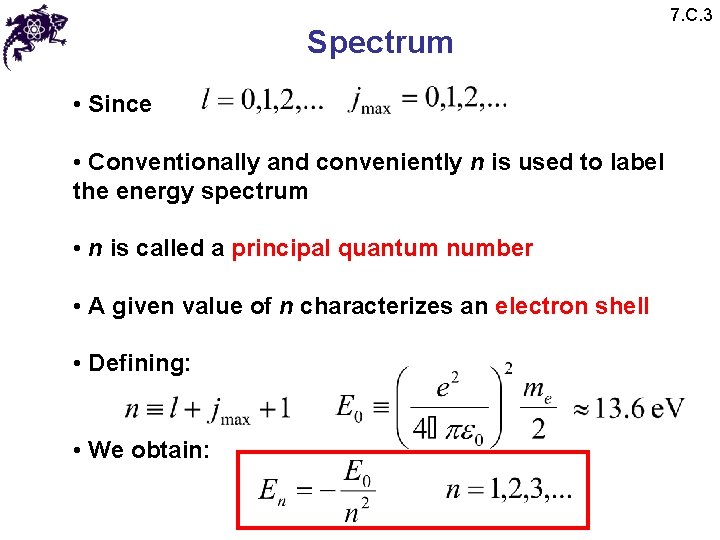
Spectrum • Since • Conventionally and conveniently n is used to label the energy spectrum • n is called a principal quantum number • A given value of n characterizes an electron shell • Defining: • We obtain: 7. C. 3
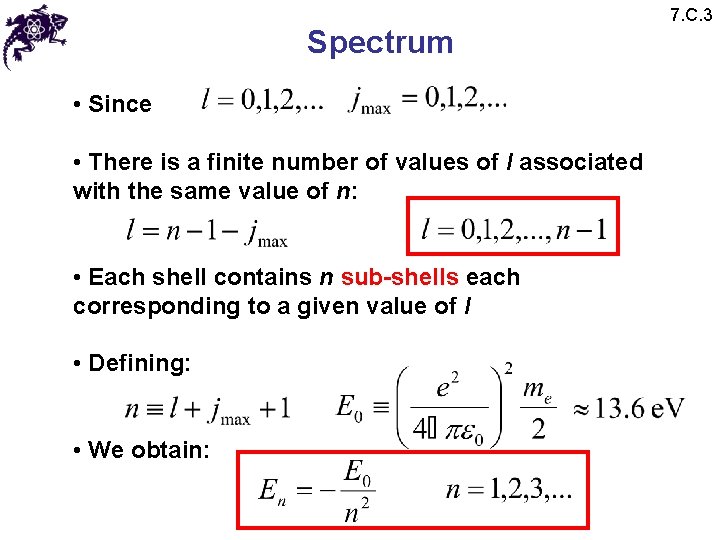
Spectrum • Since • There is a finite number of values of l associated with the same value of n: • Each shell contains n sub-shells each corresponding to a given value of l • Defining: • We obtain: 7. C. 3
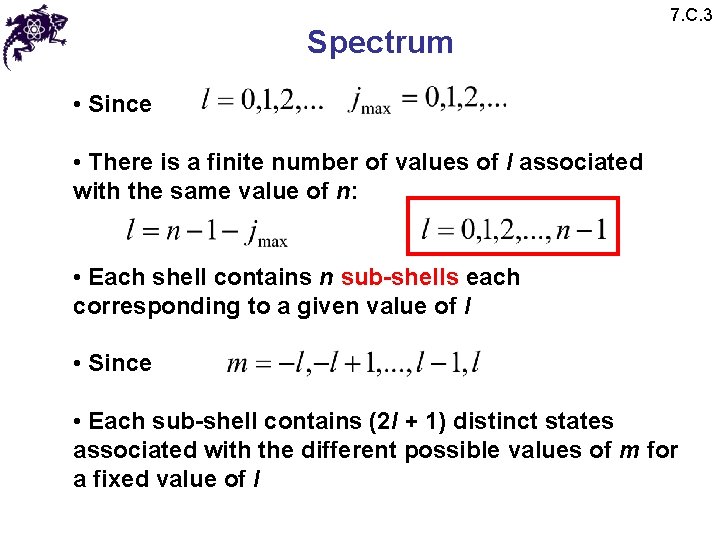
Spectrum 7. C. 3 • Since • There is a finite number of values of l associated with the same value of n: • Each shell contains n sub-shells each corresponding to a given value of l • Since • Each sub-shell contains (2 l + 1) distinct states associated with the different possible values of m for a fixed value of l
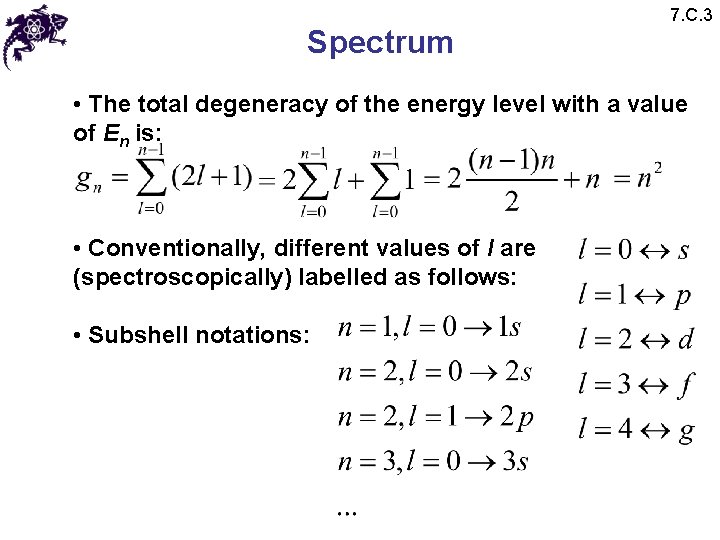
Spectrum 7. C. 3 • The total degeneracy of the energy level with a value of En is: • Conventionally, different values of l are (spectroscopically) labelled as follows: • Subshell notations:
Spectrum 7. C. 3
Spectrum 7. C. 3
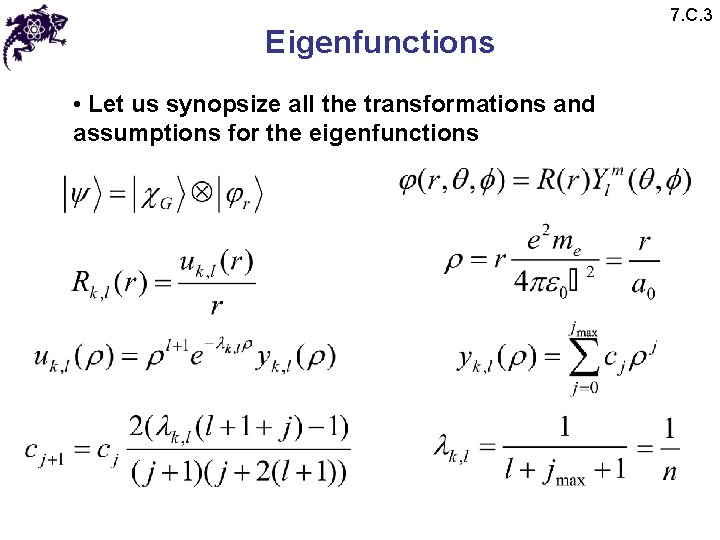
Eigenfunctions • Let us synopsize all the transformations and assumptions for the eigenfunctions 7. C. 3
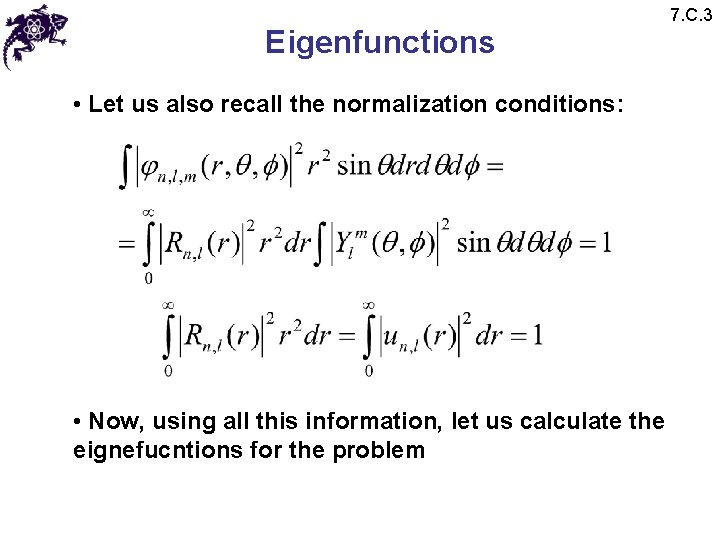
Eigenfunctions • Let us also recall the normalization conditions: • Now, using all this information, let us calculate the eignefucntions for the problem 7. C. 3
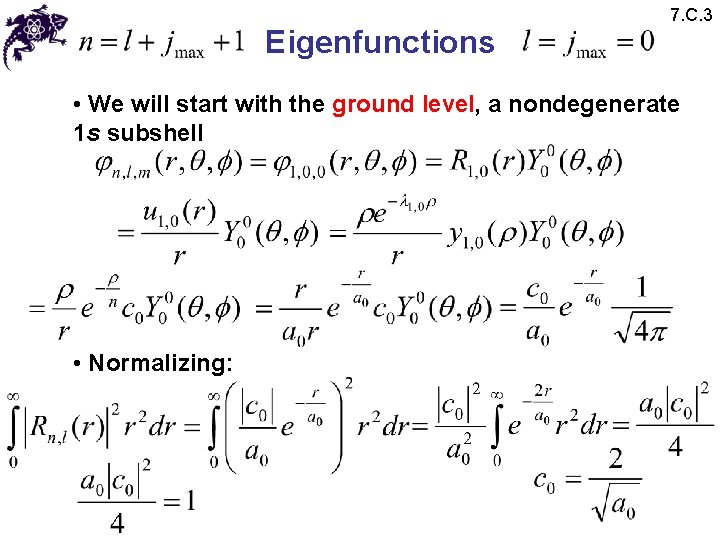
Eigenfunctions 7. C. 3 • We will start with the ground level, a nondegenerate 1 s subshell • Normalizing:
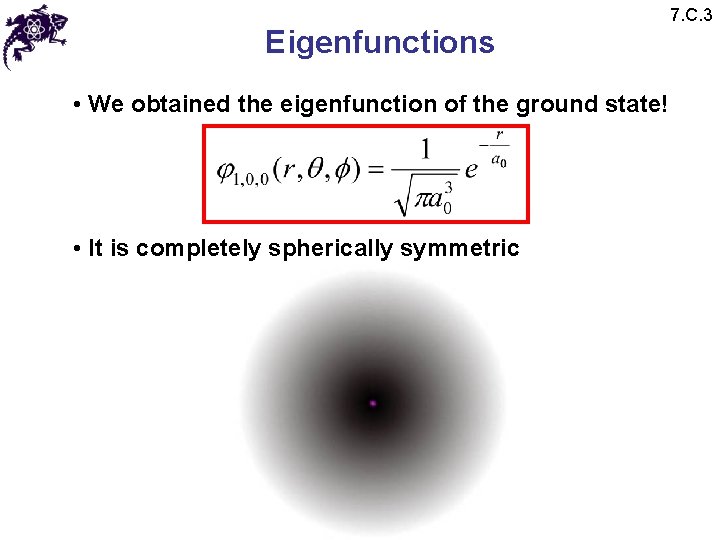
Eigenfunctions • We obtained the eigenfunction of the ground state! • It is completely spherically symmetric 7. C. 3
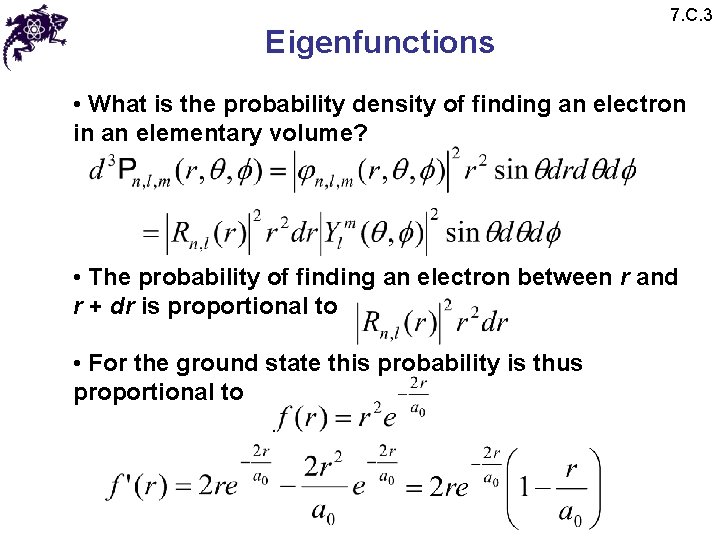
Eigenfunctions 7. C. 3 • What is the probability density of finding an electron in an elementary volume? • The probability of finding an electron between r and r + dr is proportional to • For the ground state this probability is thus proportional to
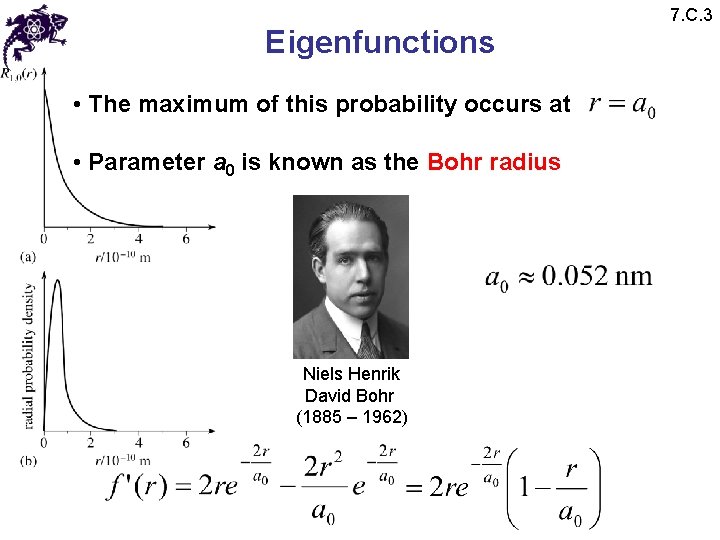
Eigenfunctions • The maximum of this probability occurs at • Parameter a 0 is known as the Bohr radius Niels Henrik David Bohr (1885 – 1962) 7. C. 3
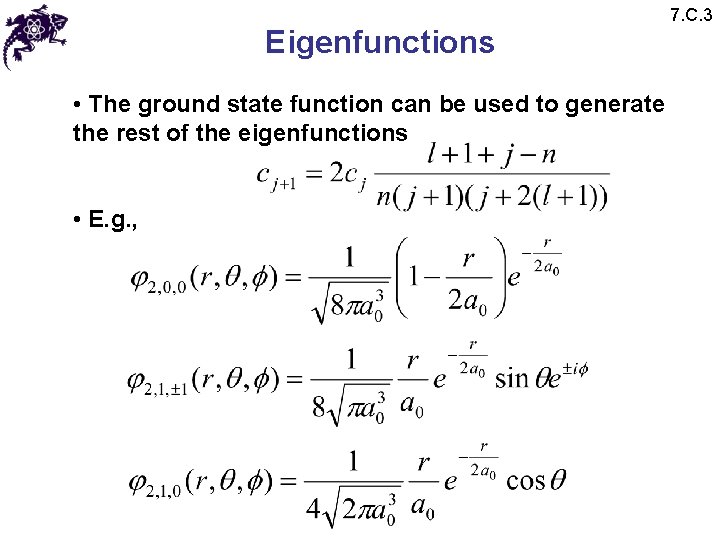
Eigenfunctions • The ground state function can be used to generate the rest of the eigenfunctions • E. g. , 7. C. 3
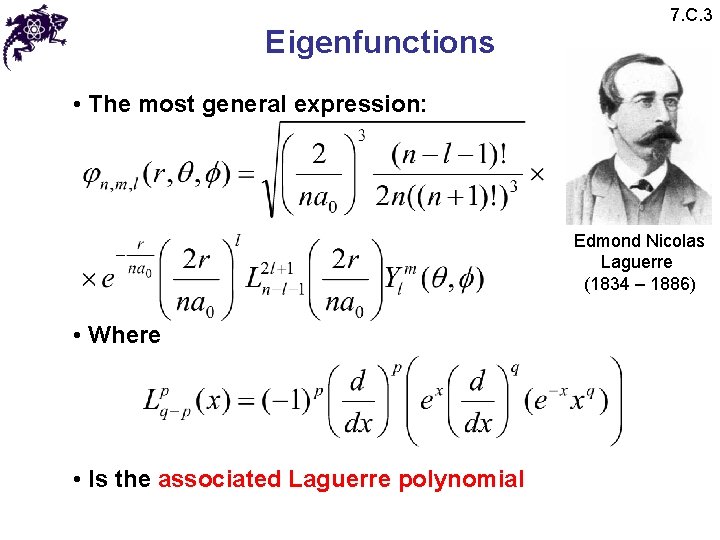
Eigenfunctions 7. C. 3 • The most general expression: Edmond Nicolas Laguerre (1834 – 1886) • Where • Is the associated Laguerre polynomial
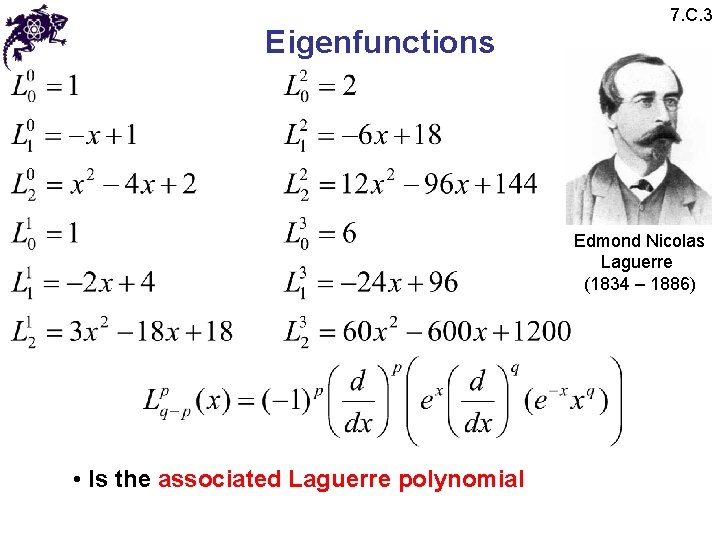
Eigenfunctions 7. C. 3 Edmond Nicolas Laguerre (1834 – 1886) • Is the associated Laguerre polynomial
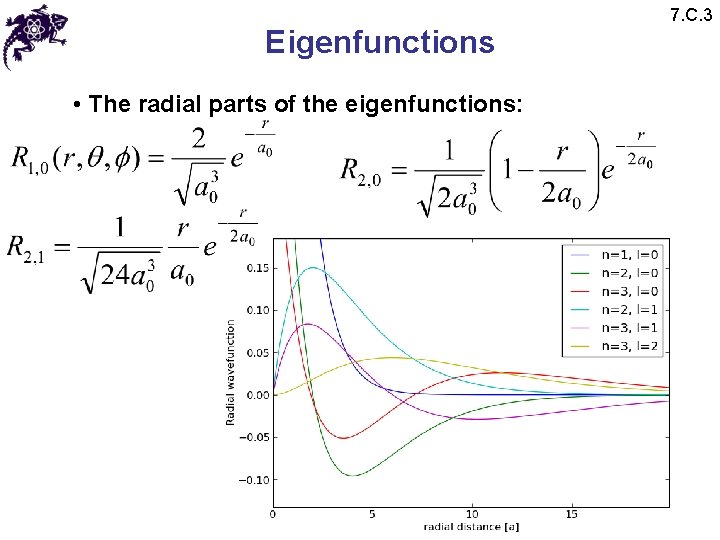
Eigenfunctions • The radial parts of the eigenfunctions: 7. C. 3
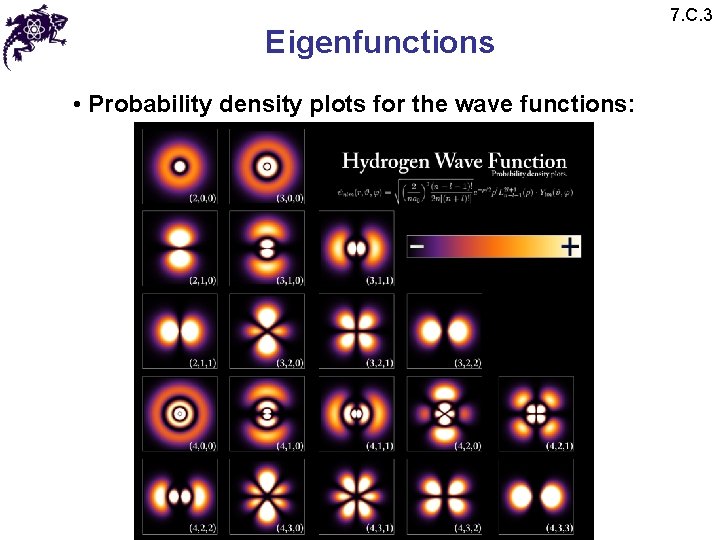
Eigenfunctions • Probability density plots for the wave functions: 7. C. 3
- Slides: 49