Chapter 7 Enzyme Mechanisms 7 1 Overview of























![Steady-State Conditions n [ES] is constant after the initial reaction time. Copyright © 2017 Steady-State Conditions n [ES] is constant after the initial reaction time. Copyright © 2017](https://slidetodoc.com/presentation_image_h/229b4825b1bdeab5921bd961e0e0a48b/image-39.jpg)













- Slides: 65

Chapter 7 Enzyme Mechanisms

7. 1 Overview of Enzymes n 1926 – James Sumner discovered that enzymes are proteins. n Urease was the first enzyme to be crystallized and purified. n 1929 – John Northrop purified pepsin. Copyright © 2017 W. W. Norton & Company

Enzyme Specificity n Lock and key n Induced fit Copyright © 2017 W. W. Norton & Company

Hexokinase: An Induced Fit Mechanism Copyright © 2017 W. W. Norton & Company

Critical Aspects of Enzyme Structure and Function, Part 1 Copyright © 2017 W. W. Norton & Company

Critical Aspects of Enzyme Structure and Function, Part 2 Copyright © 2017 W. W. Norton & Company

Critical Aspects of Enzyme Structure and Function, Part 3 Copyright © 2017 W. W. Norton & Company

Enzymes Are Chemical Catalysts Copyright © 2017 W. W. Norton & Company

Reaction Coordinate Diagram Copyright © 2017 W. W. Norton & Company

Cofactors and Coenzymes n Cofactors n Coenzyme n Prosthetic groups – coenzymes that are permanently associated with enzymes Copyright © 2017 W. W. Norton & Company

Common Cofactors Used in Catalysis Cofactor Representative enzymes Role in catalysis Positive 2 iron ion Cytochrome oxidase oxidation-reduction Positive 2 magnesium ion Hexokinase Helps bind ATP Positive 2 Manganese ion Ribonucleotide reductase oxidation-reduction Positive 2 copper ion Nitrate reductase oxidation-reduction Positive 2 zinc ion Alcohol dehydrogenase Helps bind the substrate Positive 2 nickel ion Urease Required in the catalytic site Positive 2 potassium ion Pyruvate kinase Increase enzyme activity Selenium Glutathione peroxidase oxidation-reduction Molybdenum Xanthine oxidase oxidation-reduction Copyright © 2017 W. W. Norton & Company

NAD+/NADH Redox Reaction Copyright © 2017 W. W. Norton & Company

Lipoamide as a Coenzyme Copyright © 2017 W. W. Norton & Company

Enzyme Nomenclature n Most enzymes end in “-ase. ” n The substrate is usually included in the name. Number Enzyme class Type of reaction Generic enzymes 1 Oxidoreductase Oxidation-reduction, transfer of upper H or upper O atoms Oxidases, dehydrogenases 2 Transferase Transfer of functional groups; e. g. , methyl, acyl, amino, phosphoryl Kinase, transaminases 3 Hydrolase Formation of two products by hydrolyzing a substrate Peptidases, lipases 4 Lyase Cleavage of Carbon carbon single bond, carbon oxygen single bond, carbon nitrogen single bond and other bonds by means other than hydrolysis or oxidation Decarboxylases, carboxylases 5 Isomeracse Intramolecular rearrangements, transfer of groups within molecules Mutases, isomerases 6 Ligase Formation of carbon single bond, carbon oxygen single bond, carbon sulfur single bond or carbon nitrogen single bond using ATP cleavage Synthetases Copyright © 2017 W. W. Norton & Company

7. 2 Enzyme Structure and Function n Enzymes increase the rate of reaction in three major ways: Copyright © 2017 W. W. Norton & Company

Physical and Chemical Properties of Active Sites Copyright © 2017 W. W. Norton & Company

Active Sites Contribute to Catalytic Properties 1. Sequestered microenvironment of the active site 2. Binding interactions between the substrate and the enzyme to create a transition state 3. Presence of catalytic functional groups Copyright © 2017 W. W. Norton & Company

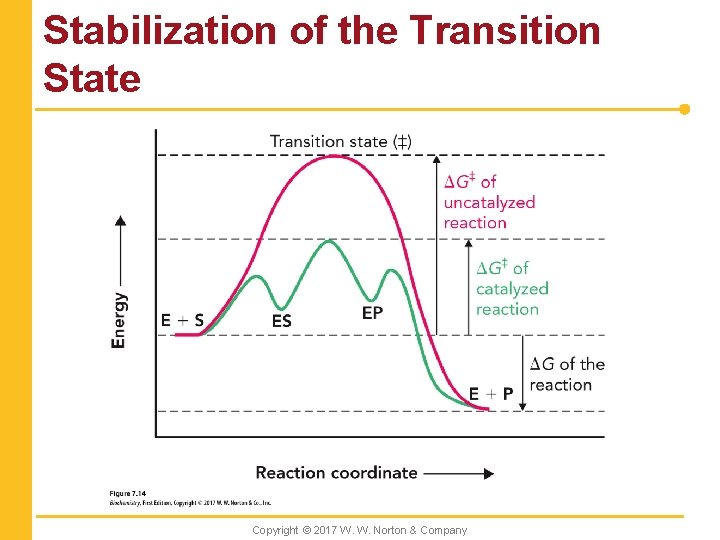
Stabilization of the Transition State Copyright © 2017 W. W. Norton & Company

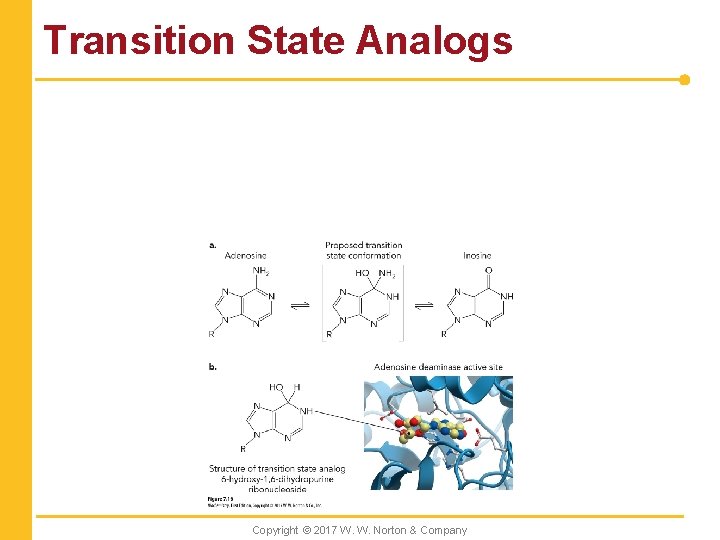
Transition State Analogs Copyright © 2017 W. W. Norton & Company

Catalytic Functional Groups Amino acid General acid form General base form His (The imidazole part of the side chain. One of the nitrogen in the fivemembered ring is positive and has an extra hydrogen) (The imidazole part of the side chain. One of the nitrogen in the fivemembered ring is neutral and has no hydrogen) Asp, Glu (The carboxylic acid group of the side chain for asp and glu) (The deprotonated carboxylic acid group of the side chain with a negative charge on one of the oxygen) Ser, Thr (The hydroxyl group from the side chain for Ser and Thr) (the deprotonated hydroxyl group from the side chain with a negative charge on the oxygen) Tyr (The side chain of Tyr with the hydroxyl group on the ring is neutral) (The side chain of Tyr with a deprotenated hydroxyl group so that the oxygen has a negative charge) Cys (The thiol side chain on the Cyc ) (The deprotonated thiol side chain on Cyc so that there is a negative charge on the sulfur) Lys (The protonated amino group of the side chain with three hydrogens and a positive charge on the nitrogen) (The amino group part of the side chain from lys) Arg (The side chain of Arg with two hydrogens on the nitrogen double bonded to the carbon so that the nitrogen has a positive charge) (the side chain of Arg which has an overall neutral charge) Copyright © 2017 W. W. Norton & Company

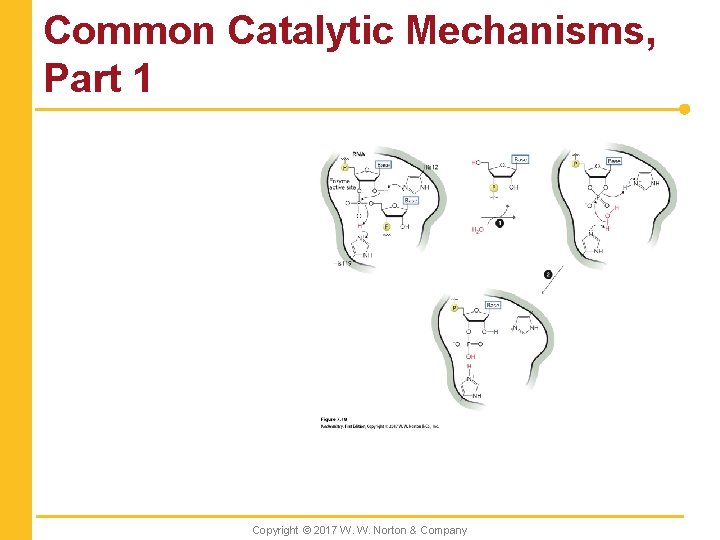
Common Catalytic Mechanisms, Part 1 Copyright © 2017 W. W. Norton & Company

Common Catalytic Mechanisms, Part 2 Copyright © 2017 W. W. Norton & Company

Common Catalytic Mechanisms, Part 3 Copyright © 2017 W. W. Norton & Company

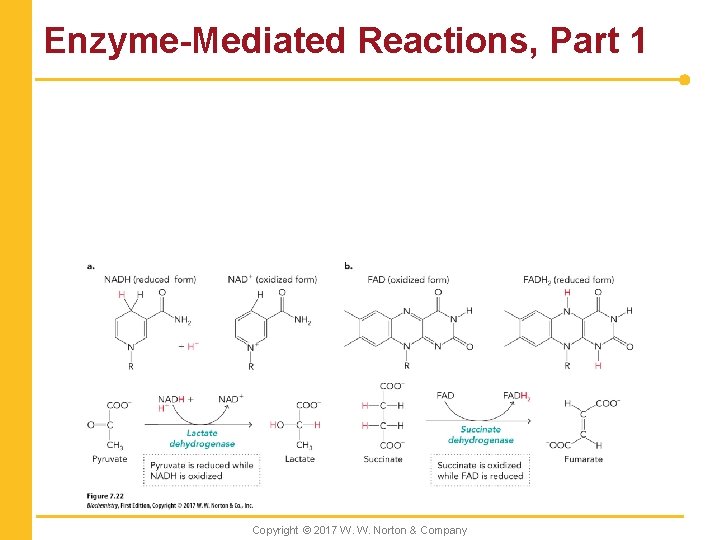
Enzyme-Mediated Reactions, Part 1 Copyright © 2017 W. W. Norton & Company

Enzyme-Mediated Reactions, Part 2 Copyright © 2017 W. W. Norton & Company

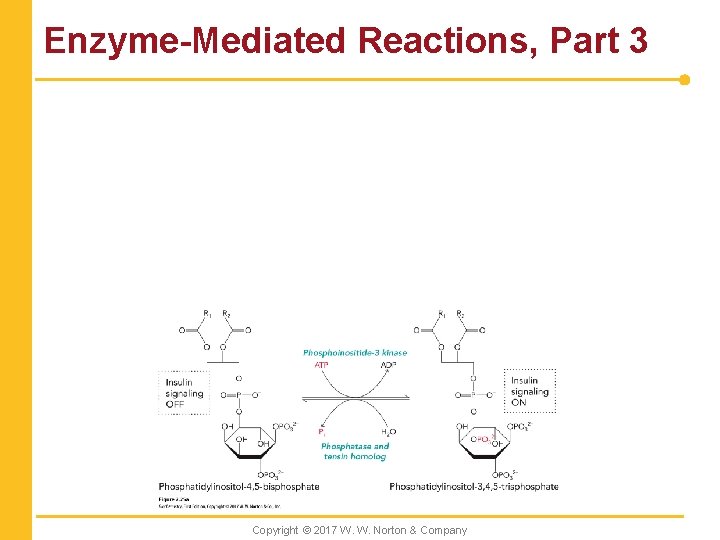
Enzyme-Mediated Reactions, Part 3 Copyright © 2017 W. W. Norton & Company

7. 3 Enzyme Reaction Mechanism Copyright © 2017 W. W. Norton & Company

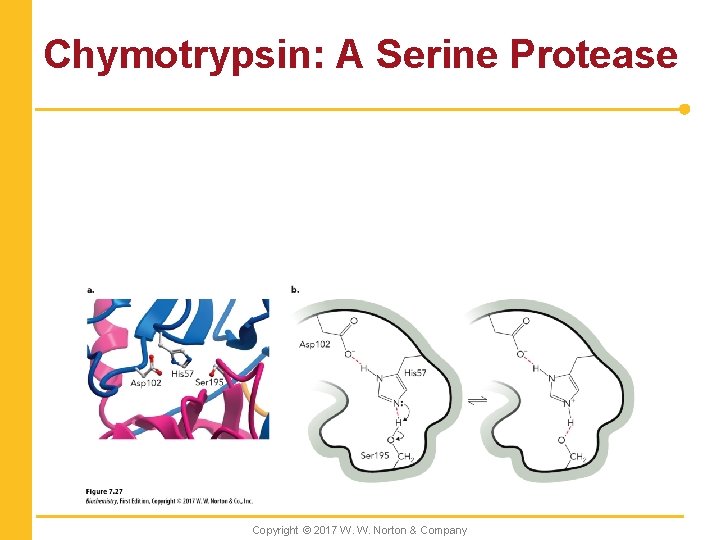
Chymotrypsin: A Serine Protease Copyright © 2017 W. W. Norton & Company

Catalytic Mechanism for Chymotrypsin Copyright © 2017 W. W. Norton & Company

Enzyme Specificity for Binding Pockets Copyright © 2017 W. W. Norton & Company

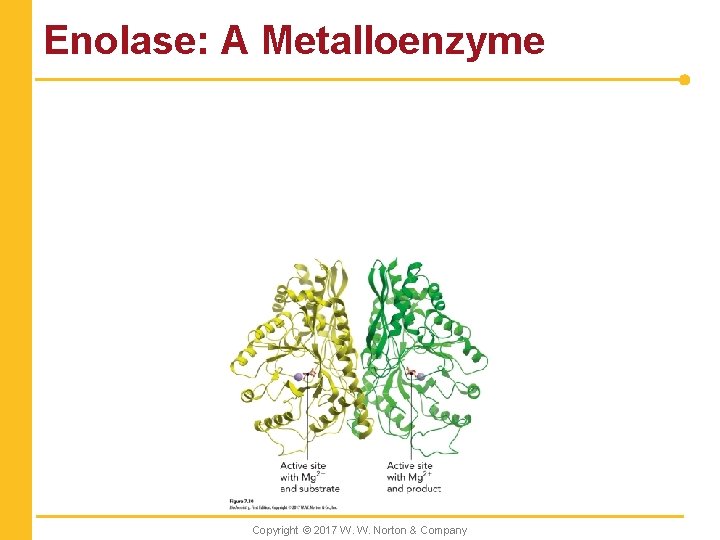
Enolase: A Metalloenzyme Copyright © 2017 W. W. Norton & Company

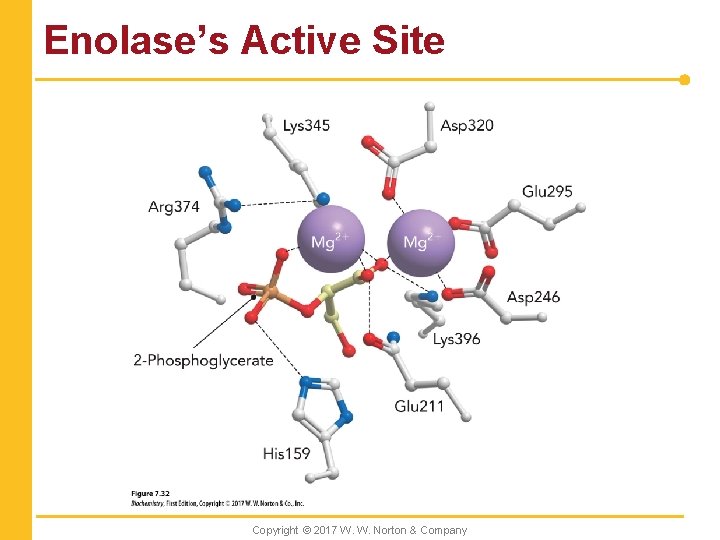
Enolase’s Active Site Copyright © 2017 W. W. Norton & Company

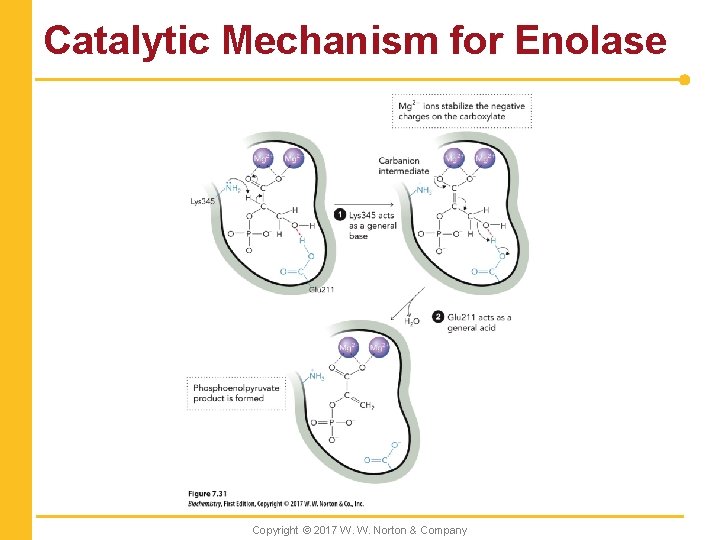
Catalytic Mechanism for Enolase Copyright © 2017 W. W. Norton & Company

HMG-Co. A Reductase Copyright © 2017 W. W. Norton & Company

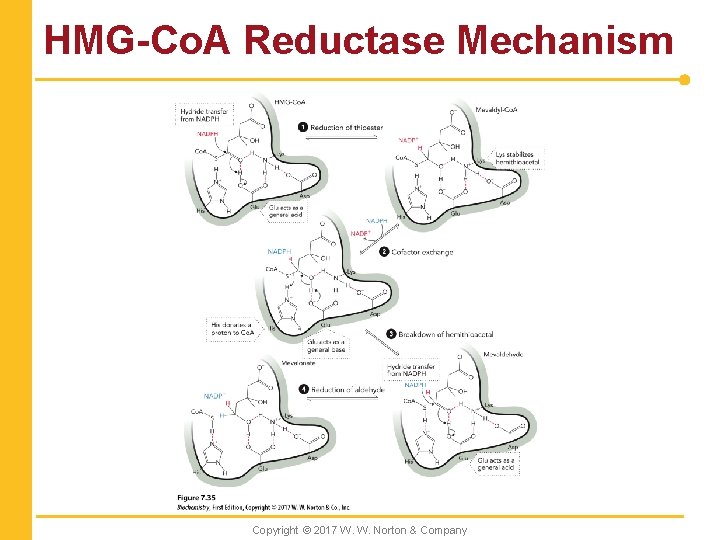
HMG-Co. A Reductase Mechanism Copyright © 2017 W. W. Norton & Company

7. 4 Enzyme Kinetics Copyright © 2017 W. W. Norton & Company

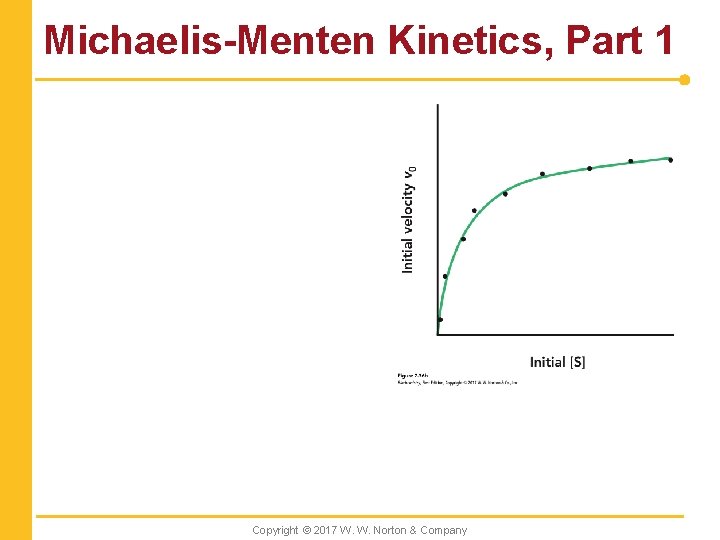
Michaelis-Menten Kinetics, Part 1 Copyright © 2017 W. W. Norton & Company

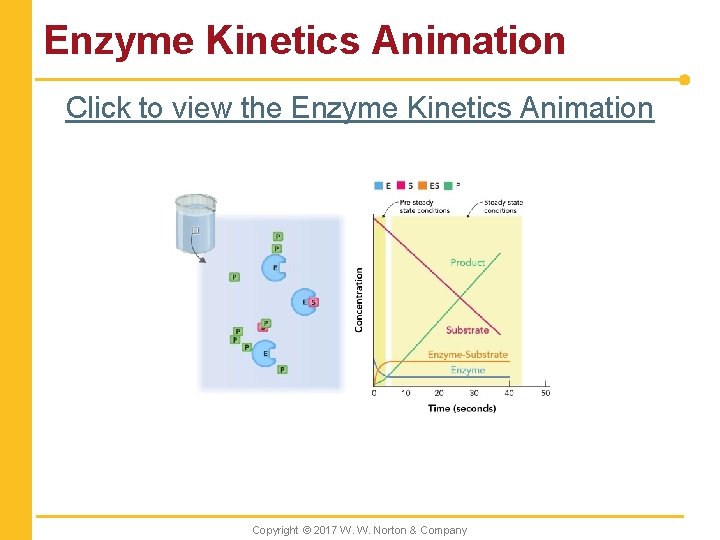
Enzyme Kinetics Animation Click to view the Enzyme Kinetics Animation Copyright © 2017 W. W. Norton & Company
![SteadyState Conditions n ES is constant after the initial reaction time Copyright 2017 Steady-State Conditions n [ES] is constant after the initial reaction time. Copyright © 2017](https://slidetodoc.com/presentation_image_h/229b4825b1bdeab5921bd961e0e0a48b/image-39.jpg)
Steady-State Conditions n [ES] is constant after the initial reaction time. Copyright © 2017 W. W. Norton & Company

Michaelis-Menten Kinetics, Part 2 n Michaelis-Menten equation Copyright © 2017 W. W. Norton & Company

Typical Michaelis–Menten Enzyme Copyright © 2017 W. W. Norton & Company

Lineweaver-Burk Equation Copyright © 2017 W. W. Norton & Company

Catalytic Efficiency n Turnover number (kcat) Copyright © 2017 W. W. Norton & Company

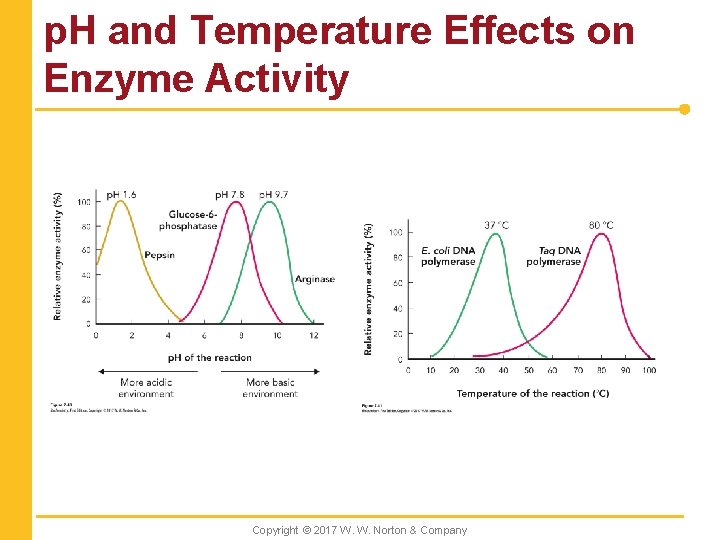
p. H and Temperature Effects on Enzyme Activity Copyright © 2017 W. W. Norton & Company

7. 5 Regulation of Enzyme Activity Copyright © 2017 W. W. Norton & Company

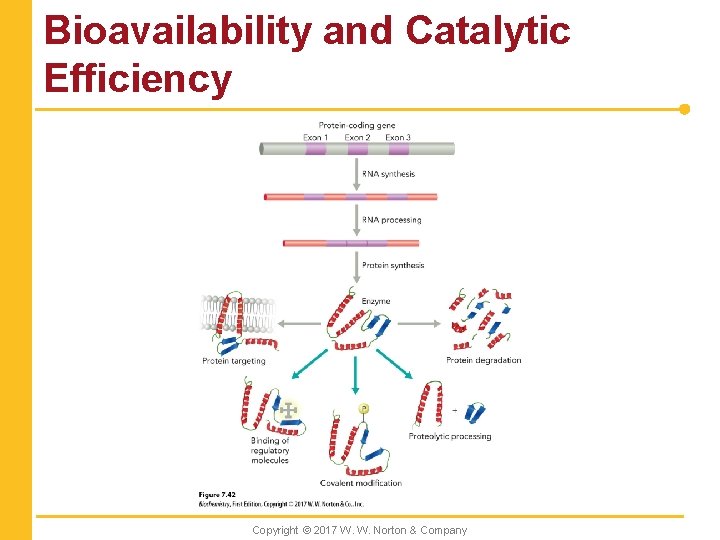
Bioavailability and Catalytic Efficiency Copyright © 2017 W. W. Norton & Company

Enzyme Inhibition n Reversible n Irreversible Copyright © 2017 W. W. Norton & Company

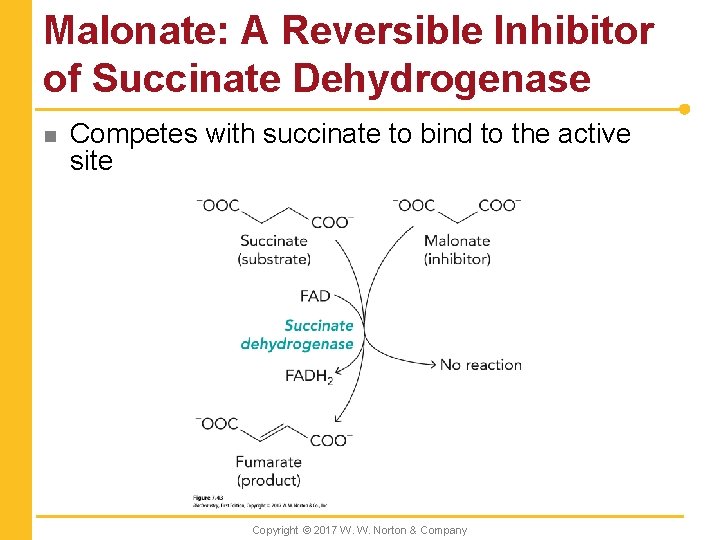
Malonate: A Reversible Inhibitor of Succinate Dehydrogenase n Competes with succinate to bind to the active site Copyright © 2017 W. W. Norton & Company

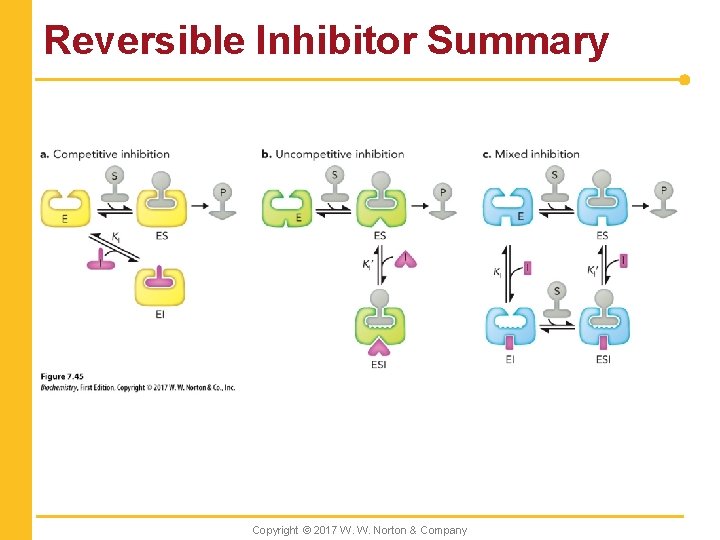
Reversible Inhibitor Summary Copyright © 2017 W. W. Norton & Company

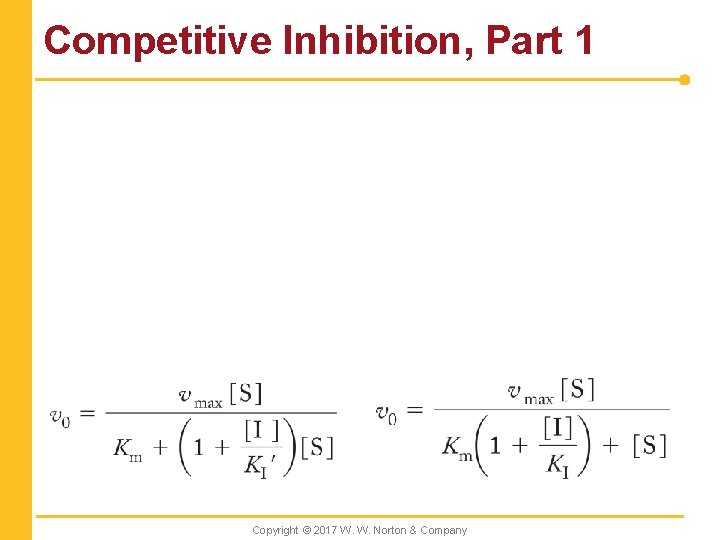
Competitive Inhibition, Part 1 Copyright © 2017 W. W. Norton & Company

Competitive Inhibition, Part 2 Copyright © 2017 W. W. Norton & Company

Saquinavir: A Competitive Inhibitor Copyright © 2017 W. W. Norton & Company

Uncompetitive Inhibition Copyright © 2017 W. W. Norton & Company

Mixed Inhibition, Part 1 Copyright © 2017 W. W. Norton & Company

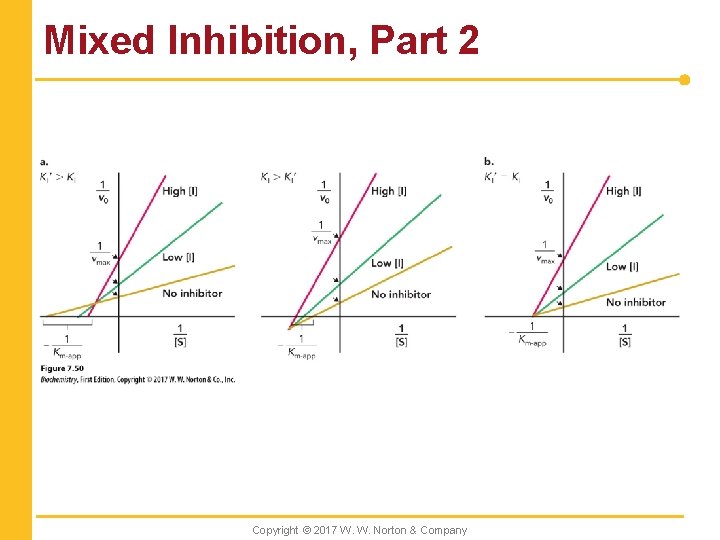
Mixed Inhibition, Part 2 Copyright © 2017 W. W. Norton & Company

Diisopropylfluorophosphate: An Irreversible Enzyme Inhibitor Copyright © 2017 W. W. Norton & Company

Allosteric Regulation Copyright © 2017 W. W. Norton & Company

Modes of Enzyme Regulation Click to view the Modes of Enzyme Regulation animation Copyright © 2017 W. W. Norton & Company

Allosteric Regulation of Catalytic Activity Copyright © 2017 W. W. Norton & Company

ATCase Copyright © 2017 W. W. Norton & Company

ATCase Activity Copyright © 2017 W. W. Norton & Company

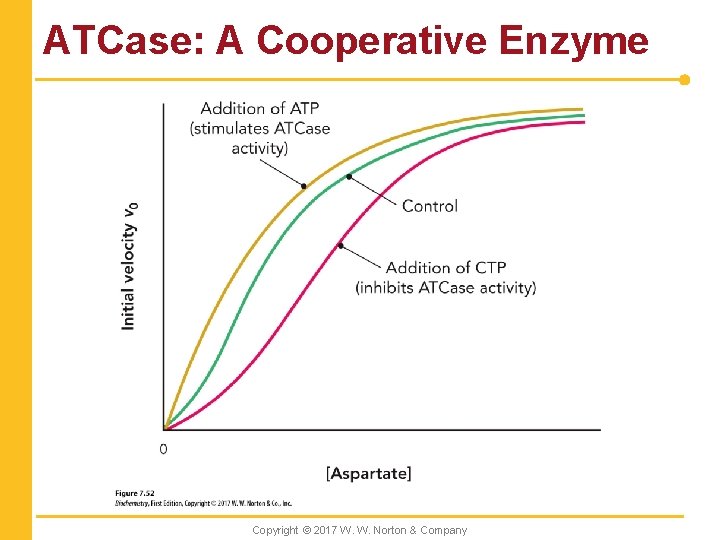
ATCase: A Cooperative Enzyme Copyright © 2017 W. W. Norton & Company

Covalent Modification Copyright © 2017 W. W. Norton & Company

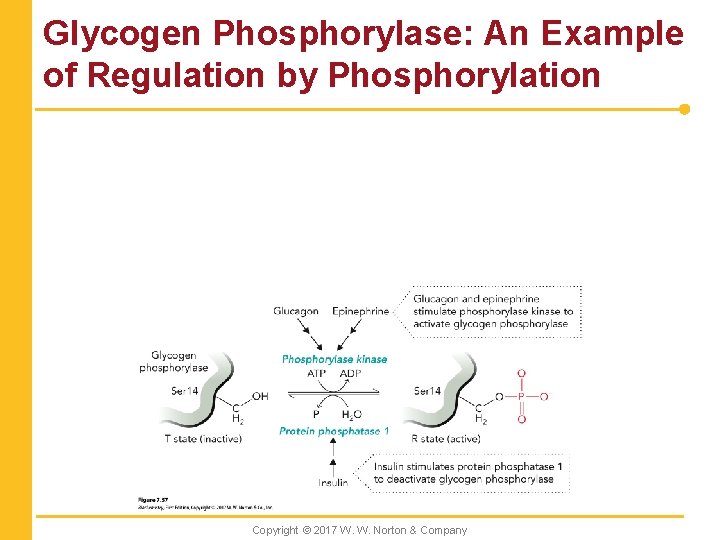
Glycogen Phosphorylase: An Example of Regulation by Phosphorylation Copyright © 2017 W. W. Norton & Company

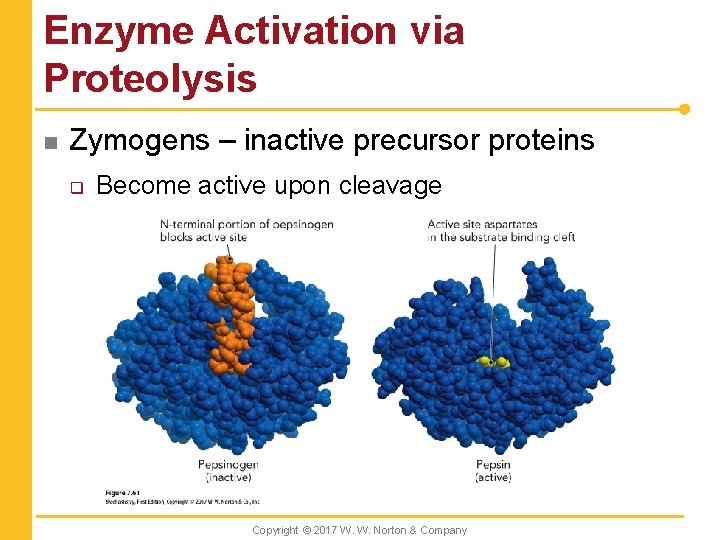
Enzyme Activation via Proteolysis n Zymogens – inactive precursor proteins q Become active upon cleavage Copyright © 2017 W. W. Norton & Company