Chapter 7 Intermolecular Forces and the Phases of








- Slides: 38

Chapter 7 – Intermolecular Forces and the Phases of Matter Please turn cell phones off 3. 5 © 2010 -13 J. E. Johnson (Worksheet available on Web. CT) 1

7. 1 Why Does Matter Exist in Different Phases? From Chapter 1, Physical Changes: Ø Changes in matter that do not result in changing the molecules present e. g. ) Phase changes: physical changes from one “phase” or “state” of matter to another © 2010 -13 J. E. Johnson 2

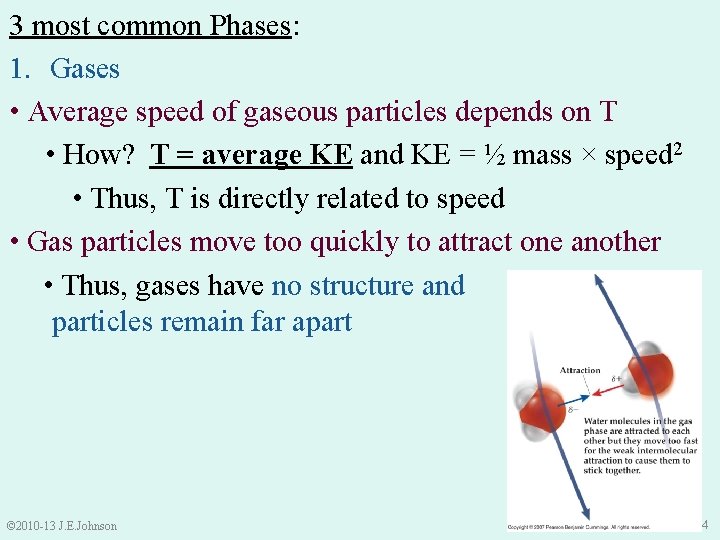
3 most common Phases: 1. Gases • Average speed of gaseous particles depends on T • How? T = average KE and KE = ½ mass × speed 2 • Thus, T is directly related to speed © 2010 -13 J. E. Johnson 3

3 most common Phases: 1. Gases • Average speed of gaseous particles depends on T • How? T = average KE and KE = ½ mass × speed 2 • Thus, T is directly related to speed • Gas particles move too quickly to attract one another • Thus, gases have no structure and particles remain far apart © 2010 -13 J. E. Johnson 4

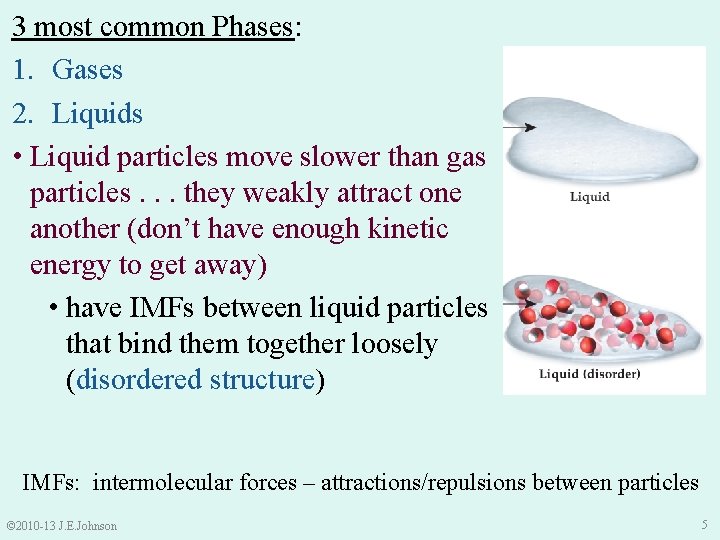
3 most common Phases: 1. Gases 2. Liquids • Liquid particles move slower than gas particles. . . they weakly attract one another (don’t have enough kinetic energy to get away) • have IMFs between liquid particles that bind them together loosely (disordered structure) IMFs: intermolecular forces – attractions/repulsions between particles © 2010 -13 J. E. Johnson 5

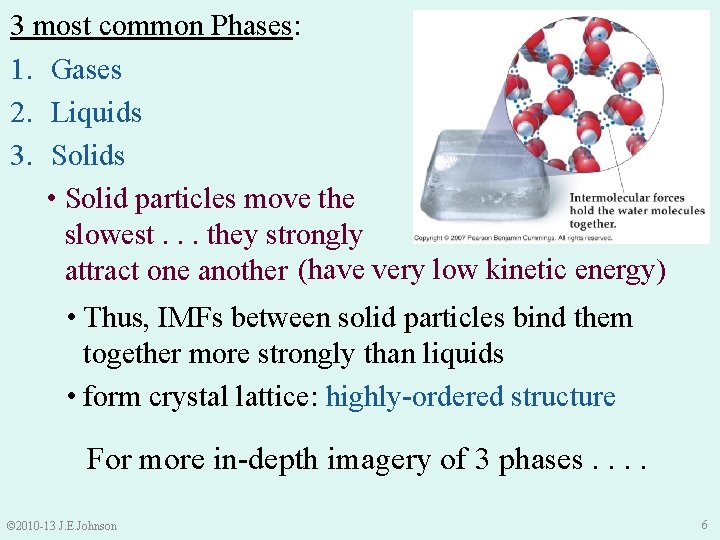
3 most common Phases: 1. Gases 2. Liquids 3. Solids • Solid particles move the slowest. . . they strongly attract one another (have very low kinetic energy) • Thus, IMFs between solid particles bind them together more strongly than liquids • form crystal lattice: highly-ordered structure For more in-depth imagery of 3 phases. . © 2010 -13 J. E. Johnson 6

Ø Moving among phases (Phase Changes): 1. Vaporization (l → g) • reverse: Condensation (g → l) 2. Fusion (s → l) • reverse: Crystallization (l → s) 3. Sublimation (s → g) • reverse: Deposition (g → s) fusion deposition sublimation condensation © 2010 -13 J. E. Johnson 7

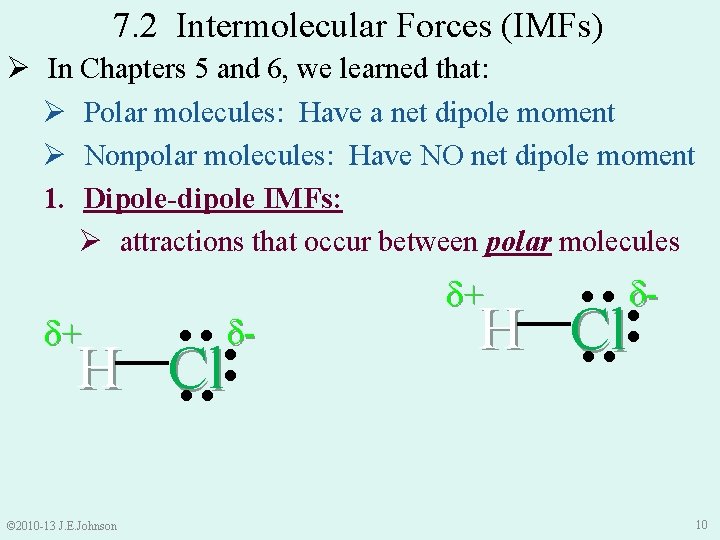
7. 2 Intermolecular Forces (IMFs) Ø In Chapters 5 and 6, we learned that: Ø Polar molecules: Have a net dipole moment Ø Nonpolar molecules: Have NO net dipole moment 1. Dipole-dipole IMFs: Ø attractions that occur between polar molecules ●● ●● © 2010 -13 J. E. Johnson ●● H Cl δ- ●● H Cl ●● δ- ●● δ+ δ+ 10

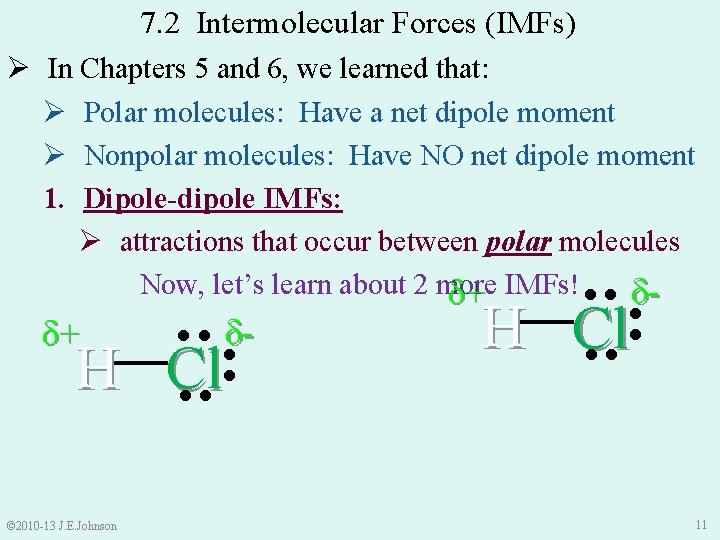
7. 2 Intermolecular Forces (IMFs) Ø In Chapters 5 and 6, we learned that: Ø Polar molecules: Have a net dipole moment Ø Nonpolar molecules: Have NO net dipole moment 1. Dipole-dipole IMFs: Ø attractions that occur between polar molecules Now, let’s learn about 2 more δ+ IMFs! ● ● δ●● ●● © 2010 -13 J. E. Johnson ●● H Cl δ- H Cl ●● ●● δ+ 11

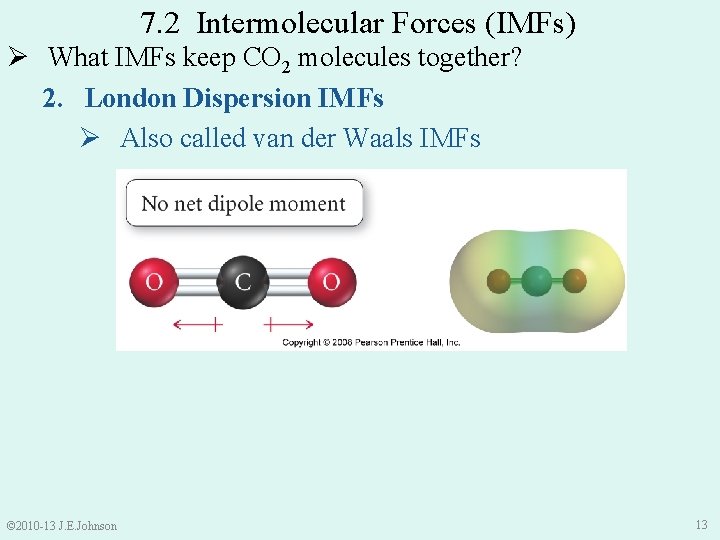
7. 2 Intermolecular Forces (IMFs) Ø In Chapter 6, we discovered that: Ø CO 2 is a nonpolar molecule So, what IMFs keep CO 2(s) molecules together? © 2010 -13 J. E. Johnson 12

7. 2 Intermolecular Forces (IMFs) Ø What IMFs keep CO 2 molecules together? 2. London Dispersion IMFs Ø Also called van der Waals IMFs © 2010 -13 J. E. Johnson 13

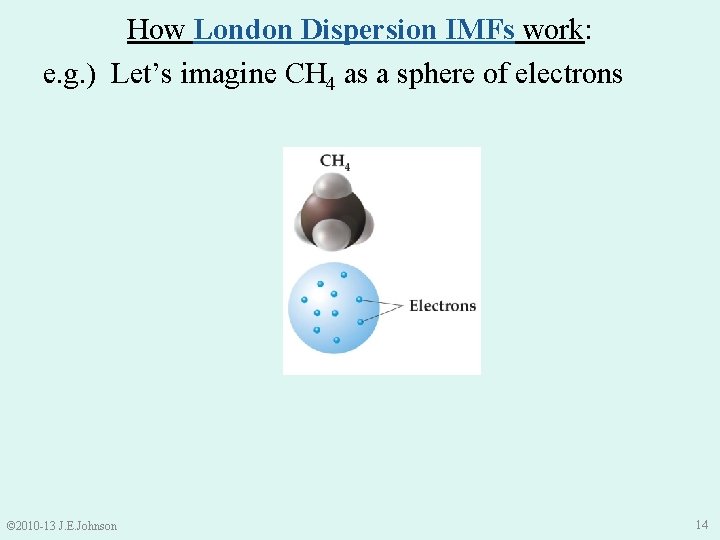
How London Dispersion IMFs work: e. g. ) Let’s imagine CH 4 as a sphere of electrons © 2010 -13 J. E. Johnson 14

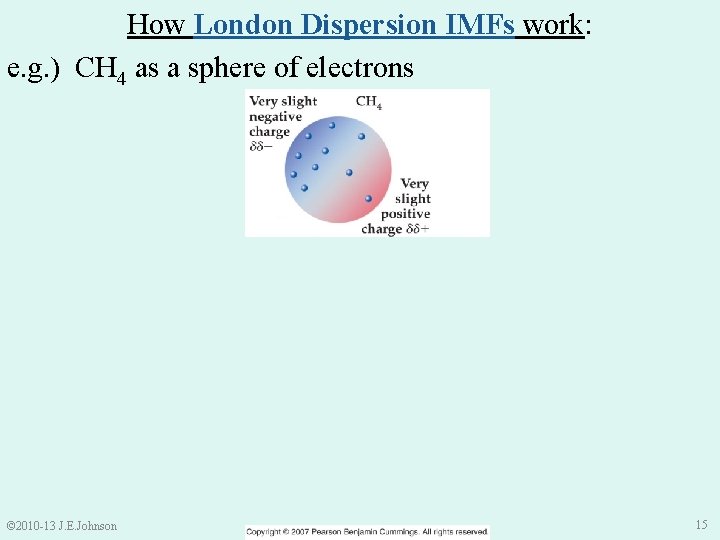
How London Dispersion IMFs work: e. g. ) CH 4 as a sphere of electrons © 2010 -13 J. E. Johnson 15

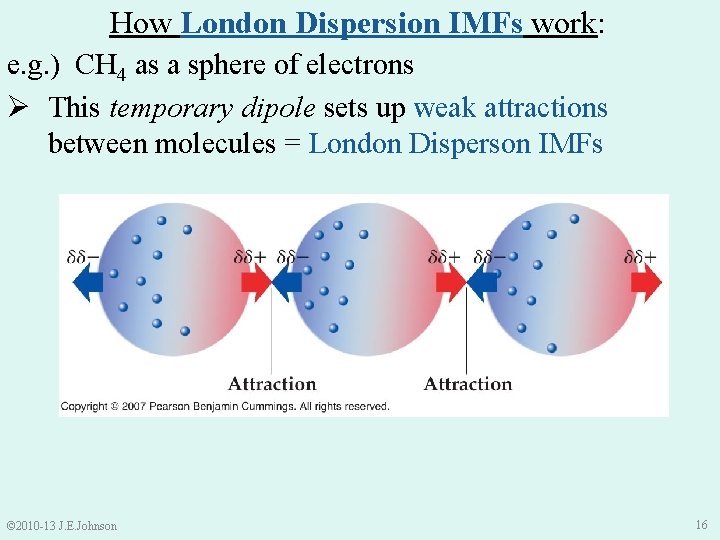
How London Dispersion IMFs work: e. g. ) CH 4 as a sphere of electrons Ø This temporary dipole sets up weak attractions between molecules = London Disperson IMFs © 2010 -13 J. E. Johnson 16

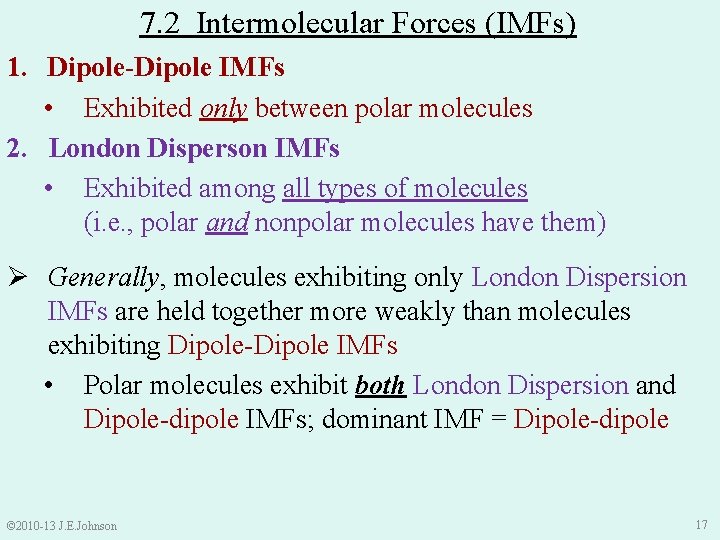
7. 2 Intermolecular Forces (IMFs) 1. Dipole-Dipole IMFs • Exhibited only between polar molecules 2. London Disperson IMFs • Exhibited among all types of molecules (i. e. , polar and nonpolar molecules have them) Ø Generally, molecules exhibiting only London Dispersion IMFs are held together more weakly than molecules exhibiting Dipole-Dipole IMFs • Polar molecules exhibit both London Dispersion and Dipole-dipole IMFs; dominant IMF = Dipole-dipole © 2010 -13 J. E. Johnson 17

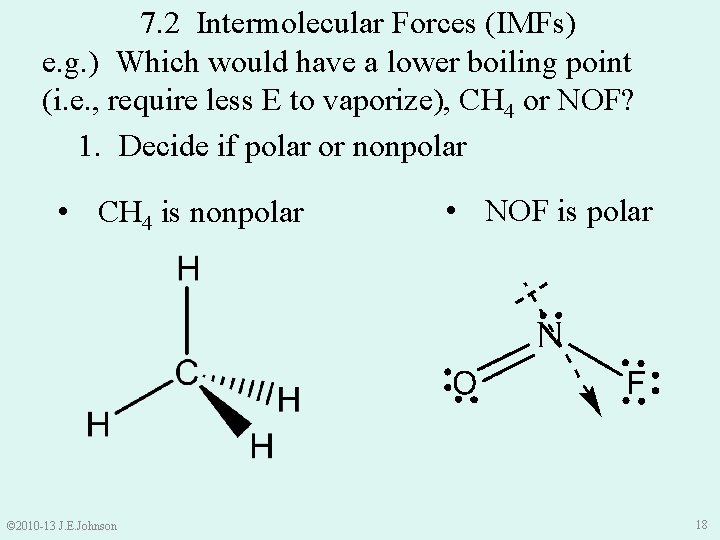
7. 2 Intermolecular Forces (IMFs) e. g. ) Which would have a lower boiling point (i. e. , require less E to vaporize), CH 4 or NOF? 1. Decide if polar or nonpolar • CH 4 is nonpolar © 2010 -13 J. E. Johnson • NOF is polar 18

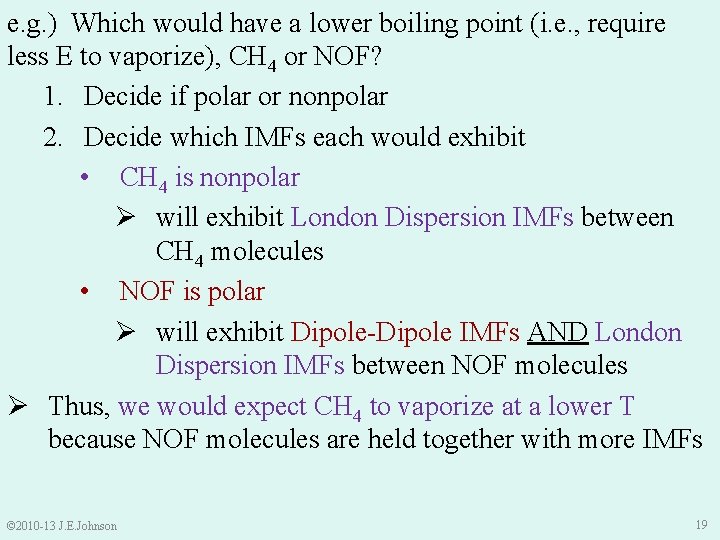
e. g. ) Which would have a lower boiling point (i. e. , require less E to vaporize), CH 4 or NOF? 1. Decide if polar or nonpolar 2. Decide which IMFs each would exhibit • CH 4 is nonpolar Ø will exhibit London Dispersion IMFs between CH 4 molecules • NOF is polar Ø will exhibit Dipole-Dipole IMFs AND London Dispersion IMFs between NOF molecules Ø Thus, we would expect CH 4 to vaporize at a lower T because NOF molecules are held together with more IMFs © 2010 -13 J. E. Johnson 19

1. Dipole-dipole IMFs • Exhibited only between polar molecules 2. London Disperson IMFs • Exhibited between all types of molecules 3. Hydrogen Bonding IMFs • A unique dipole-dipole interaction that is exhibited only between polar molecules possessing H attached to F, O, or N • Due to large ∆EN and small size of atoms involved • Thus, molecules can get close © 2010 -13 J. E. Johnson 20

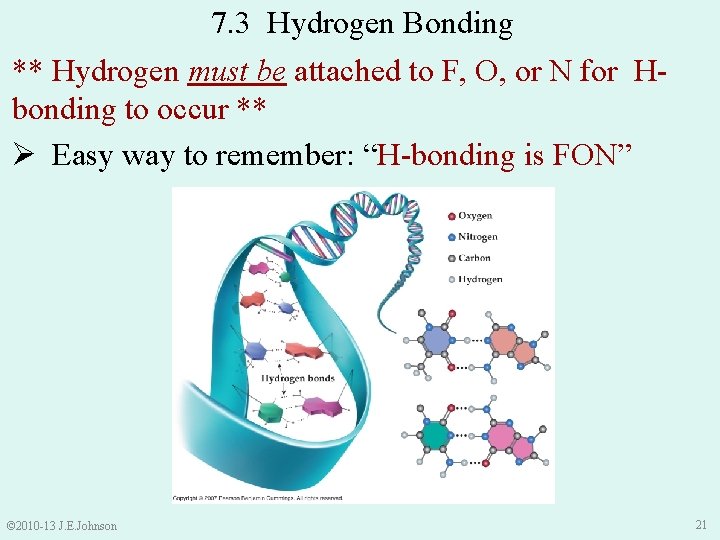
7. 3 Hydrogen Bonding ** Hydrogen must be attached to F, O, or N for Hbonding to occur ** Ø Easy way to remember: “H-bonding is FON” © 2010 -13 J. E. Johnson 21

1. Dipole-dipole IMFs: Exhibited only between polar molecules 2. London Disperson IMFs: Exhibited between all types of molecules 3. Hydrogen Bonding IMFs: Exhibited only between polar molecules possessing H attached to F, O, or N Ø Generally, molecules exhibiting only London Dispersion or Dipole-Dipole IMFs are held more weakly than molecules exhibiting Hydrogen Bonding IMFs • Polar molecules with H attached to F, O, or N are held together by London Dispersion, Dipole-Dipole, AND Hydrogen Bonding IMFs; dominant IMF = Hydrogen Bonding © 2010 -13 J. E. Johnson 22

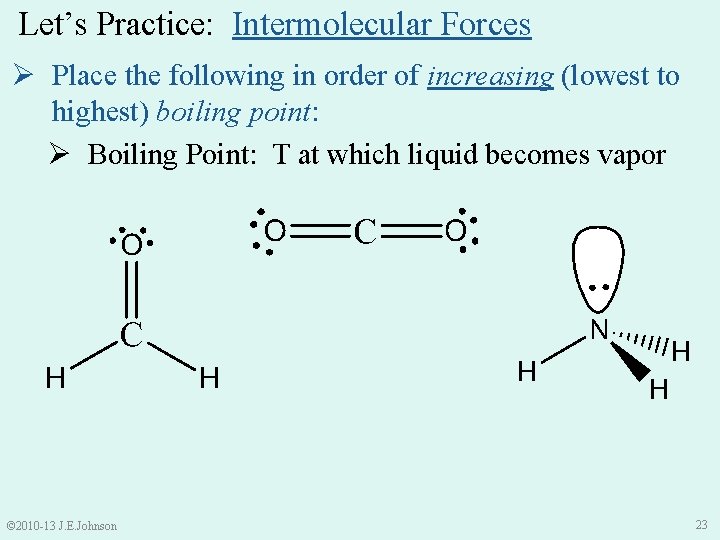
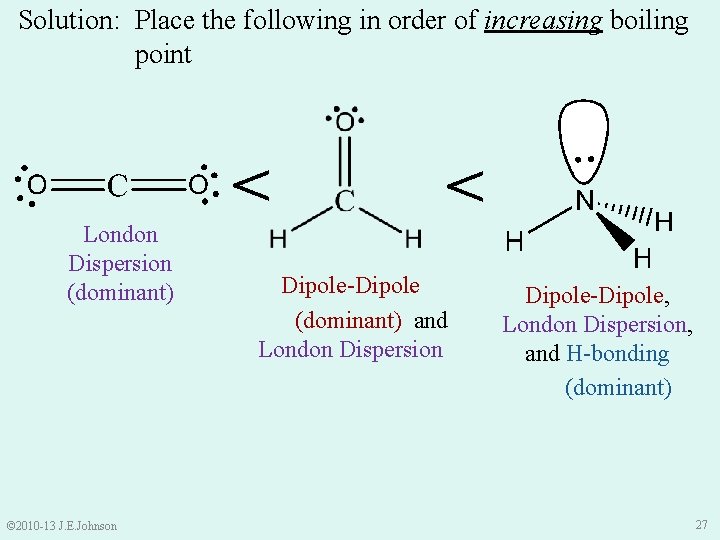
Let’s Practice: Intermolecular Forces Ø Place the following in order of increasing (lowest to highest) boiling point: Ø Boiling Point: T at which liquid becomes vapor © 2010 -13 J. E. Johnson 23

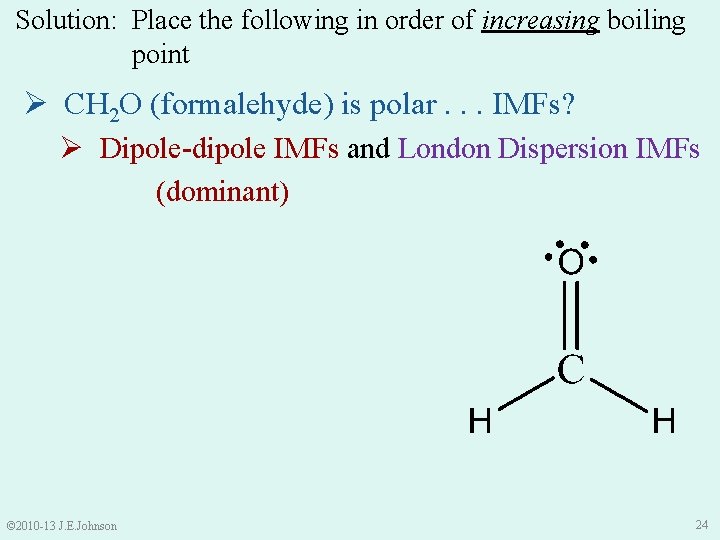
Solution: Place the following in order of increasing boiling point Ø CH 2 O (formalehyde) is polar. . . IMFs? Ø Dipole-dipole IMFs and London Dispersion IMFs (dominant) © 2010 -13 J. E. Johnson 24

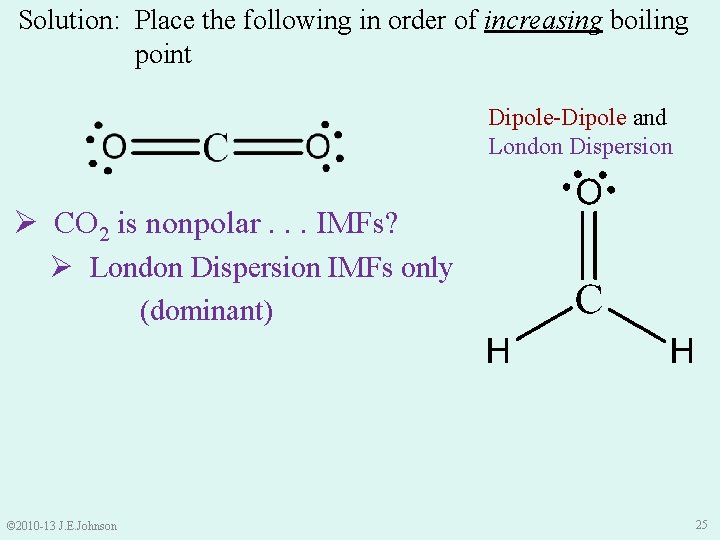
Solution: Place the following in order of increasing boiling point Dipole-Dipole and London Dispersion Ø CO 2 is nonpolar. . . IMFs? Ø London Dispersion IMFs only (dominant) © 2010 -13 J. E. Johnson 25

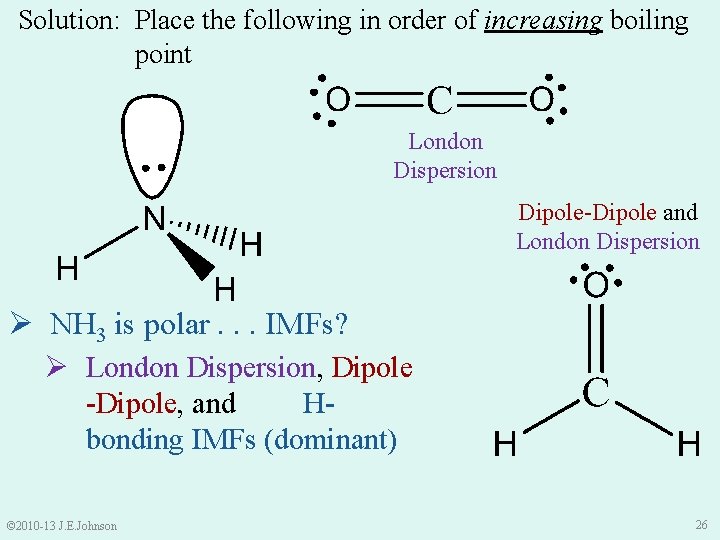
Solution: Place the following in order of increasing boiling point London Dispersion Dipole-Dipole and London Dispersion Ø NH 3 is polar. . . IMFs? Ø London Dispersion, Dipole -Dipole, and Hbonding IMFs (dominant) © 2010 -13 J. E. Johnson 26

Solution: Place the following in order of increasing boiling point > © 2010 -13 J. E. Johnson > London Dispersion (dominant) Dipole-Dipole (dominant) and London Dispersion Dipole-Dipole, London Dispersion, and H-bonding (dominant) 27

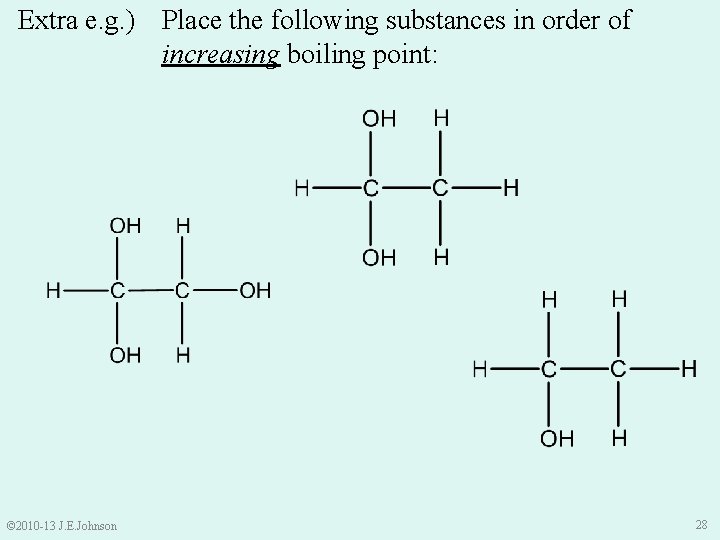
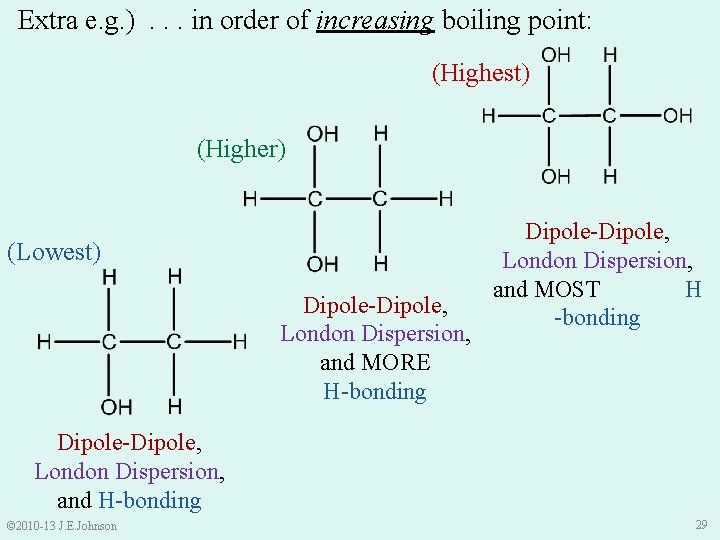
Extra e. g. ) Place the following substances in order of increasing boiling point: © 2010 -13 J. E. Johnson 28

Extra e. g. ). . . in order of increasing boiling point: (Highest) (Higher) (Lowest) Dipole-Dipole, London Dispersion, and MORE H-bonding Dipole-Dipole, London Dispersion, and MOST H -bonding Dipole-Dipole, London Dispersion, and H-bonding © 2010 -13 J. E. Johnson 29

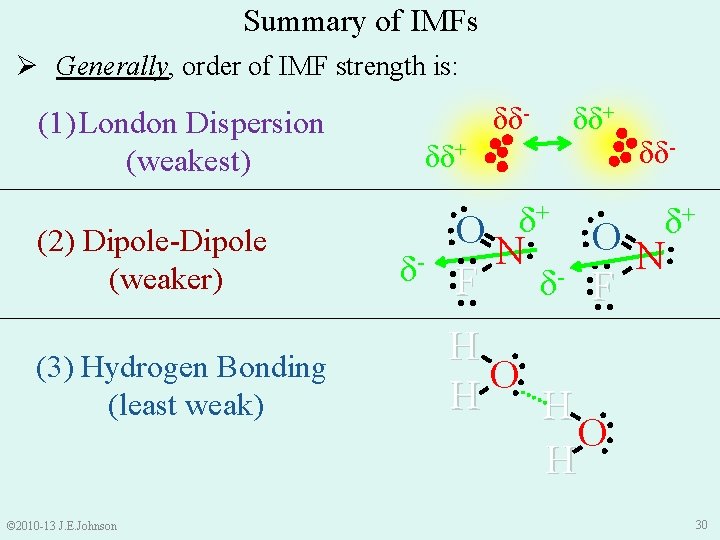
Summary of IMFs Ø Generally, order of IMF strength is: (1) London Dispersion (weakest) © 2010 -13 J. E. Johnson δδ- + + δ δ : O N: : O : N δ- : F : : : (3) Hydrogen Bonding (least weak) δδ+ : : : (2) Dipole-Dipole (weaker) δδ- H : O: H 30

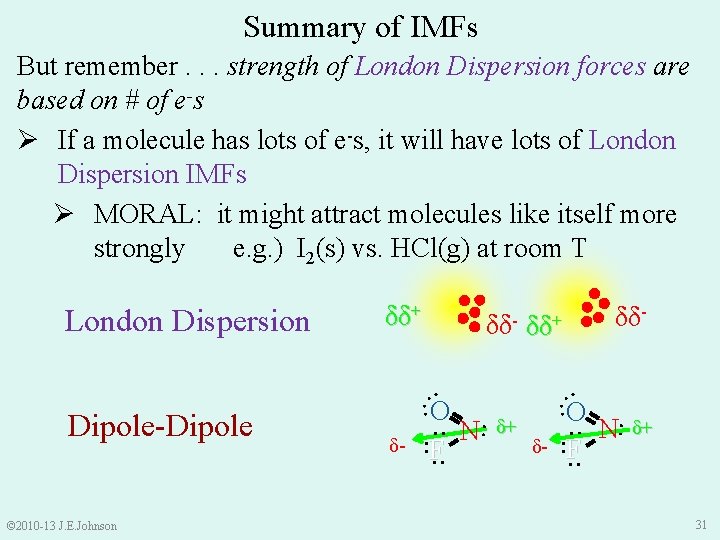
Summary of IMFs But remember. . . strength of London Dispersion forces are based on # of e-s Ø If a molecule has lots of e-s, it will have lots of London Dispersion IMFs Ø MORAL: it might attract molecules like itself more strongly e. g. ) I 2(s) vs. HCl(g) at room T London Dispersion δδ+ δδ- : : O : F N: δ+ δ- : : © 2010 -13 J. E. Johnson δ- : : Dipole-Dipole δδ- δδ+ : F N: δ+ 31

Two types of Solids From Chapter 5: 1. Molecular solids: made up of individual molecules and are held together by weak IMFs • melting/boiling points dependent on relative IMF strengths ice melts ~ 0 o. C CO 2(s) sublimes – 79 o. C Se 8 melts ~221 o. C © 2010 -13 J. E. Johnson 32

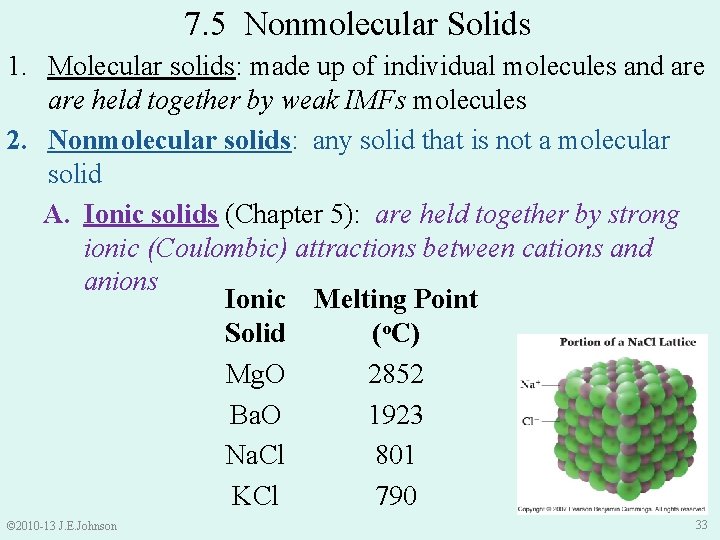
7. 5 Nonmolecular Solids 1. Molecular solids: made up of individual molecules and are held together by weak IMFs molecules 2. Nonmolecular solids: any solid that is not a molecular solid A. Ionic solids (Chapter 5): are held together by strong ionic (Coulombic) attractions between cations and anions Ionic Melting Point Solid (o. C) Mg. O 2852 Ba. O 1923 Na. Cl 801 KCl 790 © 2010 -13 J. E. Johnson 33

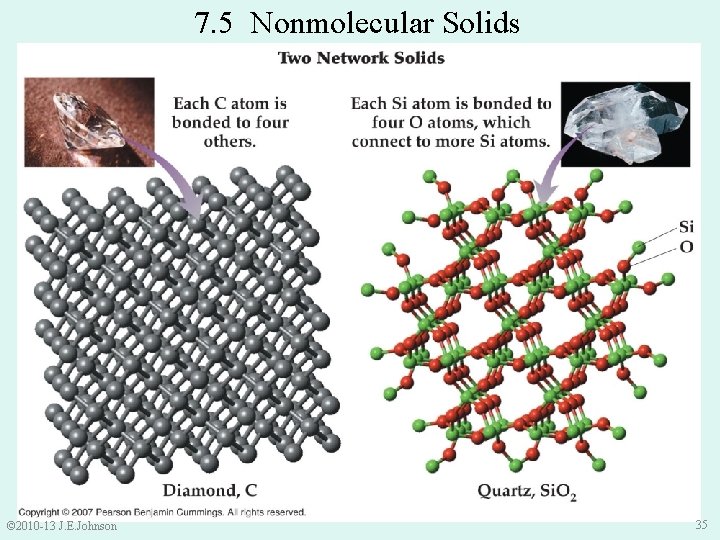
1. Molecular solids: made up of individual molecules and are held together by IMFs molecules 2. Nonmolecular solids: any solid that is not a molecular solid A. Ionic solids: are held together by strong ionic (Coulombic) attractions between cations and anions (high melting points > 1000 o. C) B. Network – covalent solids: held together by covalent forces between atoms • have even higher melting/boiling points e. g) diamond melts ~ 3550 o. C Si. O 2 melts ~ 1610 o. C © 2010 -13 J. E. Johnson 34

7. 5 Nonmolecular Solids © 2010 -13 J. E. Johnson 35

1. Molecular solids: made up of individual molecules and are held together by IMFs molecules 2. Nonmolecular solids: any solid that is not a molecular solid A. Ionic solids: are held together by strong ionic (Coulombic) attractions between cations and anions (high melting points > 1000 o. C) B. Network – covalent solids: held together by covalent forces between atoms (even higher melting/boiling pts) C. Metallic solid: held together by metallic bonding between atoms • have variable melting and boiling points © 2010 -13 J. E. Johnson 36

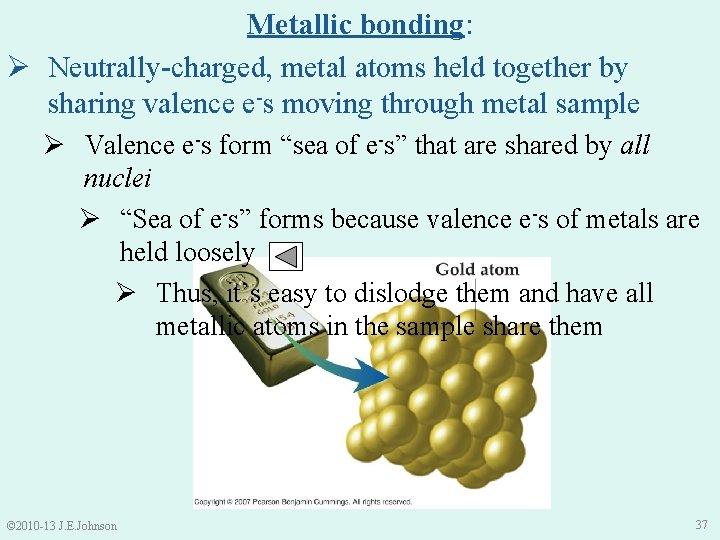
Metallic bonding: Ø Neutrally-charged, metal atoms held together by sharing valence e-s moving through metal sample Ø Valence e-s form “sea of e-s” that are shared by all nuclei Ø “Sea of e-s” forms because valence e-s of metals are held loosely Ø Thus, it’s easy to dislodge them and have all metallic atoms in the sample share them © 2010 -13 J. E. Johnson 37

Let’s Practice: Types of Solids Ø Classify the following solids as MOLECULAR, IONIC, or METALLIC. Which would conduct electricity? A) I 2(s) B) Ca. Br 2 (s) C) NH 4 NO 3(s) D) K(s) © 2010 -13 J. E. Johnson 38

Solution: Classify the following solids. Which would conduct electricity? A) I 2(s) • Molecular solid B) Ca. Br 2 (s) • Ionic solid C) NH 4 NO 3(s) • Ionic solid D) K(s) • Metallic solid © 2010 -13 J. E. Johnson 39

** DON’T FORGET ** Take the Chapter 7 Quiz - it’s available on Web. CT after 7 pm tonight! © 2010 -13 J. E. Johnson 40