Chapter 7 Ionic and Metallic Bonding Section 1
Chapter 7: Ionic and Metallic Bonding Section 1: Ions
Valence Electrons • Valence Electrons – Electrons. . – Determines … • Representative elements only – Group number = – Exception:

Practice For each element, state the number of valence electrons: Cl: Ba: Ga: N: K:
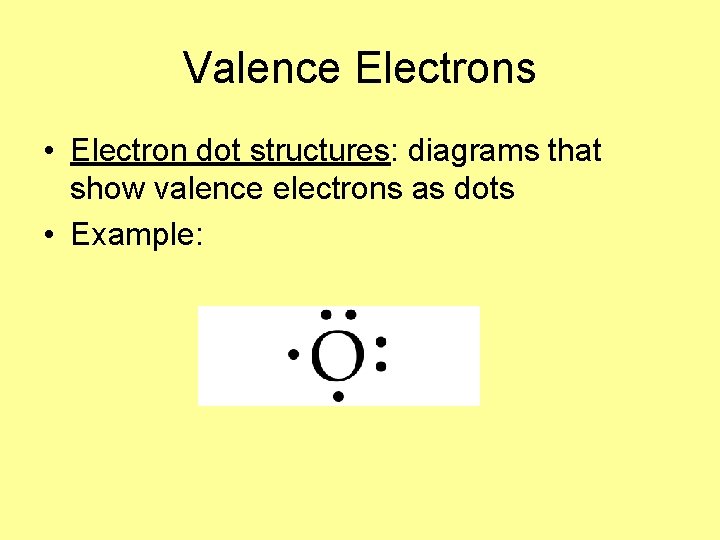
Valence Electrons • Electron dot structures: diagrams that show valence electrons as dots • Example:

Valence electrons • Valence electrons – Correspond to the… – All dot structures in a group are… • Example: Boron – Group: – Valence electrons: – Short form: – Electron dot structure:
Practice Write the electron dot structures for the following elements: Ar H Sn Mg
The Octet Rule • Octet Rule – Elements tend to form the electron configuration of a __________. – Electron configuration: _____ • Atoms of metallic elements – tend to ____ valence electrons – complete octet in the …. • Atoms of nonmetallic elements – tend to _____ or _______valence electrons – Complete octet in the same _______energy level.
Formation of Cations • Atoms are _______! (electrons = protons) • Cation – Atom _____ an electron to achieve the octet rule – Becomes ion with a _____ charge • Most common in _____. • Properties _____.
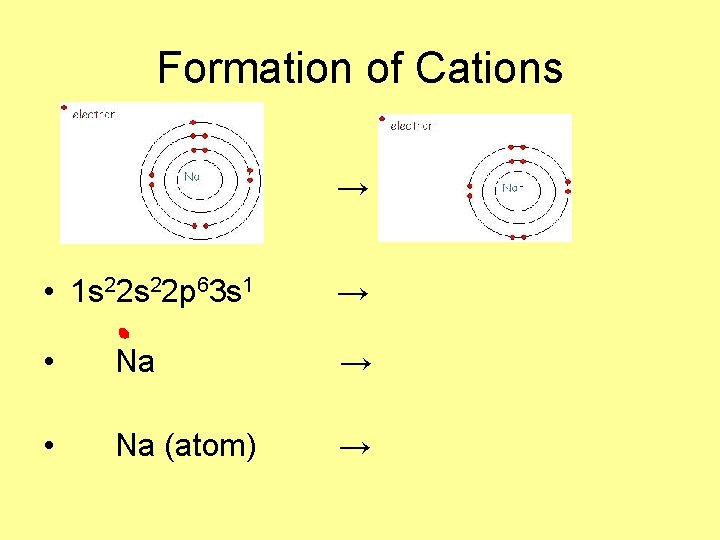
Formation of Cations → • 1 s 22 p 63 s 1 → • Na (atom) →
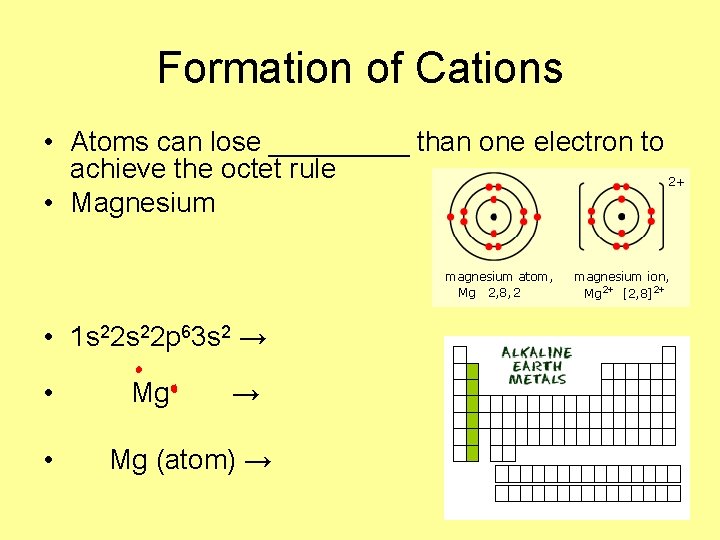
Formation of Cations • Atoms can lose _____ than one electron to achieve the octet rule • Magnesium • 1 s 22 p 63 s 2 → • • Mg → Mg (atom) →
Formation of Cations • Transition metals – Ions formed ____ • Fe → _______ or ____ – do not form _________ (s 2 p 6) – exception to the ________ – unlikely to lose or gain more than _______.

Formation of Cations • Transition metals – Lose electrons to achieve a filled ____ sublevel – Not as favorable as ________ – more stable than _______ configuration – “_______ noble-gas configuration” – lose electrons from the ____ sublevel

Practice • Ag 1 s 22 p 63 s 23 p 63 d 104 s 24 p 64 d 105 s 1 – What does silver need to do to acquire a more stable configuration? – What would be the charge of silver?

Formation of Cations • Naming Cations – _____ change their name – Add “______” to the name of the element – For example: • Na+: ______ • Mg 2+: ______
Formation of Anions • Atoms are ____ (electrons = protons) • Anions – Atoms that _____ an electron – Become ions with a ______ charge • Most common in ______
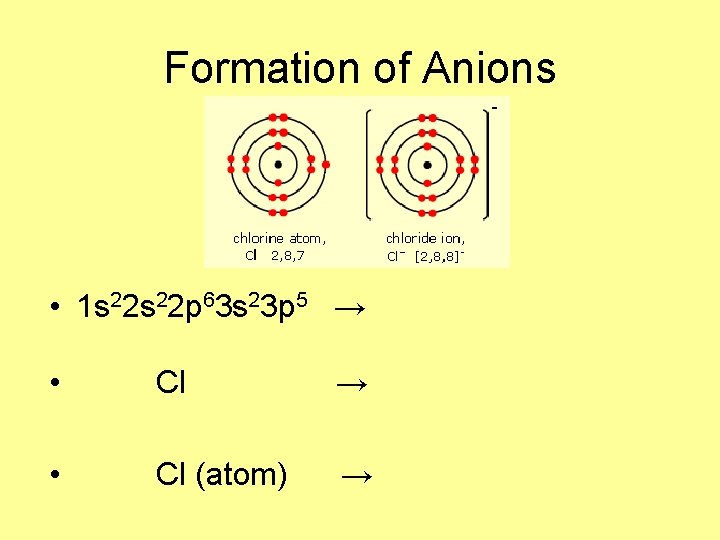
Formation of Anions • 1 s 22 p 63 s 23 p 5 → • Cl → • Cl (atom) →
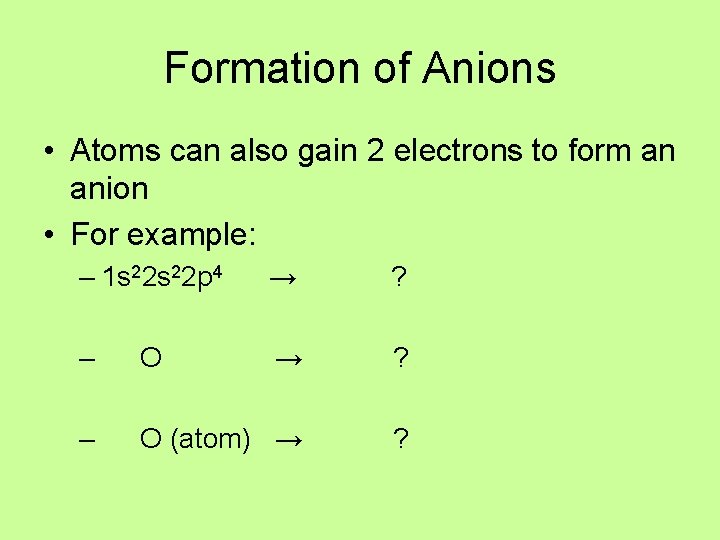
Formation of Anions • Atoms can also gain 2 electrons to form an anion • For example: – 1 s 22 p 4 → ? – O (atom) → ?
Naming of Anions • Naming Anions – Generally end in the suffix “____” – Examples • Chlorine atom (Cl) → • Oxygen atom (O) → • Ions formed from _____ – “halide ions” – charge of _______
- Slides: 18