Chapter 7 Formulas Compounds 7 2 Using Chemical
- Slides: 17

Chapter 7 – Formulas & Compounds 7. 2 – Using Chemical Formulas Main Ideas: a. Formula mass is the sum of the atomic masses of a compound’s atoms. b. The molar mass of a compound is numerically equal to its formula mass. c. Molar mass is used to convert from moles to grams d. Percentage composition is the mass ratio of an element present in a compound to the molar mass of the compound.

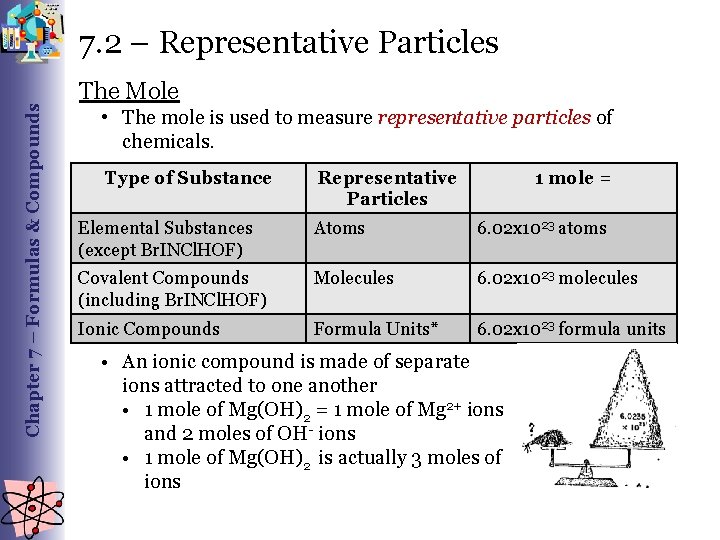
Chapter 7 – Formulas & Compounds 7. 2 – Representative Particles The Mole • The mole is used to measure representative particles of chemicals. Type of Substance Representative Particles 1 mole = Elemental Substances (except Br. INCl. HOF) Atoms 6. 02 x 1023 atoms Covalent Compounds (including Br. INCl. HOF) Molecules 6. 02 x 1023 molecules Ionic Compounds Formula Units* 6. 02 x 1023 formula units • An ionic compound is made of separate ions attracted to one another • 1 mole of Mg(OH)2 = 1 mole of Mg 2+ ions and 2 moles of OH- ions • 1 mole of Mg(OH)2 is actually 3 moles of ions

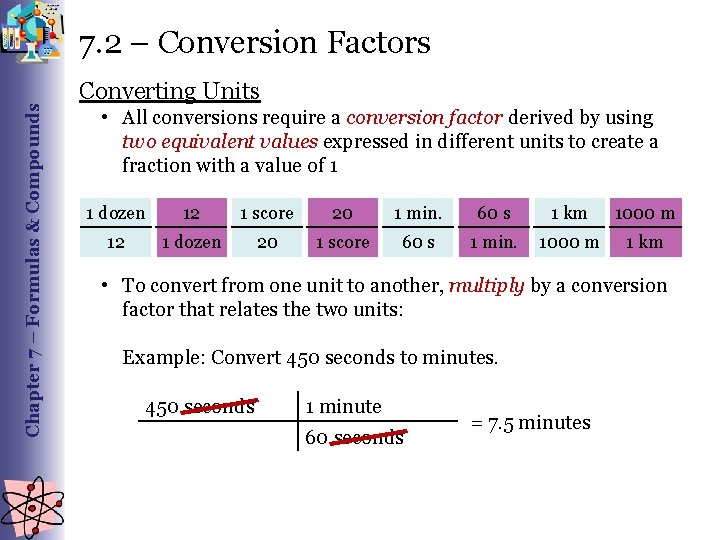
Chapter 7 – Formulas & Compounds 7. 2 – Conversion Factors Converting Units • All conversions require a conversion factor derived by using two equivalent values expressed in different units to create a fraction with a value of 1 1 dozen 12 1 score 20 1 min. 60 s 1 km 1000 m 12 1 dozen 20 1 score 60 s 1 min. 1000 m 1 km • To convert from one unit to another, multiply by a conversion factor that relates the two units: Example: Convert 450 seconds to minutes. 450 seconds 1 minute 60 seconds = 7. 5 minutes

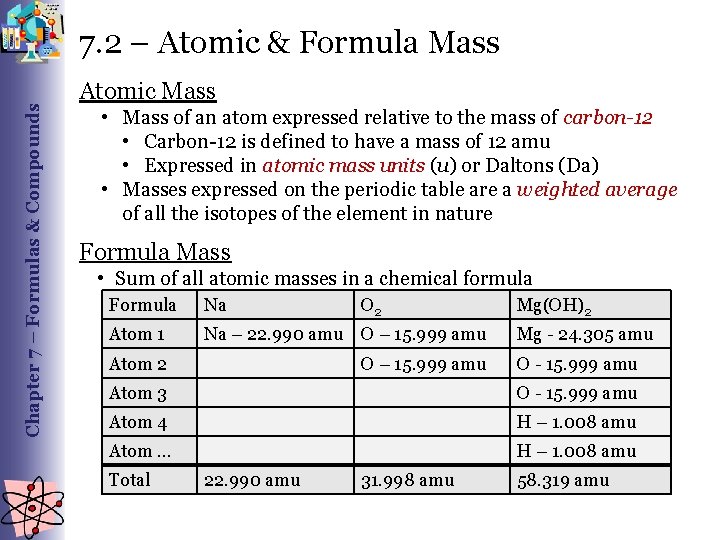
Chapter 7 – Formulas & Compounds 7. 2 – Atomic & Formula Mass Atomic Mass • Mass of an atom expressed relative to the mass of carbon-12 • Carbon-12 is defined to have a mass of 12 amu • Expressed in atomic mass units (u) or Daltons (Da) • Masses expressed on the periodic table are a weighted average of all the isotopes of the element in nature Formula Mass • Sum of all atomic masses in a chemical formula Formula Na O 2 Atom 1 Na – 22. 990 amu O – 15. 999 amu Atom 2 O – 15. 999 amu Mg(OH)2 Mg - 24. 305 amu O - 15. 999 amu Atom 3 O - 15. 999 amu Atom 4 H – 1. 008 amu Atom … H – 1. 008 amu Total 22. 990 amu 31. 998 amu 58. 319 amu

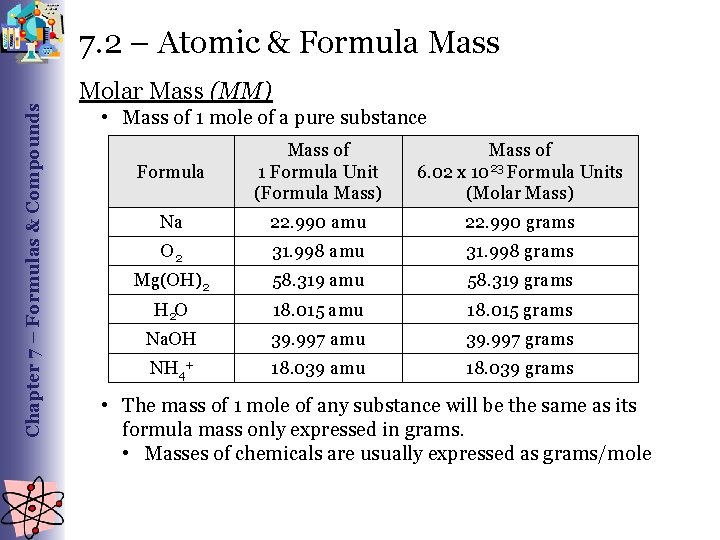
Chapter 7 – Formulas & Compounds 7. 2 – Atomic & Formula Mass Molar Mass (MM) • Mass of 1 mole of a pure substance Formula Mass of 1 Formula Unit (Formula Mass) Mass of 6. 02 x 1023 Formula Units (Molar Mass) Na 22. 990 amu 22. 990 grams O 2 31. 998 amu 31. 998 grams Mg(OH)2 58. 319 amu 58. 319 grams H 2 O 18. 015 amu 18. 015 grams Na. OH 39. 997 amu 39. 997 grams NH 4+ 18. 039 amu 18. 039 grams • The mass of 1 mole of any substance will be the same as its formula mass only expressed in grams. • Masses of chemicals are usually expressed as grams/mole

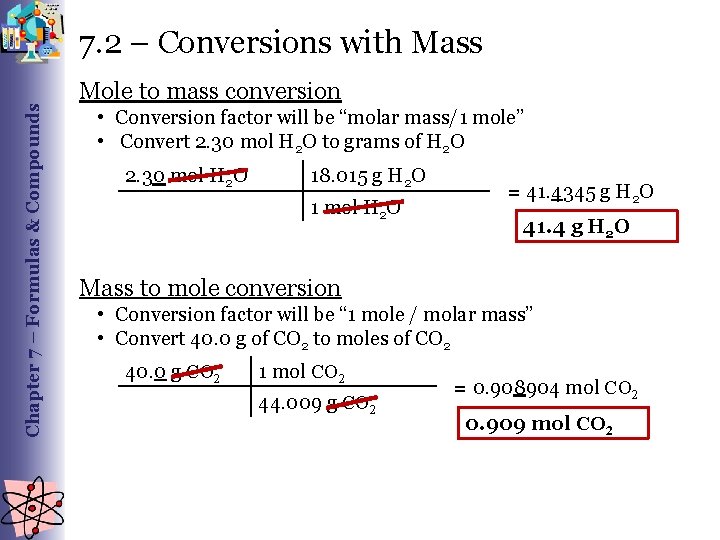
Chapter 7 – Formulas & Compounds 7. 2 – Conversions with Mass Mole to mass conversion • Conversion factor will be “molar mass/1 mole” • Convert 2. 30 mol H 2 O to grams of H 2 O 2. 30 mol H 2 O 18. 015 g H 2 O 1 mol H 2 O = 41. 4345 g H 2 O 41. 4 g H 2 O Mass to mole conversion • Conversion factor will be “ 1 mole / molar mass” • Convert 40. 0 g of CO 2 to moles of CO 2 40. 0 g CO 2 1 mol CO 2 44. 009 g CO 2 = 0. 908904 mol CO 2 0. 909 mol CO 2

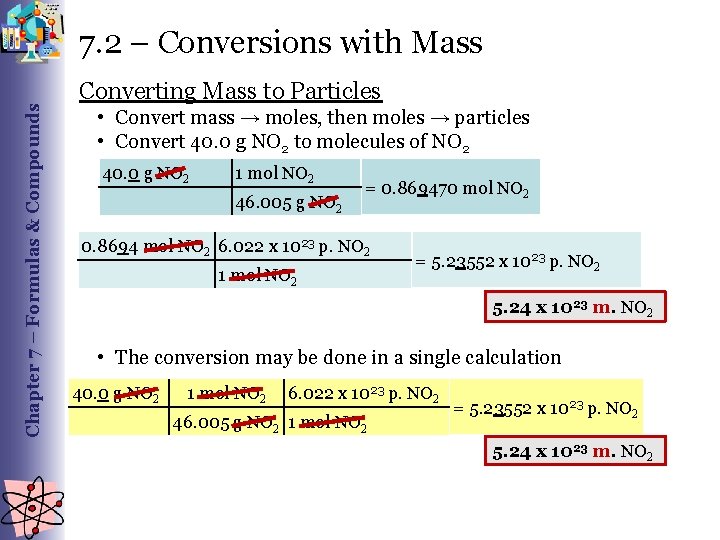
Chapter 7 – Formulas & Compounds 7. 2 – Conversions with Mass Converting Mass to Particles • Convert mass → moles, then moles → particles • Convert 40. 0 g NO 2 to molecules of NO 2 40. 0 g NO 2 1 mol NO 2 46. 005 g NO 2 = 0. 869470 mol NO 2 0. 8694 mol NO 2 6. 022 x 1023 p. NO 2 1 mol NO 2 = 5. 23552 x 1023 p. NO 2 5. 24 x 1023 m. NO 2 • The conversion may be done in a single calculation 40. 0 g NO 2 1 mol NO 2 6. 022 x 1023 p. NO 2 46. 005 g NO 2 1 mol NO 2 = 5. 23552 x 1023 p. NO 2 5. 24 x 1023 m. NO 2

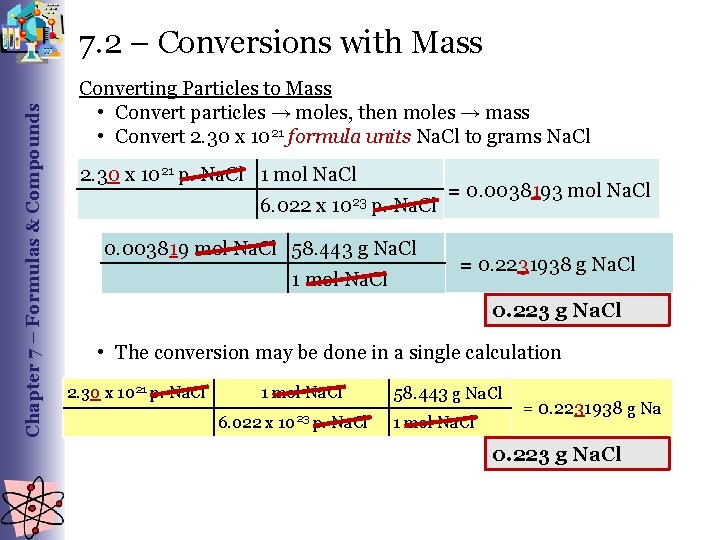
Chapter 7 – Formulas & Compounds 7. 2 – Conversions with Mass Converting Particles to Mass • Convert particles → moles, then moles → mass • Convert 2. 30 x 1021 formula units Na. Cl to grams Na. Cl 2. 30 x 1021 p. Na. Cl 1 mol Na. Cl 6. 022 x 1023 p. Na. Cl 0. 003819 mol Na. Cl 58. 443 g Na. Cl 1 mol Na. Cl = 0. 0038193 mol Na. Cl = 0. 2231938 g Na. Cl 0. 223 g Na. Cl • The conversion may be done in a single calculation 2. 30 x 1021 p. Na. Cl 1 mol Na. Cl 6. 022 x 1023 p. Na. Cl 58. 443 g Na. Cl 1 mol Na. Cl = 0. 2231938 g Na 0. 223 g Na. Cl

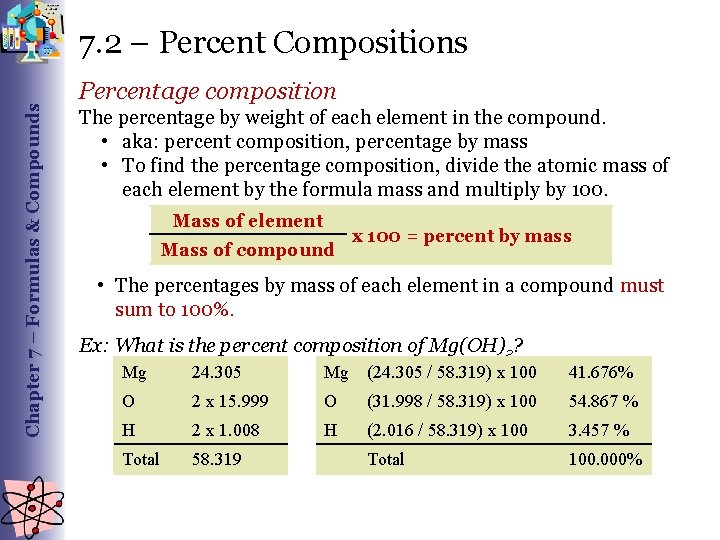
Chapter 7 – Formulas & Compounds 7. 2 – Percent Compositions Percentage composition The percentage by weight of each element in the compound. • aka: percent composition, percentage by mass • To find the percentage composition, divide the atomic mass of each element by the formula mass and multiply by 100. Mass of element Mass of compound x 100 = percent by mass • The percentages by mass of each element in a compound must sum to 100%. Ex: What is the percent composition of Mg(OH)2? Mg 24. 305 Mg (24. 305 / 58. 319) x 100 41. 676% O 2 x 15. 999 O (31. 998 / 58. 319) x 100 54. 867 % H 2 x 1. 008 H (2. 016 / 58. 319) x 100 3. 457 % Total 58. 319 Total 100. 000%

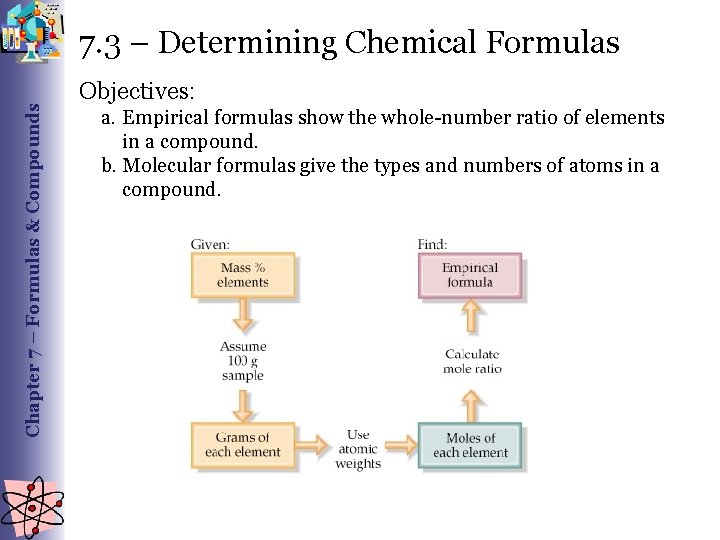
Chapter 7 – Formulas & Compounds 7. 3 – Determining Chemical Formulas Objectives: a. Empirical formulas show the whole-number ratio of elements in a compound. b. Molecular formulas give the types and numbers of atoms in a compound.

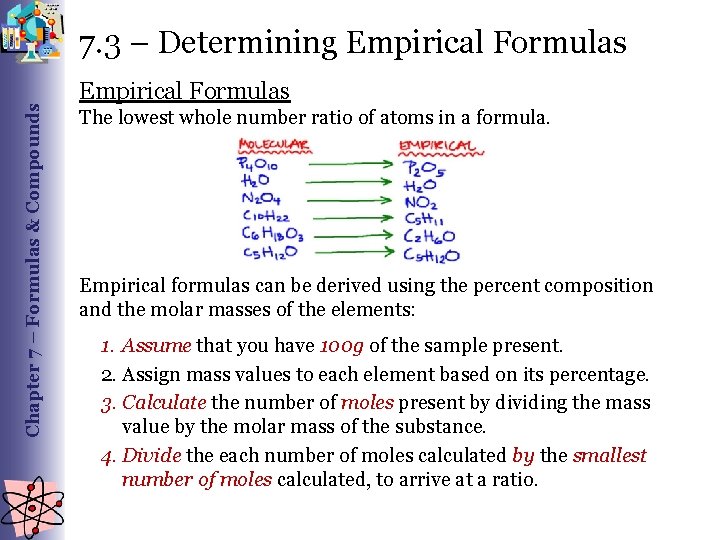
Chapter 7 – Formulas & Compounds 7. 3 – Determining Empirical Formulas The lowest whole number ratio of atoms in a formula. Empirical formulas can be derived using the percent composition and the molar masses of the elements: 1. Assume that you have 100 g of the sample present. 2. Assign mass values to each element based on its percentage. 3. Calculate the number of moles present by dividing the mass value by the molar mass of the substance. 4. Divide the each number of moles calculated by the smallest number of moles calculated, to arrive at a ratio.

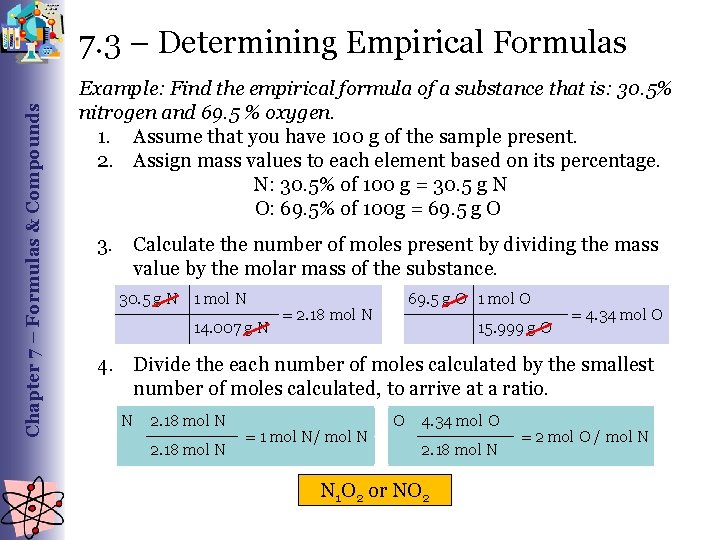
Chapter 7 – Formulas & Compounds 7. 3 – Determining Empirical Formulas Example: Find the empirical formula of a substance that is: 30. 5% nitrogen and 69. 5 % oxygen. 1. Assume that you have 100 g of the sample present. 2. Assign mass values to each element based on its percentage. N: 30. 5% of 100 g = 30. 5 g N O: 69. 5% of 100 g = 69. 5 g O 3. Calculate the number of moles present by dividing the mass value by the molar mass of the substance. 30. 5 g N 1 mol N 14. 007 g N 69. 5 g O 1 mol O = 2. 18 mol N 15. 999 g O = 4. 34 mol O 4. Divide the each number of moles calculated by the smallest number of moles calculated, to arrive at a ratio. N 2. 18 mol N = 1 mol N/ mol N O 4. 34 mol O 2. 18 mol N N 1 O 2 or NO 2 = 2 mol O / mol N

Chapter 7 – Formulas & Compounds 7. 3 – Determining Molecular Formulas The actual number of atoms of each element present in a molecular compound. • The molecular formula is a whole number multiple of the empirical formula. • To determine the molecular formula, divide the molar mass of the substance (which will be given in the problem) by the empirical formula mass. This gives the multiple by which you multiply the subscripts in the empirical formula. Example: A compound with the empirical formula CH 2 O has a molecular mass of 180. 156 grams. What is the molecular formula? Molar mass of CH 2 O is only 30. 026 g/mol. 180. 156 ÷ 30. 026 = 6 Multiply the subscripts by 6 to get: C 6 H 12 O 6.

Chapter 7 – Formulas & Compounds 7. 3 – Determining Molecular Formulas Shortcut for determining molecular formula from a % composition: • Assume 1 mole of the molecular substance. • Multiply the molar mass by the percentage of each element to determine the mass of that element in the 1 mole of molecules. • Divide the mass of each element present in the sample by its molar mass. (% of element x molecular mass) ÷ atomic weight = subscript Example: Find the molecular formula for a compound that is 40. 9% carbon, 4. 58 % hydrogen, and 54. 5% oxygen. The molar mass is 176. 124 g/mol. C = 0. 409 x 176. 124 ÷ 12. 011 = 6. 00 mol C H = 0. 0458 x 176. 124 ÷ 1. 008 = 8. 00 mol H O = 0. 545 x 176. 124 ÷ 15. 999 = 6. 00 mol O } C 6 H 8 O 6

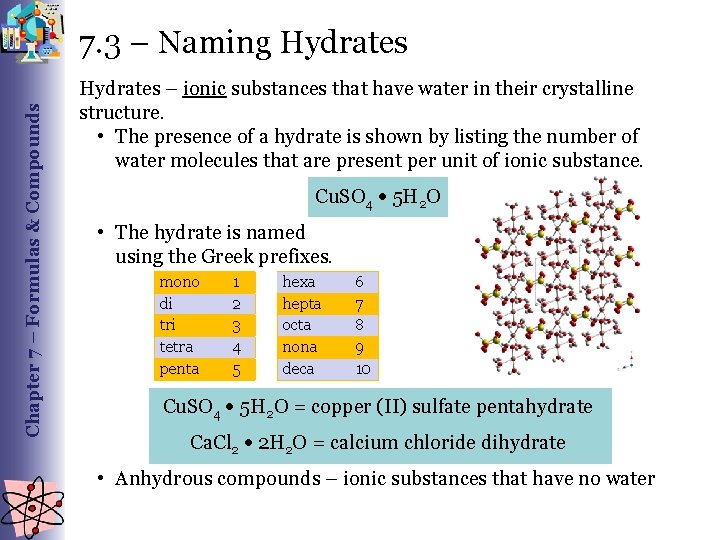
Chapter 7 – Formulas & Compounds 7. 3 – Naming Hydrates – ionic substances that have water in their crystalline structure. • The presence of a hydrate is shown by listing the number of water molecules that are present per unit of ionic substance. Cu. SO 4 5 H 2 O • The hydrate is named using the Greek prefixes. mono di tri tetra penta 1 2 3 4 5 hexa hepta octa nona deca 6 7 8 9 10 Cu. SO 4 5 H 2 O = copper (II) sulfate pentahydrate Ca. Cl 2 2 H 2 O = calcium chloride dihydrate • Anhydrous compounds – ionic substances that have no water

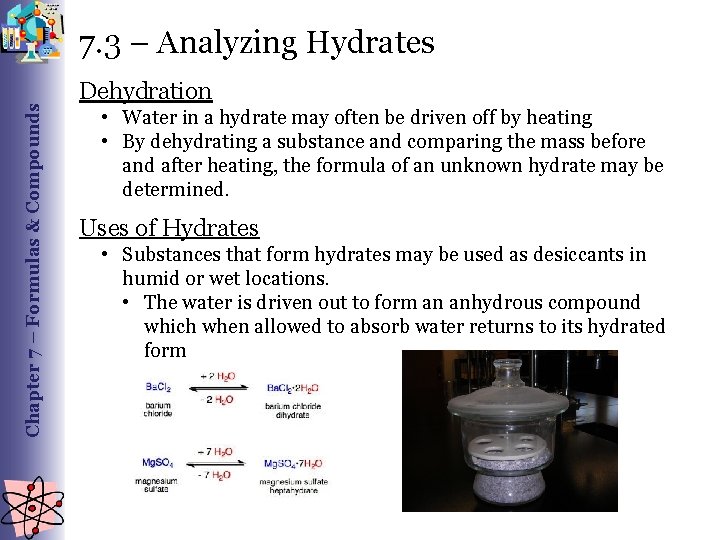
Chapter 7 – Formulas & Compounds 7. 3 – Analyzing Hydrates Dehydration • Water in a hydrate may often be driven off by heating • By dehydrating a substance and comparing the mass before and after heating, the formula of an unknown hydrate may be determined. Uses of Hydrates • Substances that form hydrates may be used as desiccants in humid or wet locations. • The water is driven out to form an anhydrous compound which when allowed to absorb water returns to its hydrated form

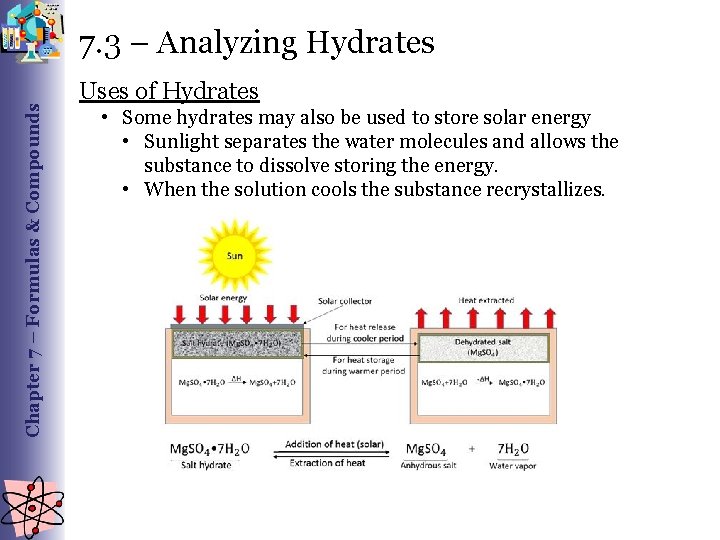
Chapter 7 – Formulas & Compounds 7. 3 – Analyzing Hydrates Uses of Hydrates • Some hydrates may also be used to store solar energy • Sunlight separates the water molecules and allows the substance to dissolve storing the energy. • When the solution cools the substance recrystallizes.