Chapter 7 Microbial Metabolism FFY CONTENT An Overview
Chapter 7 Microbial Metabolism FFY 農歷 丁亥 年 丙午月癸巳日


CONTENT • An Overview of Metabolism • Biological Oxidation of Microorganisms • Metabolism: energy release and conservation • Breakdown of Glucose • Destination of glycolic products • An Overview of Alternate Modes of Energy Generation • Metabolism: the use of energy in biosynthesis • peptidoglycan • Nitrogen fixation

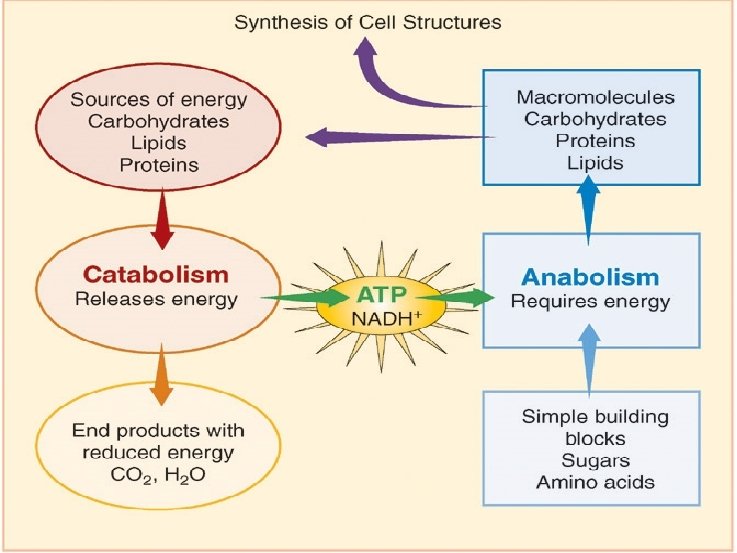
1. An Overview of Metabolism • Metabolism is the total of all chemical reactions occurring in the cell. A simplified view of cell metabolism depicts how catabolic degradative reactions supply energy needed for cell functions and how anabolic reactions bring about the synthesis of cell components from nutrients. • Note that in anabolism, nutrients from the environment or those generated from catabolic reactions are converted to cell components, whereas in catabolism, energy sources from the environment are converted to waste products • Energy producing and using is the central of
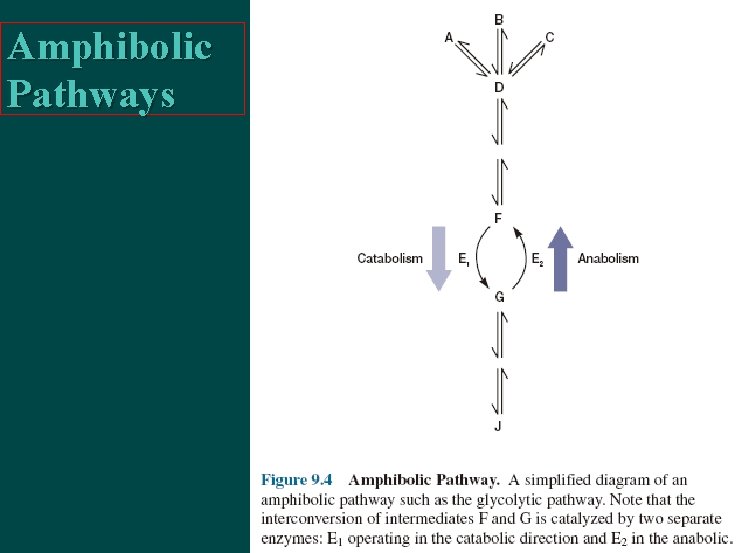
Amphibolic Pathways

ATP Energy currency: ATP, as a coupling agent

Energy source Ø Primary energy source Organic substance l Light energy l Reductive inorganic substance Ø Universal energy source l ATP l osmotic potential ? l
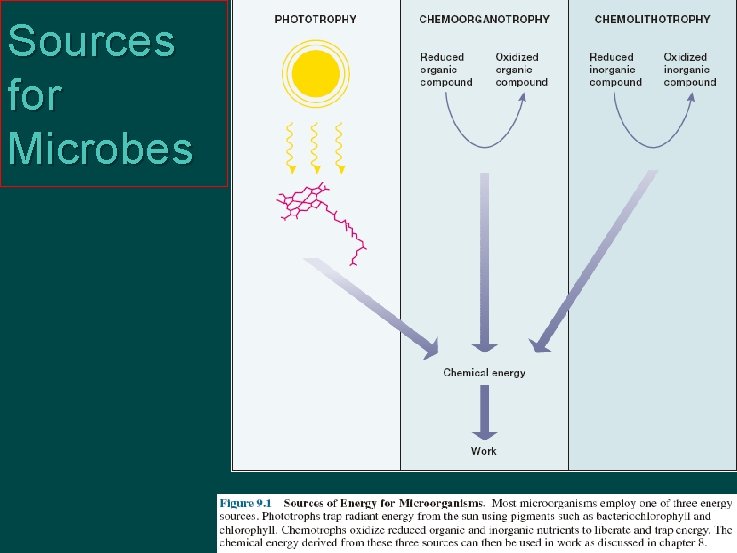
Sources for Microbes

Patterns of Energy Release

2. Energy producing metabolism in microorganisms ØBiological oxidation ØPhotosynthesis

Biological Oxidation in heterotrophic microorganisms Ø Biological Oxidation l l l Concept Stages Substrates Functions Types

Concept of biological oxidation (micro)organisms Matters Energy Oxidation-reduction reactions

Stages of biological oxidation

Types of biological oxidation Ø Fermentation Ø Respiration Aerobic respiration l Anaerobic respiration l
Dehydrogen Pathway ---upstream of fermentation and respiration ØGlucose → pyruvic acid Øpyruvic acid →TCA
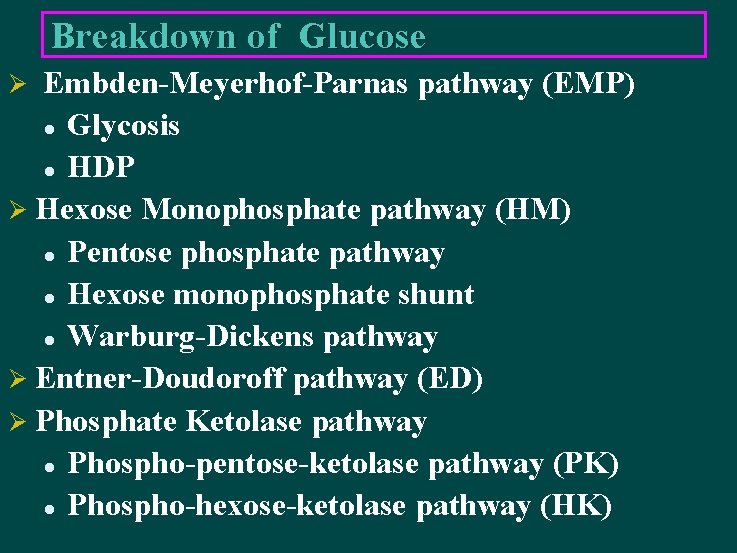
Breakdown of Glucose Embden-Meyerhof-Parnas pathway (EMP) l Glycosis l HDP Ø Hexose Monophosphate pathway (HM) l Pentose phosphate pathway l Hexose monophosphate shunt l Warburg-Dickens pathway Ø Entner-Doudoroff pathway (ED) Ø Phosphate Ketolase pathway l Phospho-pentose-ketolase pathway (PK) l Phospho-hexose-ketolase pathway (HK) Ø
EMP pathway Glycolysis: A common biochemical pathway for the fermentation of glucose is glycolysis, also named the Embden. Meyerh-Parnas of pathway for its major discoverers. Can be divided into two major stages.

Stages I and II: Preparatory and Redox Reactions Stage I : A series of preparatory rearrangements: reactions that do not involve oxidation-reduction and do not release energy but that lead to the production from glucose of two molecules of the key intermediate, glyceraldehyde 3 -phosphate. Stage II: Oxidation-reduction occurs, energy is conserved in the form of ATP, and two molecules of pyruvate are formed.
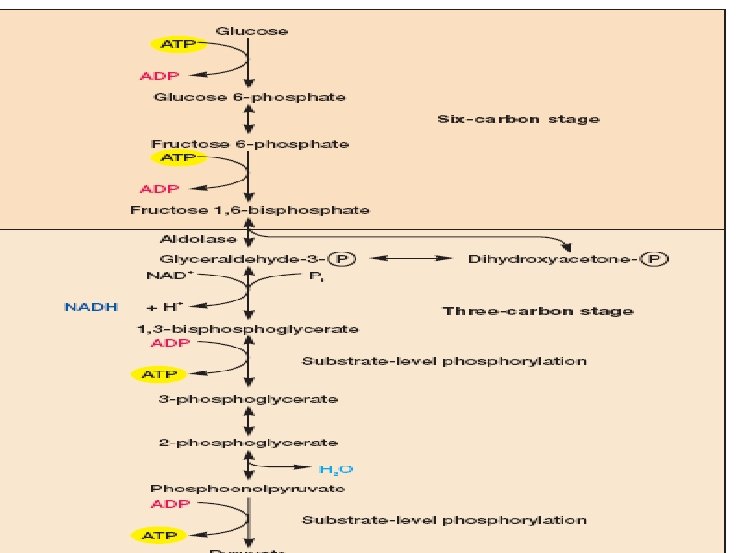
Diagram of EMP
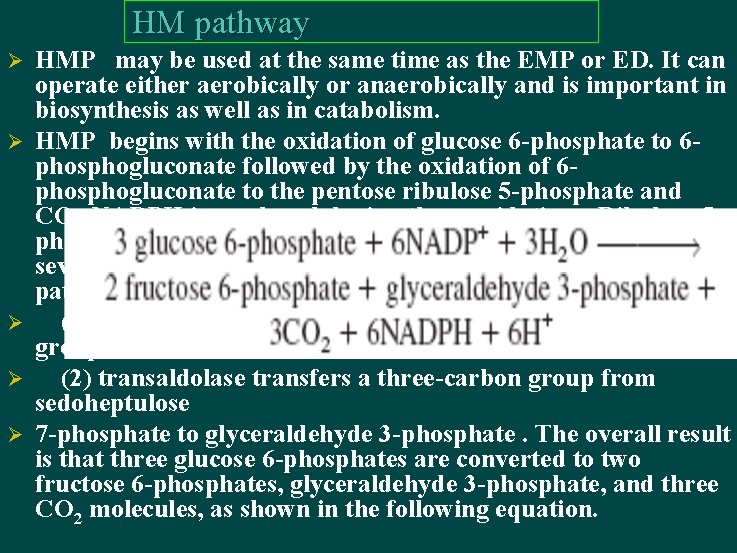
HM pathway Ø Ø Ø HMP may be used at the same time as the EMP or ED. It can operate either aerobically or anaerobically and is important in biosynthesis as well as in catabolism. HMP begins with the oxidation of glucose 6 -phosphate to 6 phosphogluconate followed by the oxidation of 6 phosphogluconate to the pentose ribulose 5 -phosphate and CO 2 NADPH is produced during these oxidations. Ribulose 5 phosphate is then converted to a mixture of three- through seven-carbon sugar phosphates. Two enzymes unique to this pathway play a central role in these transformations: (1) transketolase catalyzes the transfer of two-carbon ketol groups, (2) transaldolase transfers a three-carbon group from sedoheptulose 7 -phosphate to glyceraldehyde 3 -phosphate. The overall result is that three glucose 6 -phosphates are converted to two fructose 6 -phosphates, glyceraldehyde 3 -phosphate, and three CO 2 molecules, as shown in the following equation.

Diagram of HMP pathway

Transketolase and Transaldolase.
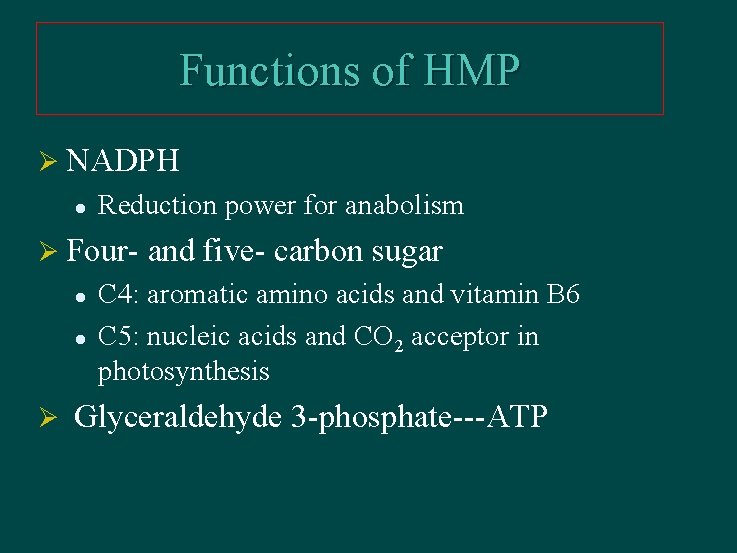
Functions of HMP Ø NADPH l Reduction power for anabolism Ø Four- and five- carbon sugar l l Ø C 4: aromatic amino acids and vitamin B 6 C 5: nucleic acids and CO 2 acceptor in photosynthesis Glyceraldehyde 3 -phosphate---ATP

Single HMP existed bacteria Ø Acetobacter suboxydans Ø Gluconobacter oxydans Ø Acetomonas oxydans
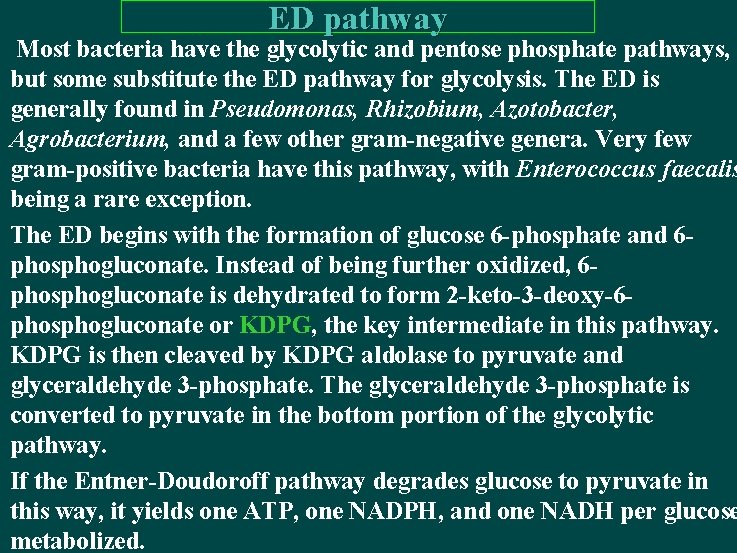
ED pathway Most bacteria have the glycolytic and pentose phosphate pathways, but some substitute the ED pathway for glycolysis. The ED is generally found in Pseudomonas, Rhizobium, Azotobacter, Agrobacterium, and a few other gram-negative genera. Very few gram-positive bacteria have this pathway, with Enterococcus faecalis being a rare exception. The ED begins with the formation of glucose 6 -phosphate and 6 phosphogluconate. Instead of being further oxidized, 6 phosphogluconate is dehydrated to form 2 -keto-3 -deoxy-6 phosphogluconate or KDPG, the key intermediate in this pathway. KDPG is then cleaved by KDPG aldolase to pyruvate and glyceraldehyde 3 -phosphate. The glyceraldehyde 3 -phosphate is converted to pyruvate in the bottom portion of the glycolytic pathway. If the Entner-Doudoroff pathway degrades glucose to pyruvate in this way, it yields one ATP, one NADPH, and one NADH per glucose metabolized.

Diagram of ED pathway

Single ED existed bacteria Ø Pseudomonas saccharophila Ø Ps. aeruginosa Ø Ps. Fluorenscens Ø Zymomonas mobilis Ø Alcaligenes eutrophus
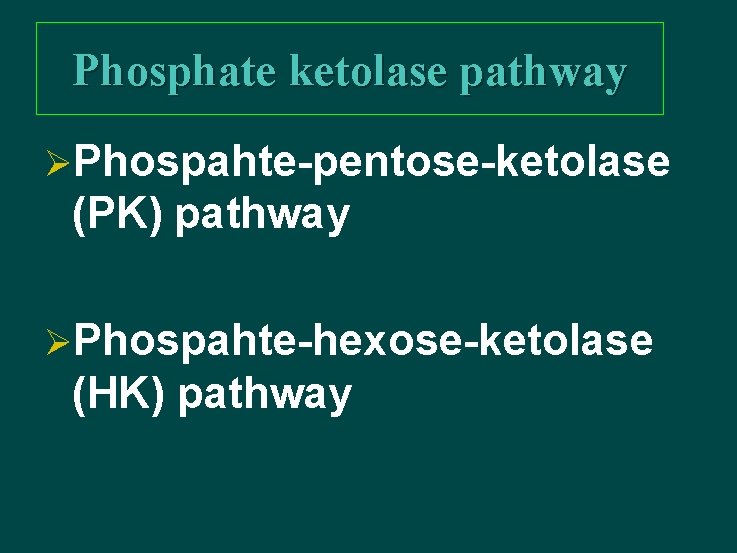
Phosphate ketolase pathway ØPhospahte-pentose-ketolase (PK) pathway ØPhospahte-hexose-ketolase (HK) pathway

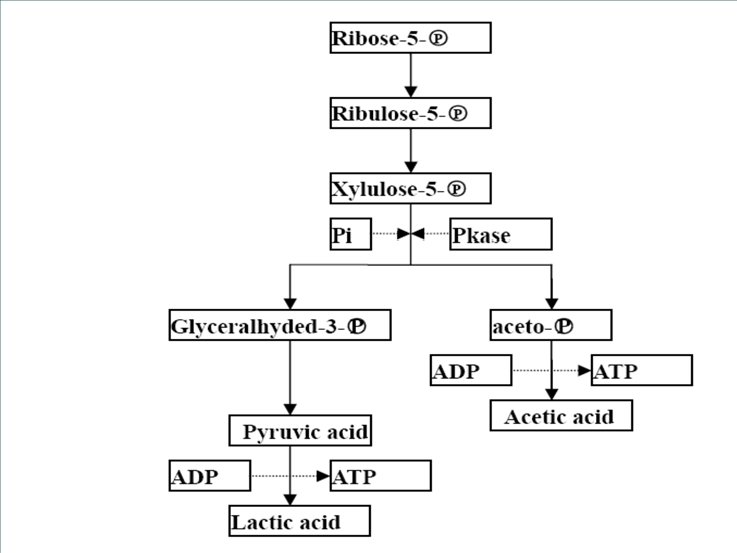
Destination of glycolic products Fermentation l When and what? Respiration l When and what?
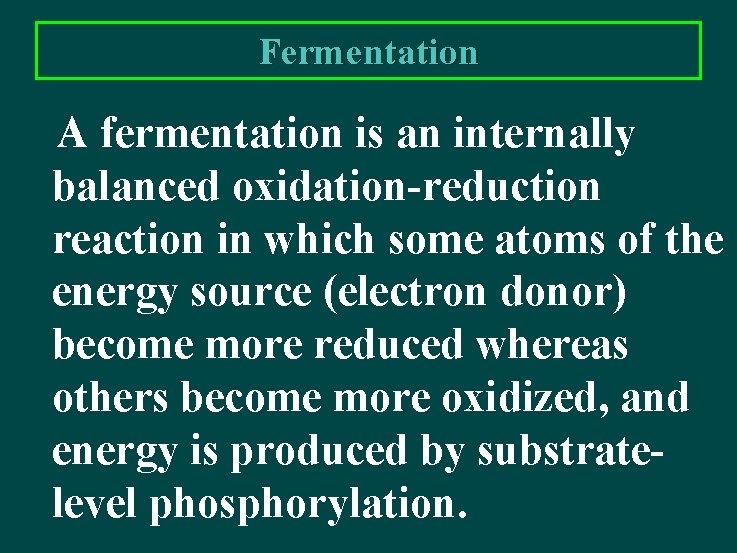
Fermentation A fermentation is an internally balanced oxidation-reduction reaction in which some atoms of the energy source (electron donor) become more reduced whereas others become more oxidized, and energy is produced by substratelevel phosphorylation.

Production of Fermentation Products A second oxidation-reduction reaction occurs and fermentation products (for example, ethanol and CO 2, or lactic acid) are formed.
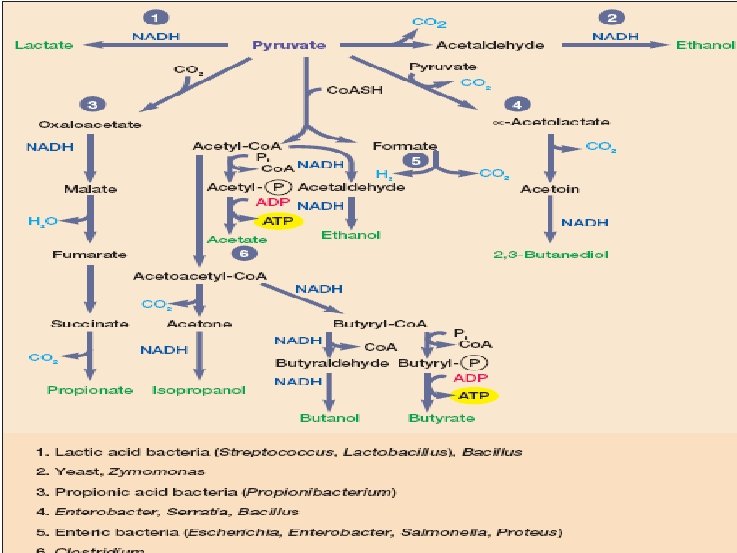
Some Common Microbial Fermentations
Glucose Fermentation: Net and Practical Results The ultimate result of glycolysis is the consumption of glucose, the net synthesis of two ATPs, and the production of fermentation products.
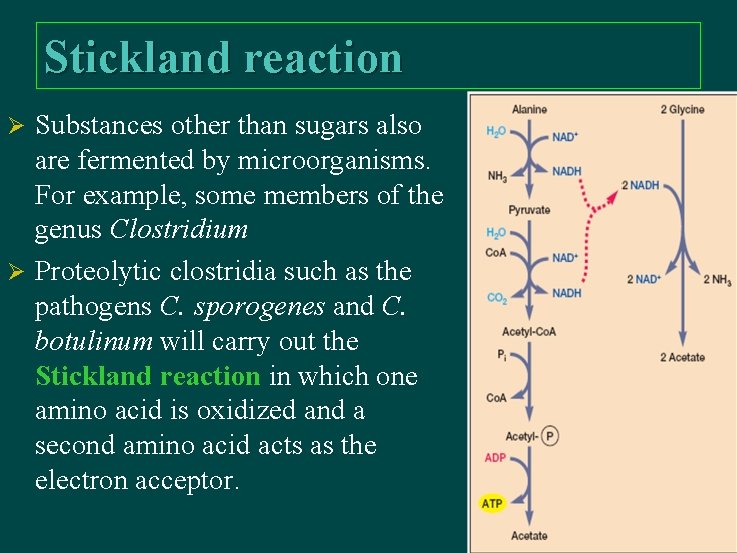
Stickland reaction Substances other than sugars also are fermented by microorganisms. For example, some members of the genus Clostridium Ø Proteolytic clostridia such as the pathogens C. sporogenes and C. botulinum will carry out the Stickland reaction in which one amino acid is oxidized and a second amino acid acts as the electron acceptor. Ø
Respiration and Electron Transport Respiration : in which molecular oxygen or some other oxidant serves as the terminal electron acceptor The discussion of respiration deals with both the carbon and electron transformations: Ø (1) the biochemical pathways involved in the transformation of organic carbon to CO 2 Ø (2) the way electrons are transferred from the organic compound to the terminal electron acceptor, driving ATP synthesis at the expense of the proton motive force.
Aerobic respiration TCA Cycle TCA cycle enzymes are widely distributed among microorganisms. The complete cycle appears to be functional in many aerobic bacteria, free-living protozoa, and most algae and fungi. This is not surprising because the cycle is such an important source of energy. However, the facultative anaerobe E. coli does not use the full TCA cycle under anaerobic conditions or when the glucose concentration is high but does at other times. Even those microorganisms that lack the complete TCA cycle usually have most of the cycle enzymes, because one of TCA cycle’s major functions is to provide carbon skeletons for use in biosynthesis.
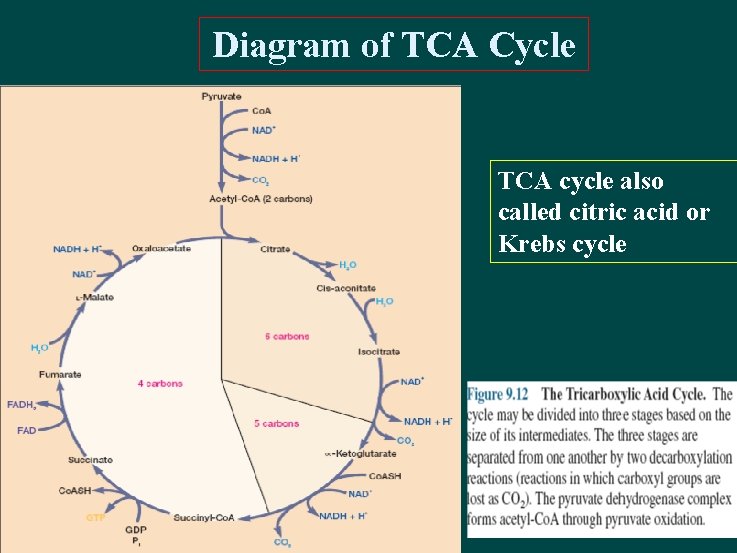
Diagram of TCA Cycle TCA cycle also called citric acid or Krebs cycle

Up to the point a little ATP produced Then time is NAD(P)H to play
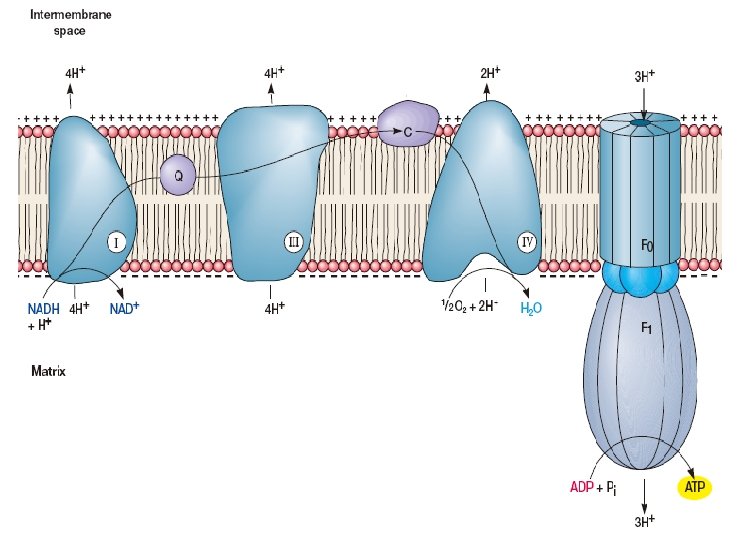
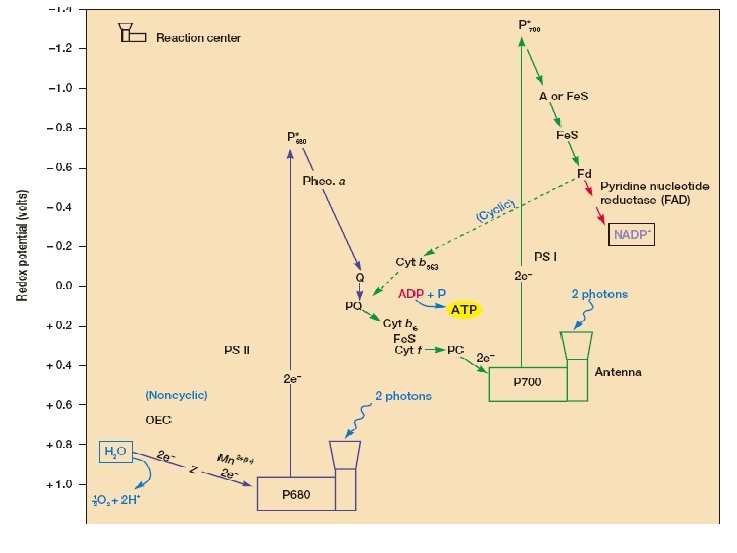
Electron Transport Electron transport systems (respiratory chain, RC) are composed of membrane associated electron carriers. These systems have two basic functions: (1) to accept electrons from an electron donor and transfer them to an electron acceptor (2) to conserve some of the energy released during electron transfer for synthesis of ATP.
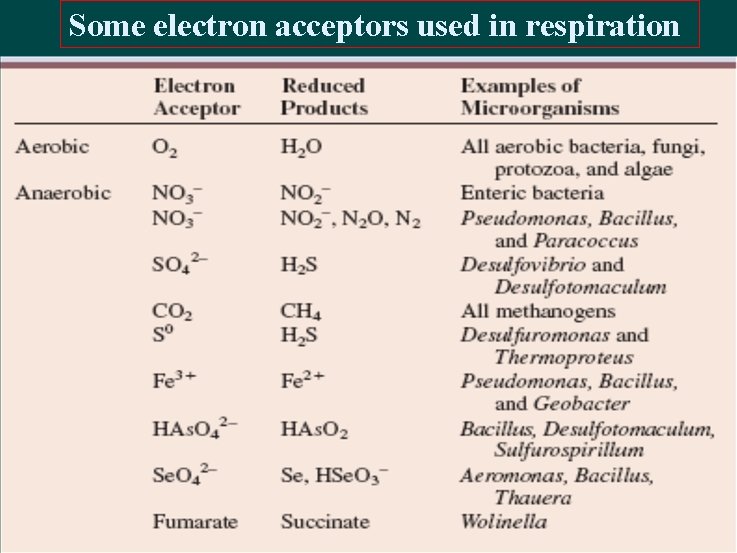
Some electron acceptors used in respiration
Types of oxidation-reduction enzymes involved in electron transport (1) NADH dehydrogenases (2) Riboflavin-containing electron carriers, generally called flavoproteins (3) iron-sulfur proteins (4) Cytochromes In addition, one class of nonprotein electron carriers is known, the lipid-soluble quinones.
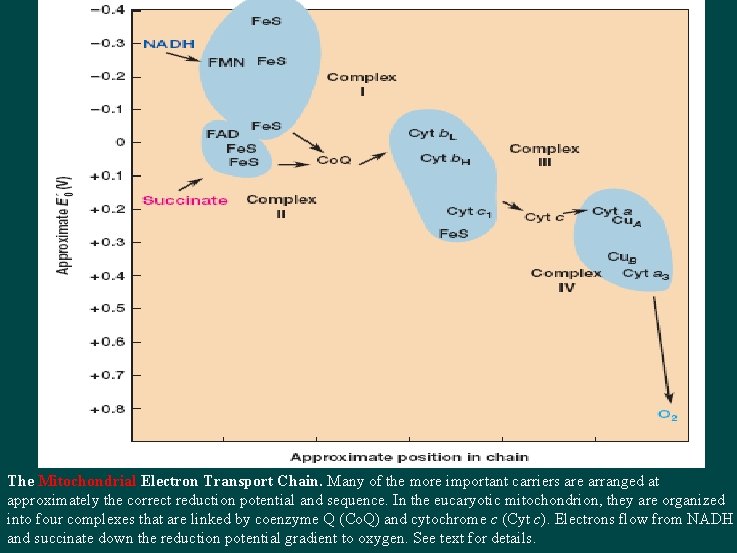
Diagram of RC The Mitochondrial Electron Transport Chain. Many of the more important carriers are arranged at approximately the correct reduction potential and sequence. In the eucaryotic mitochondrion, they are organized into four complexes that are linked by coenzyme Q (Co. Q) and cytochrome c (Cyt c). Electrons flow from NADH and succinate down the reduction potential gradient to oxygen. See text for details.
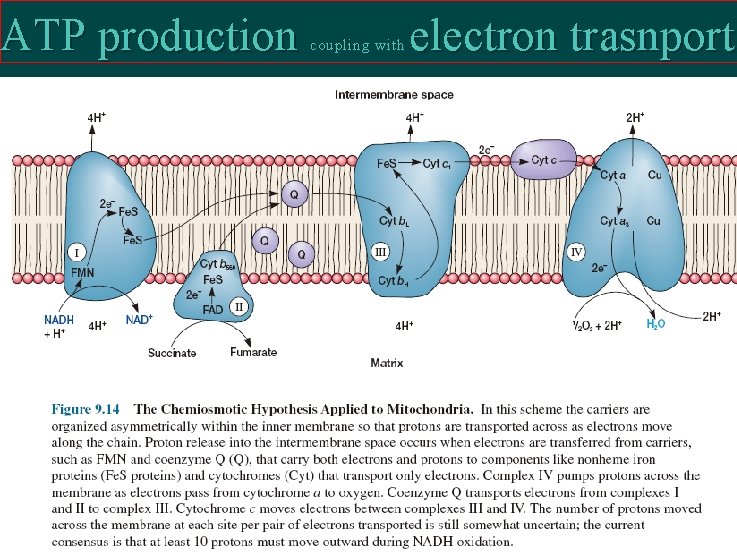
ATP production coupling with electron trasnport
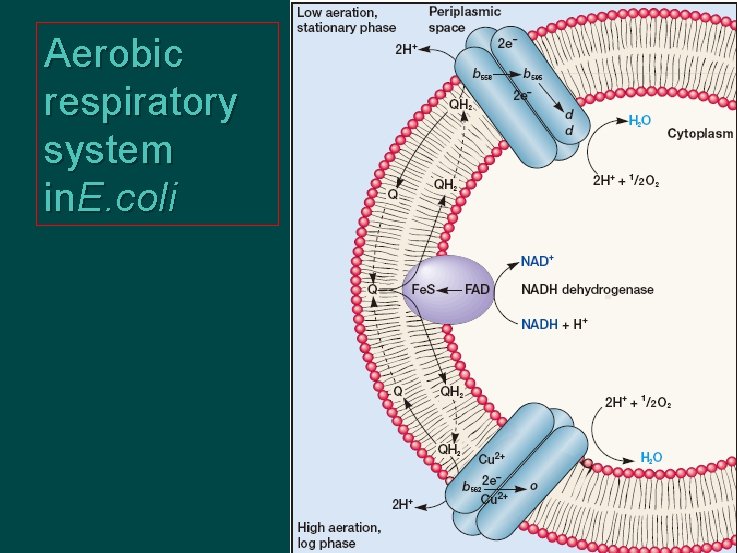
Aerobic respiratory system in. E. coli
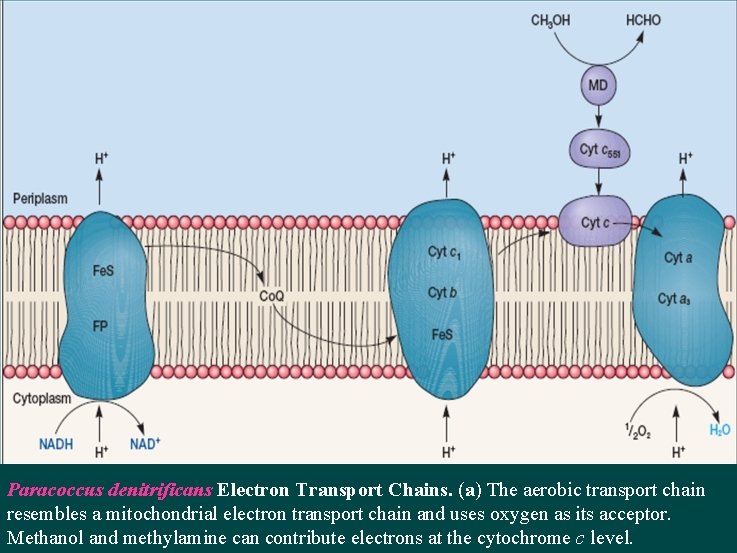
Paracoccus denitrificans Electron Transport Chains. (a) The aerobic transport chain resembles a mitochondrial electron transport chain and uses oxygen as its acceptor. Methanol and methylamine can contribute electrons at the cytochrome c level.
Anaerobic respiration Many bacteria have electron transport chains that can operate with exogenous electron acceptors other than O 2. As noted earlier, this energy-yielding process is called anaerobic respiration. The major electron acceptors are nitrate, sulfate, and CO 2, but metals and a few organic molecules can also be reduced.
Nitrate respiration Ø Nitrate respiration l Denitrification Ø Just wasting of Nitrogen?
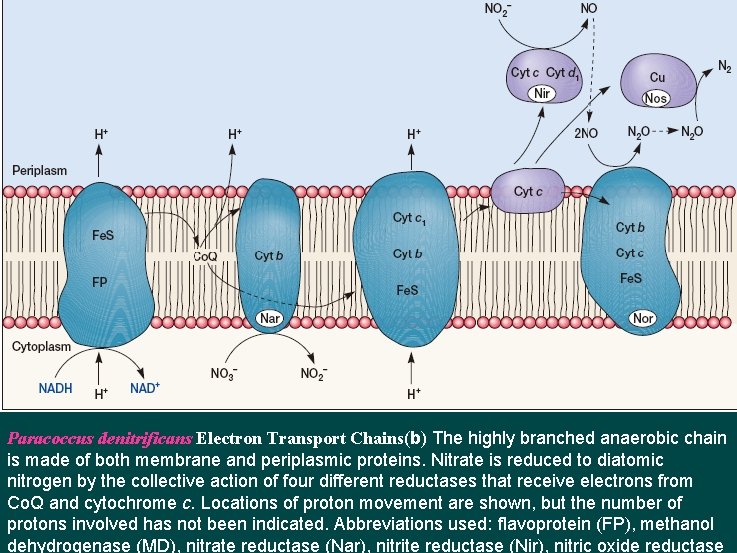
Paracoccus denitrificans Electron Transport Chains(b) The highly branched anaerobic chain is made of both membrane and periplasmic proteins. Nitrate is reduced to diatomic nitrogen by the collective action of four different reductases that receive electrons from Co. Q and cytochrome c. Locations of proton movement are shown, but the number of protons involved has not been indicated. Abbreviations used: flavoprotein (FP), methanol dehydrogenase (MD), nitrate reductase (Nar), nitrite reductase (Nir), nitric oxide reductase
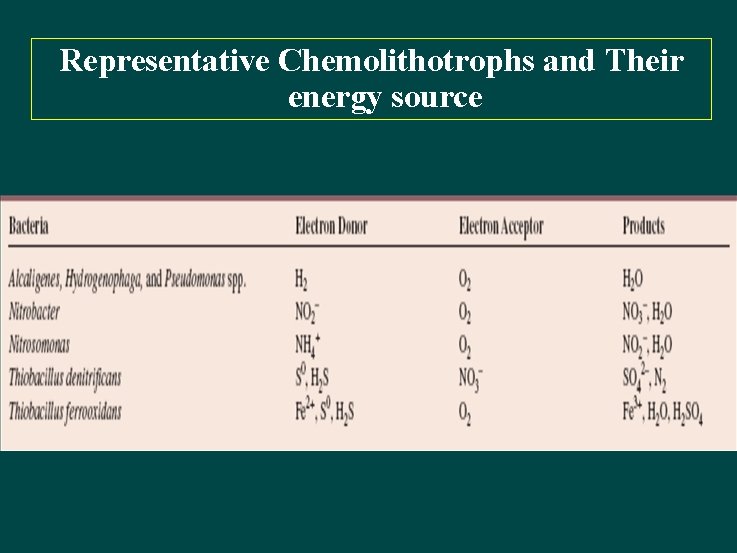
Representative Chemolithotrophs and Their energy source
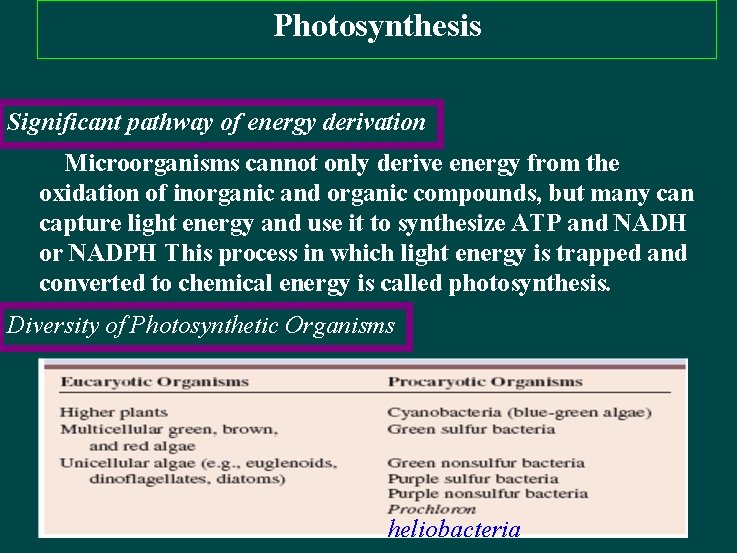
Photosynthesis Significant pathway of energy derivation Microorganisms cannot only derive energy from the oxidation of inorganic and organic compounds, but many can capture light energy and use it to synthesize ATP and NADH or NADPH This process in which light energy is trapped and converted to chemical energy is called photosynthesis. Diversity of Photosynthetic Organisms heliobacteria
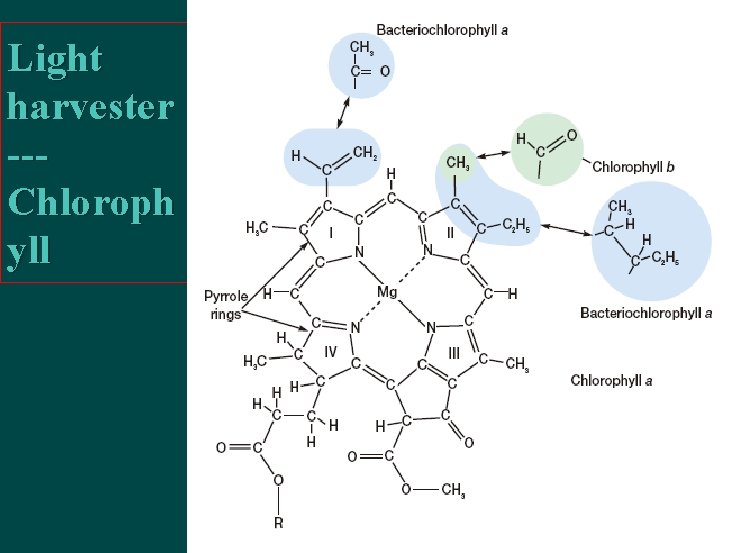
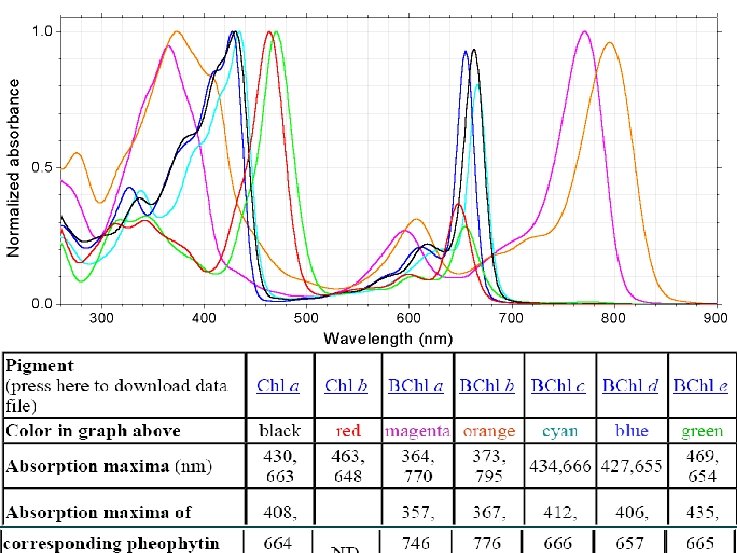
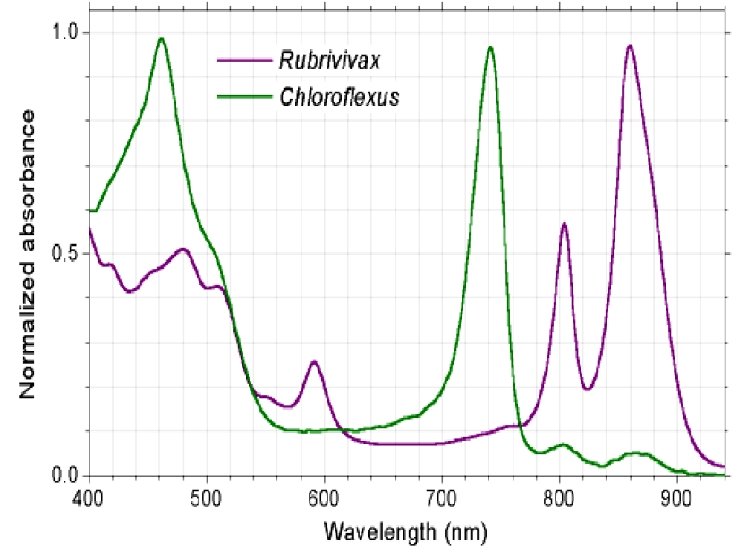
Light harvester --Chloroph yll
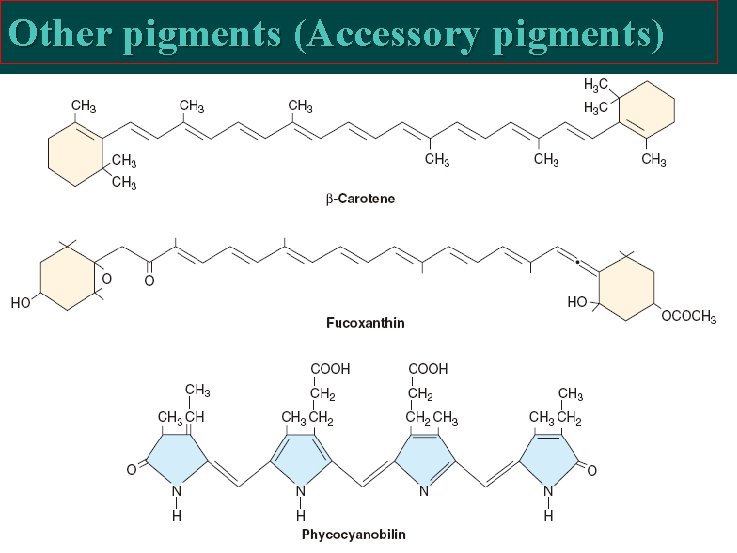
Other pigments (Accessory pigments)
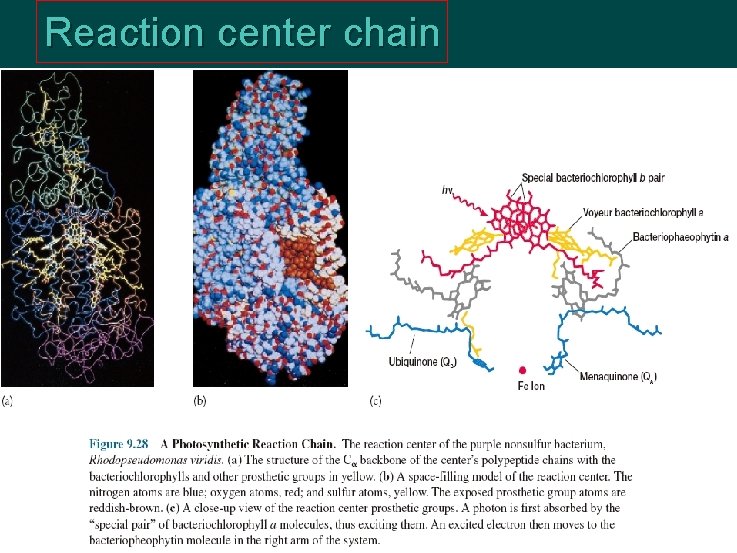
Reaction center chain
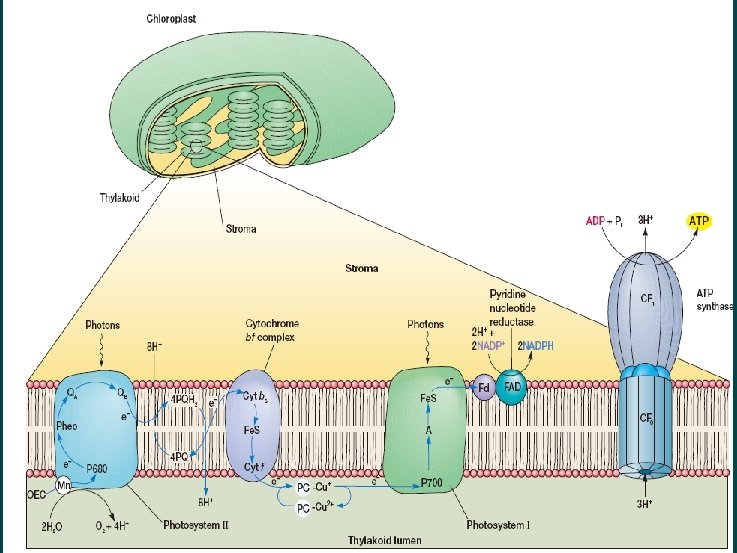
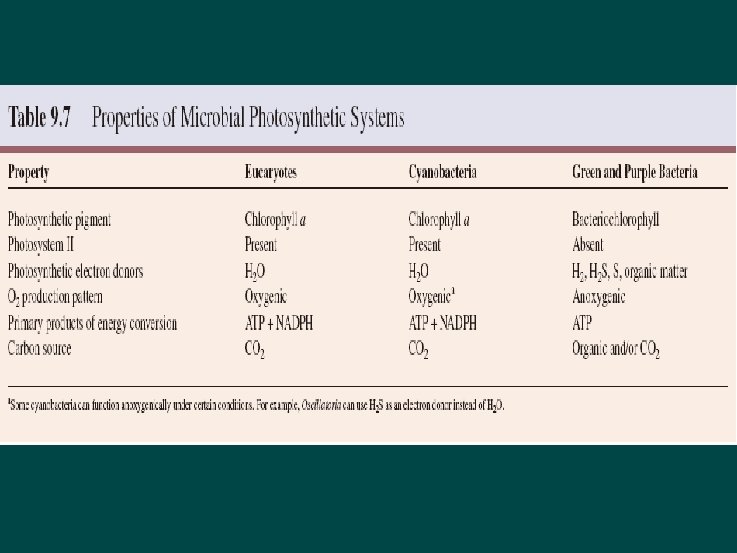
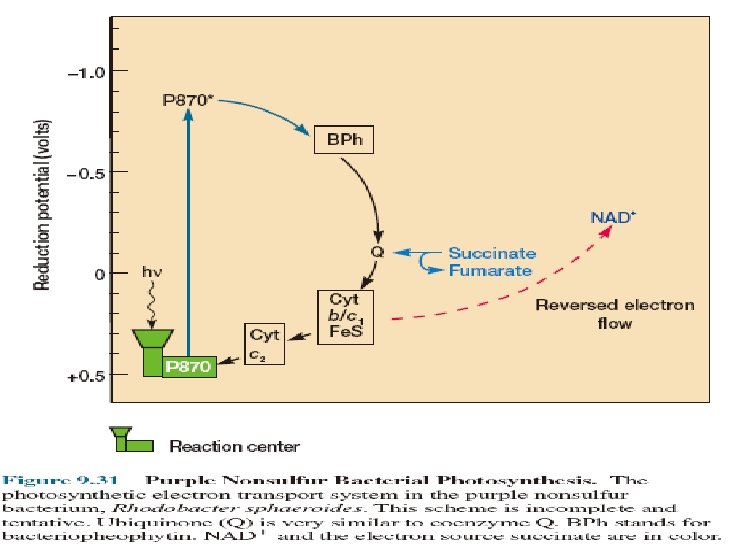
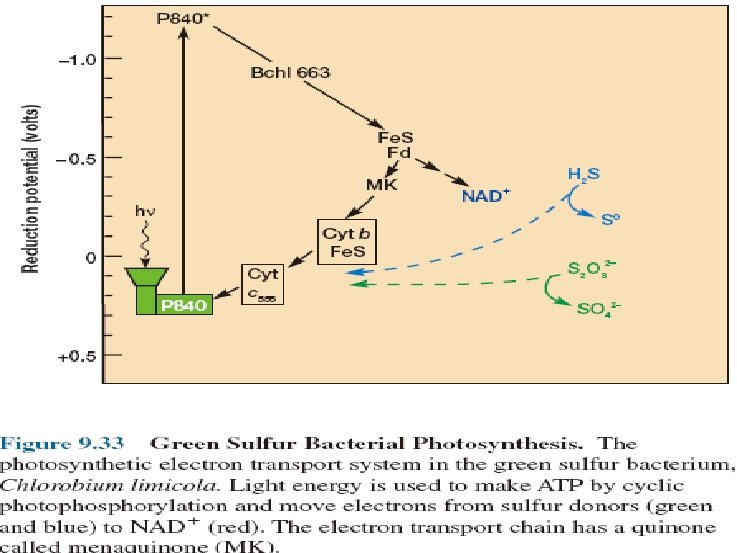
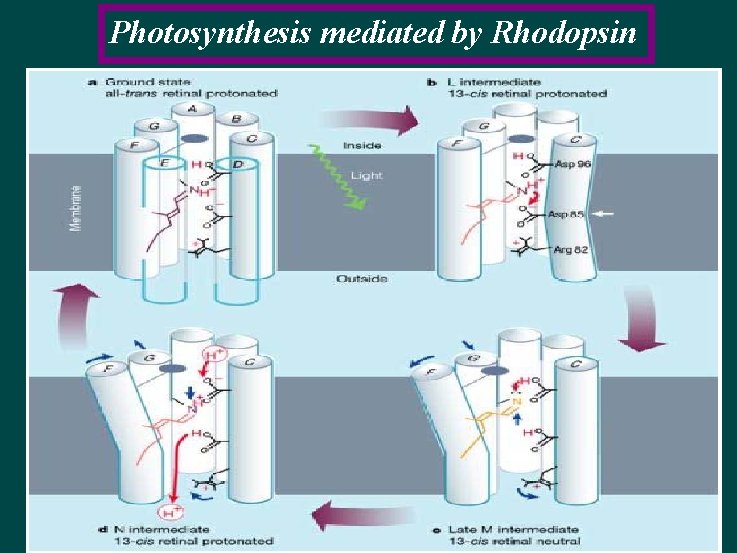
Photosynthesis mediated by Rhodopsin
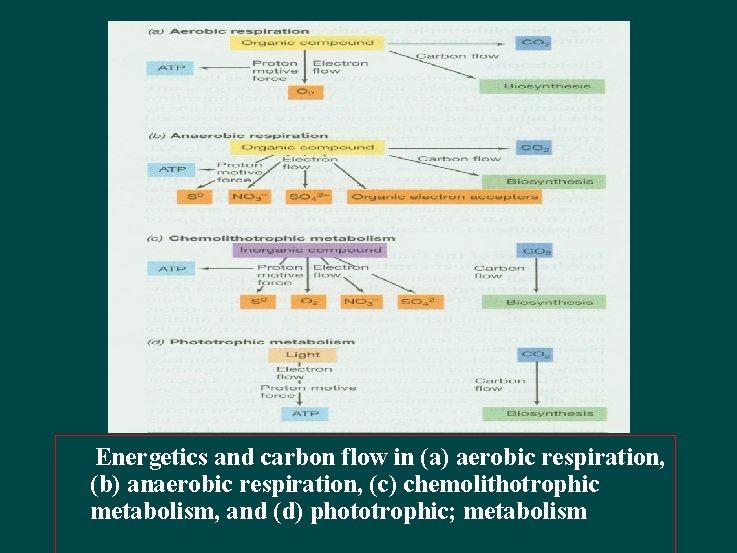
Energetics and carbon flow in (a) aerobic respiration, (b) anaerobic respiration, (c) chemolithotrophic metabolism, and (d) phototrophic; metabolism
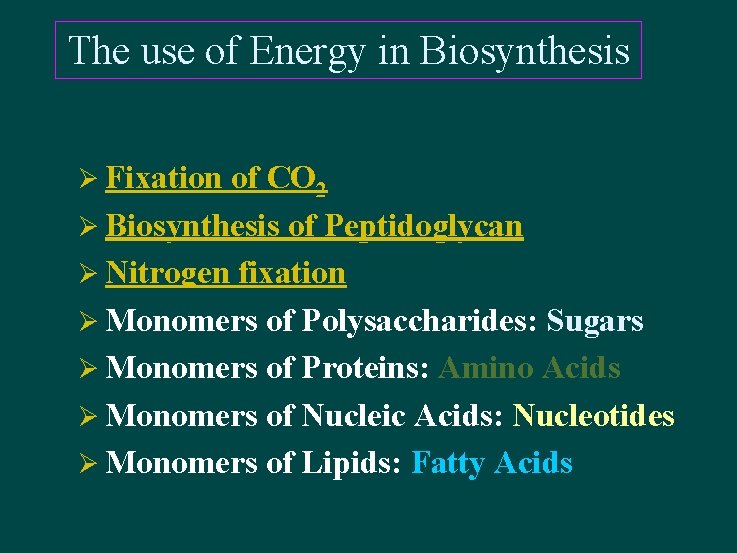
The use of Energy in Biosynthesis Ø Fixation of CO 2 Ø Biosynthesis of Peptidoglycan Ø Nitrogen fixation Ø Monomers of Polysaccharides: Sugars Ø Monomers of Proteins: Amino Acids Ø Monomers of Nucleic Acids: Nucleotides Ø Monomers of Lipids: Fatty Acids
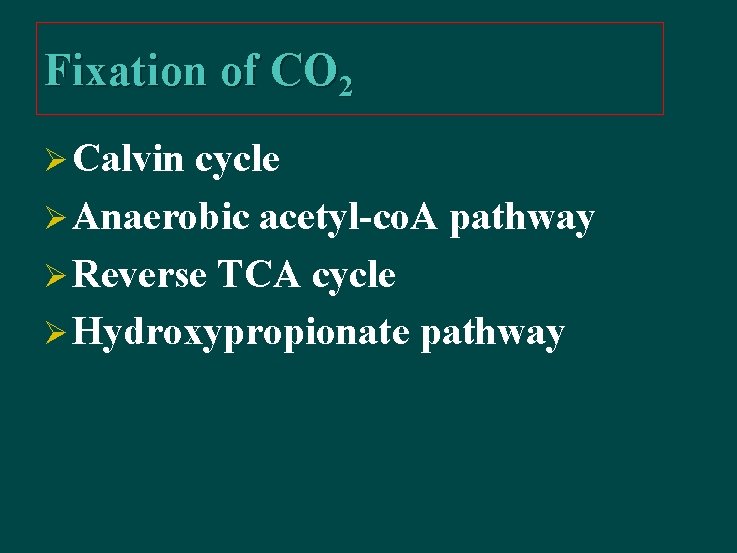
Fixation of CO 2 Ø Calvin cycle Ø Anaerobic acetyl-co. A pathway Ø Reverse TCA cycle Ø Hydroxypropionate pathway

Calvin Cycle Ø Microorganism l Photosynthetic and chemoauototrophic ones Ø Two special enzymes l l Ru. Bis. CO(ribulose biphosphate caroxylase) PRK(phosphoribulokinase) Ø Three phases l l l Carboxylation phase Reduction phase Regeneration phase Ø General equation l 6 CO 2+12 NAD(P)H 2+18 ATP C 6 H 12 O 6+12 NAD(P)+18 ADP+18 Pi


Gluconeogenesis
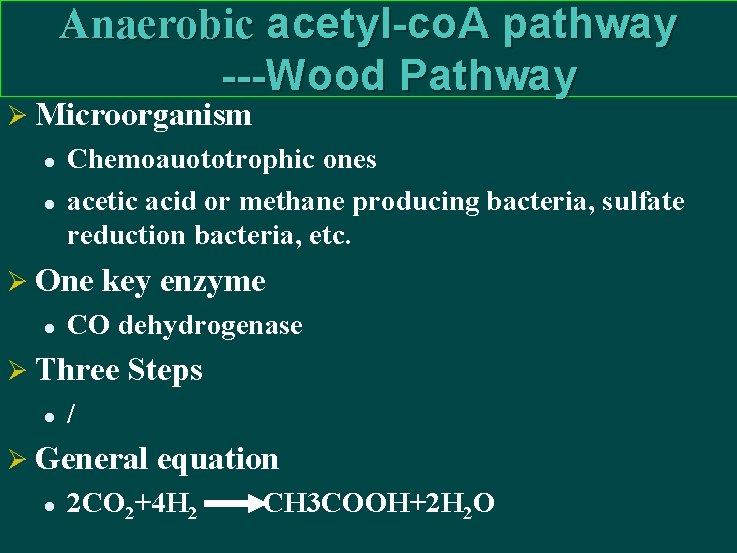
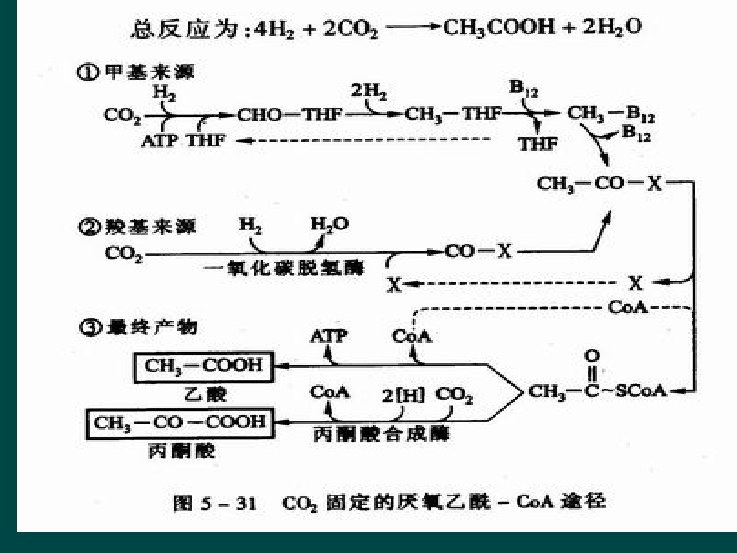
Anaerobic acetyl-co. A pathway ---Wood Pathway Ø Microorganism l l Chemoauototrophic ones acetic acid or methane producing bacteria, sulfate reduction bacteria, etc. Ø One key enzyme l CO dehydrogenase Ø Three Steps l / Ø General equation l 2 CO 2+4 H 2 CH 3 COOH+2 H 2 O
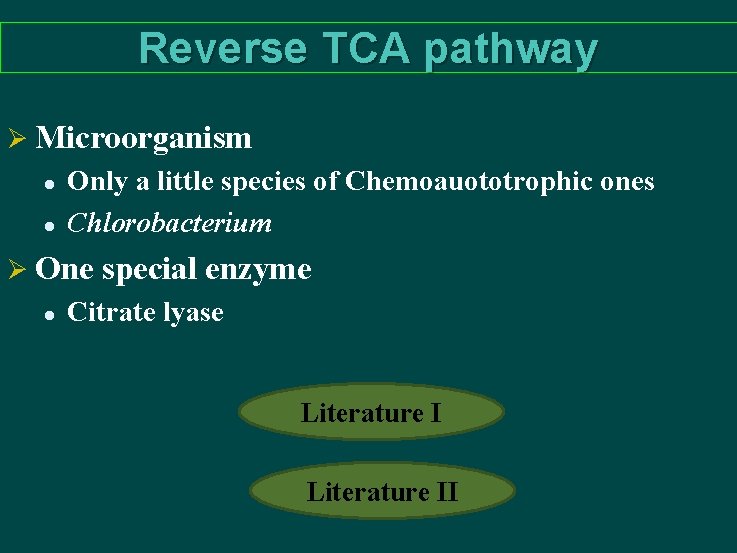
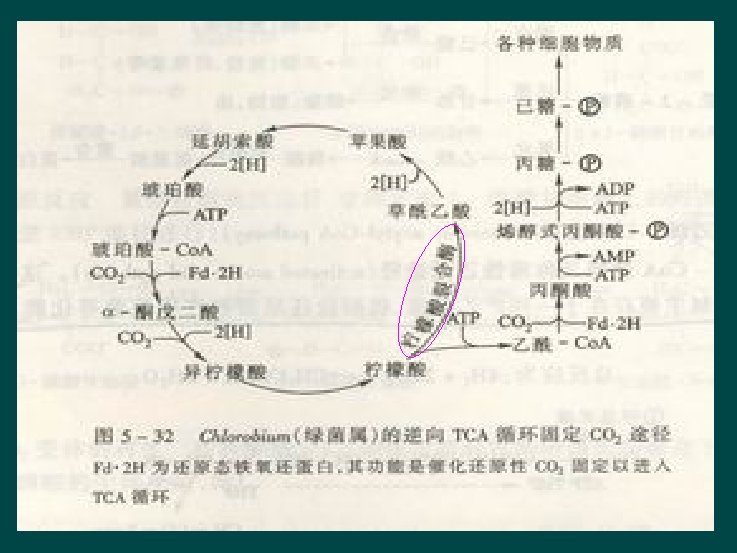
Reverse TCA pathway Ø Microorganism l l Only a little species of Chemoauototrophic ones Chlorobacterium Ø One special enzyme l Citrate lyase Literature II

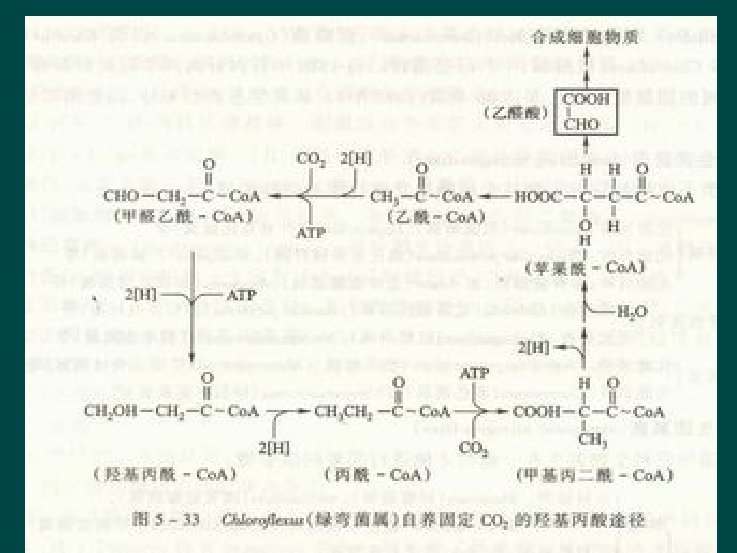
Hydroxypropionate pathway Ø Microorganism l l Only a little species of Chemoauototrophic ones Chlorflexusur Literature III
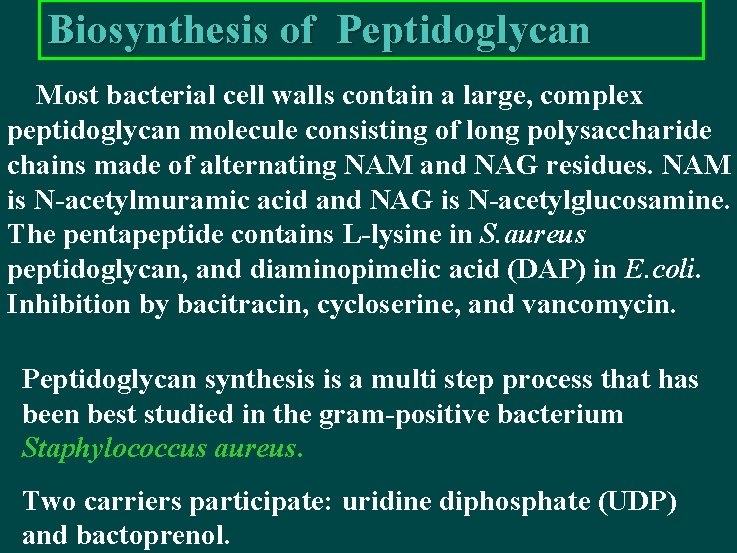
Biosynthesis of Peptidoglycan Most bacterial cell walls contain a large, complex peptidoglycan molecule consisting of long polysaccharide chains made of alternating NAM and NAG residues. NAM is N-acetylmuramic acid and NAG is N-acetylglucosamine. The pentapeptide contains L-lysine in S. aureus peptidoglycan, and diaminopimelic acid (DAP) in E. coli. Inhibition by bacitracin, cycloserine, and vancomycin. Peptidoglycan synthesis is a multi step process that has been best studied in the gram-positive bacterium Staphylococcus aureus. Two carriers participate: uridine diphosphate (UDP) and bactoprenol.
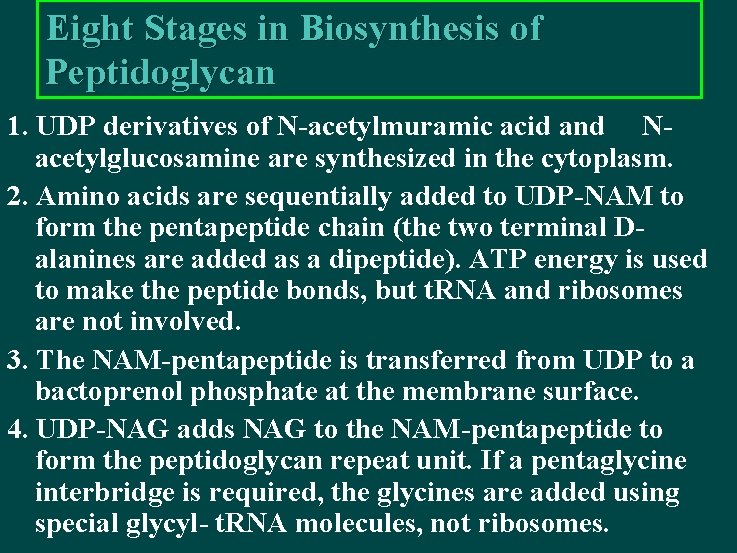
Eight Stages in Biosynthesis of Peptidoglycan 1. UDP derivatives of N-acetylmuramic acid and Nacetylglucosamine are synthesized in the cytoplasm. 2. Amino acids are sequentially added to UDP-NAM to form the pentapeptide chain (the two terminal Dalanines are added as a dipeptide). ATP energy is used to make the peptide bonds, but t. RNA and ribosomes are not involved. 3. The NAM-pentapeptide is transferred from UDP to a bactoprenol phosphate at the membrane surface. 4. UDP-NAG adds NAG to the NAM-pentapeptide to form the peptidoglycan repeat unit. If a pentaglycine interbridge is required, the glycines are added using special glycyl- t. RNA molecules, not ribosomes.
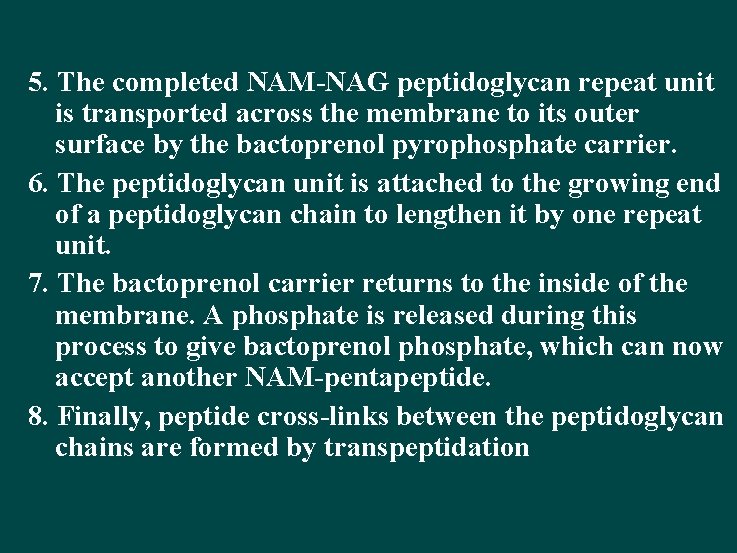
5. The completed NAM-NAG peptidoglycan repeat unit is transported across the membrane to its outer surface by the bactoprenol pyrophosphate carrier. 6. The peptidoglycan unit is attached to the growing end of a peptidoglycan chain to lengthen it by one repeat unit. 7. The bactoprenol carrier returns to the inside of the membrane. A phosphate is released during this process to give bactoprenol phosphate, which can now accept another NAM-pentapeptide. 8. Finally, peptide cross-links between the peptidoglycan chains are formed by transpeptidation
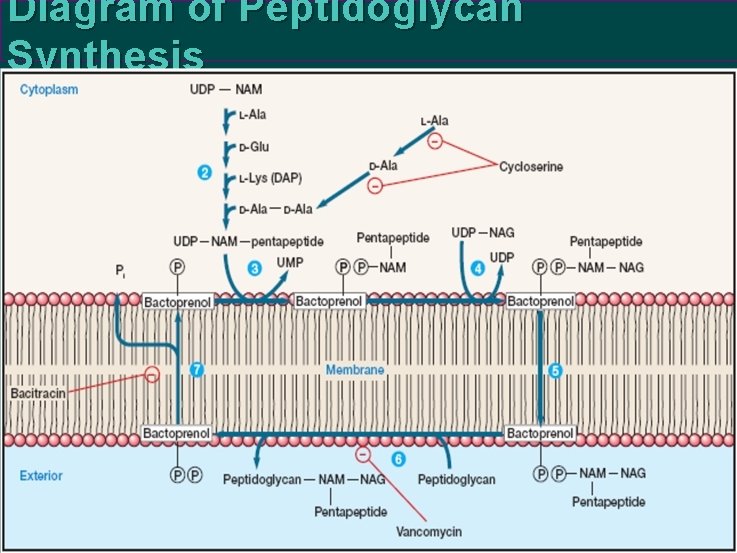
Diagram of Peptidoglycan Synthesis

Transpeptidation

Wall Synthesis Patterns

Nitrogen fixation The utilization of nitrogen gas (N 2) as a source of nitrogen is called nitrogen fixation and is a property of only certain prokaryotes. From the table below it can be seen that a variety of prokaryotes, both anaerobic and aerobic, fix nitrogen. There are some bacteria, called symbiotic, that fix nitrogen only in association with certain plants. As far as is currently known, no eukaryotic organisms fix nitrogen.

Some nitrogen-fixing organisms Free-living aerobes Chemoorganotrophs phototrophs Chemolithotrophs Azotobacter spp. Cyanobacteria Alcaligenes Azomonas (various, but not all) Thiobacillus Beijerinckia Bacillus polymyxa N 2 fixation occurs only under anoxic condition

One of the most interesting and important nitrogen-fixation bacteria is certain type , such as Rhizobium Bradyrhizobium、 Sinorhizobium or Azorhizobium, they can build up symbiosis relationship with leguminous plant.
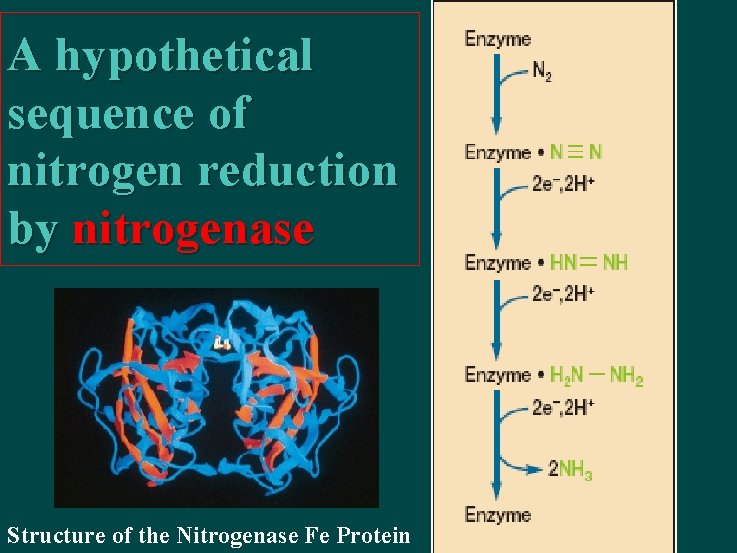
A hypothetical sequence of nitrogen reduction by nitrogenase Structure of the Nitrogenase Fe Protein
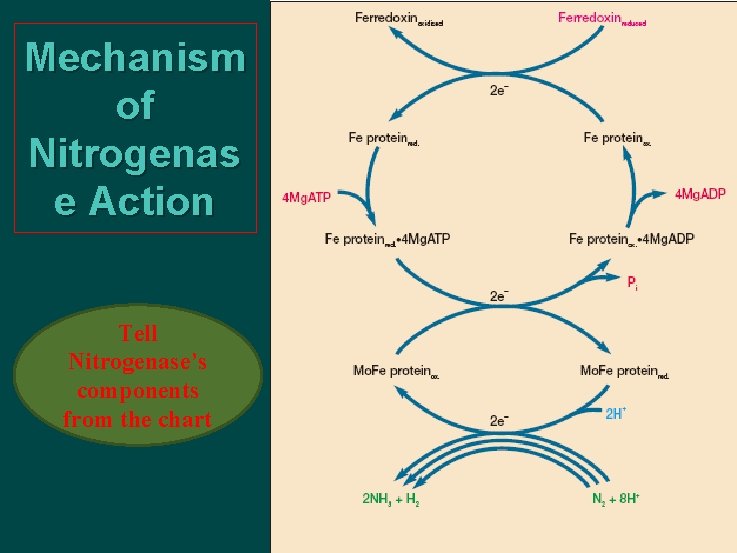
Mechanism of Nitrogenas e Action Tell Nitrogenase’s components from the chart

Protection of Nitrogenase against O 2 Ø Respiration protection l Ø Conformation protection l Ø Azotobacter vinelandii, A. chroococcum Seperation from O 2 l l Ø Azotobacteraceae Anabaena, Nostoc (space separation) Plectomena (time separation) Trichodesmium (cells less-PSIIed at the center of the population) Gloeocapsa (remove by enzymes activity-increased) Bacteriods l Azobacteraceae, Rhizobacteria (leghemoglobin)
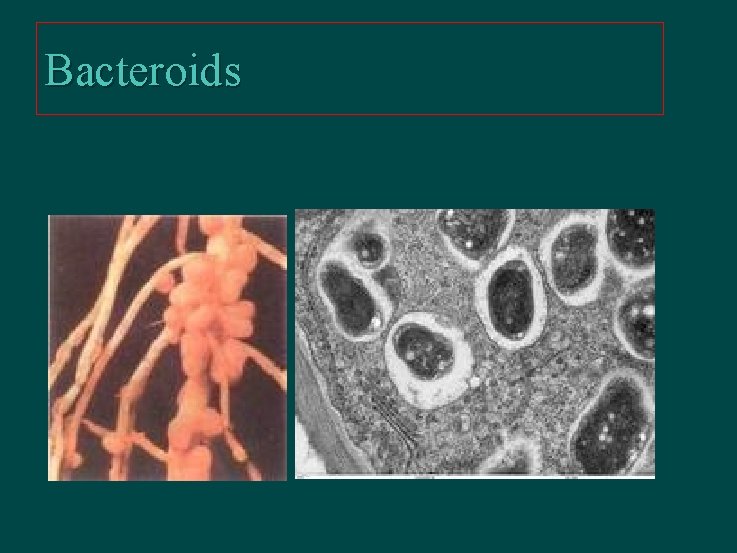
Bacteroids
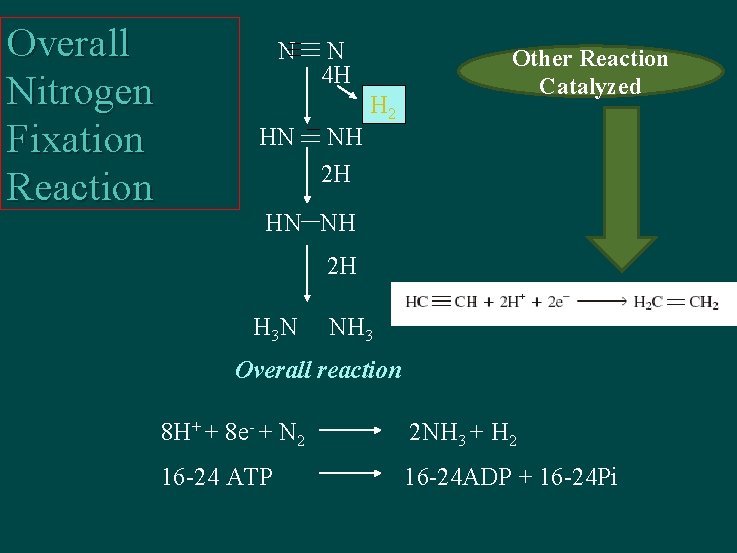
Overall Nitrogen Fixation Reaction N HN N 4 H NH 2 H H 2 Other Reaction Catalyzed HN NH 2 H H 3 N NH 3 Overall reaction 8 H+ + 8 e- + N 2 2 NH 3 + H 2 16 -24 ATP 16 -24 ADP + 16 -24 Pi
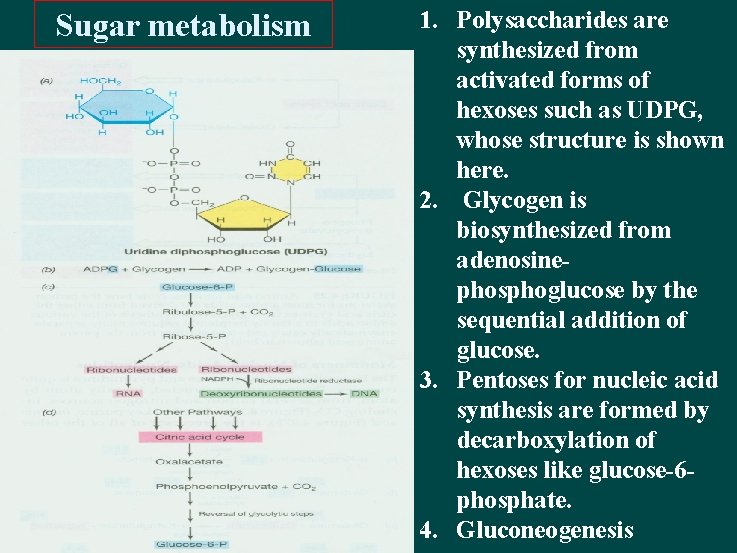
Sugar metabolism 1. Polysaccharides are synthesized from activated forms of hexoses such as UDPG, whose structure is shown here. 2. Glycogen is biosynthesized from adenosinephosphoglucose by the sequential addition of glucose. 3. Pentoses for nucleic acid synthesis are formed by decarboxylation of hexoses like glucose-6 phosphate. 4. Gluconeogenesis
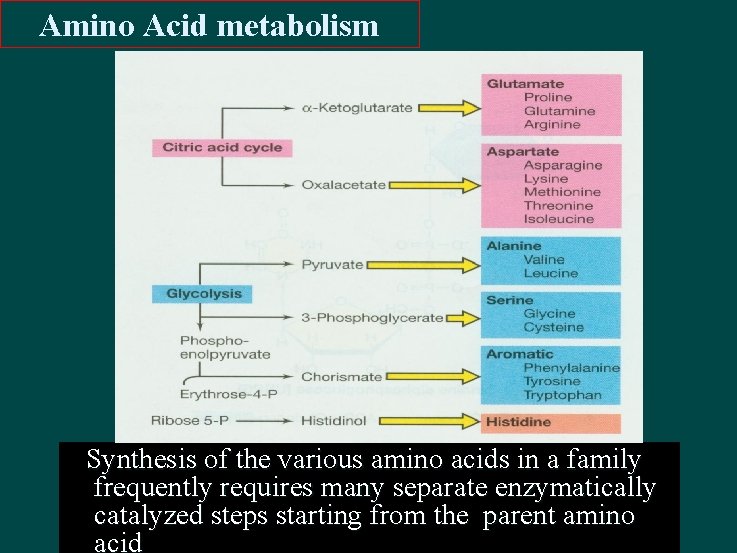
Amino Acid metabolism Synthesis of the various amino acids in a family frequently requires many separate enzymatically catalyzed steps starting from the parent amino acid
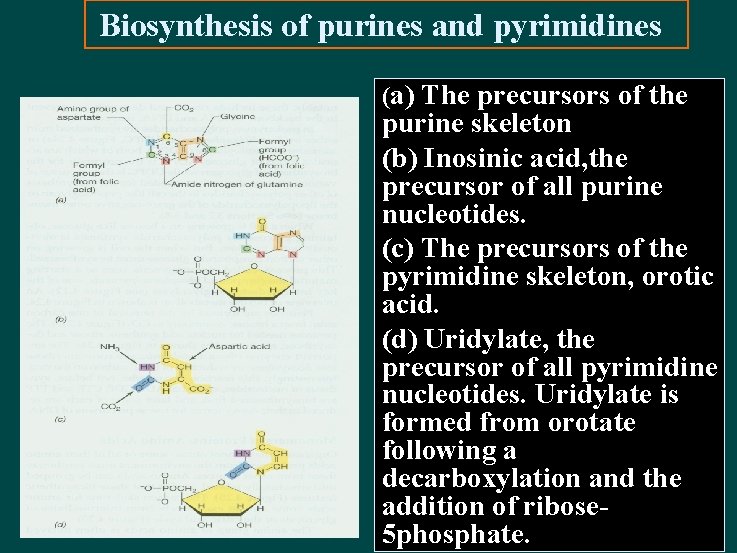
Biosynthesis of purines and pyrimidines (a) The precursors of the purine skeleton (b) Inosinic acid, the precursor of all purine nucleotides. (c) The precursors of the pyrimidine skeleton, orotic acid. (d) Uridylate, the precursor of all pyrimidine nucleotides. Uridylate is formed from orotate following a decarboxylation and the addition of ribose 5 phosphate.
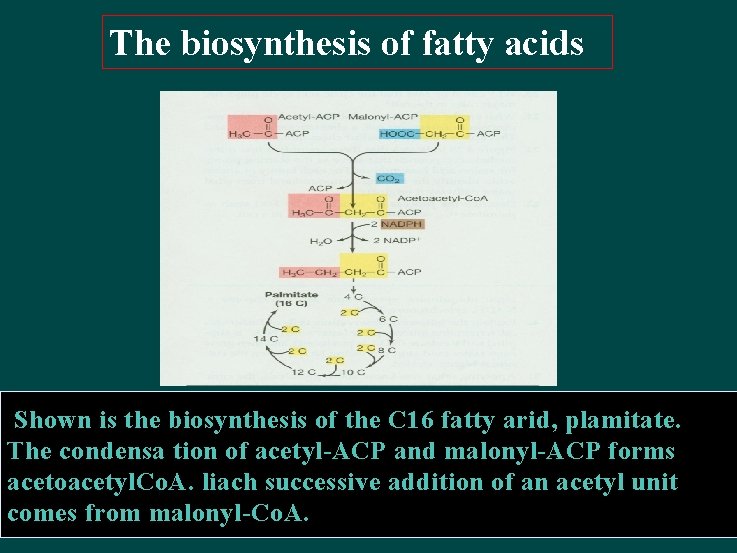
The biosynthesis of fatty acids Shown is the biosynthesis of the C 16 fatty arid, plamitate. The condensa tion of acetyl-ACP and malonyl-ACP forms acetoacetyl. Co. A. liach successive addition of an acetyl unit comes from malonyl-Co. A.

Review Questions 1. Why might it be desirable for a microorganism with the Embden-Meyerhof pathway and the TCA cycle also to have the pentose phosphate pathway? 2. How do substrate-level phosphorylation and oxidative phosphorylation differ from one another? 3. Can fermentation products be used in identifying bacteria? Give some examples if the answer is yes.

4. Suppose that you isolated a bacteria strain that carried out oxygenic photosynthesis. What photosystems would it most likely belong to? 5. Predict some conditions that would descible niches occupied by green and purple photosynthetic bacteria. 6. From an evolutionary perspective, discuss why all microorganisms (with only some rare exceptions) use aerobic respiration to generate ATP. 7. Suppose that a microorganism was growing on a medium that contained amino acids but no sugars. In general terms how would it synthesize the pentoses and hexoses it required?

7. Which two enzymes discussed in the chapter appear to be specific to the Calvin cycle? 8. What is unusual about the synthesis of peptides that takes place during peptidoglycan construction?
- Slides: 98