Chapter 7 Electrochemistry 7 4 Activity and activity







- Slides: 14

Chapter 7 Electrochemistry § 7. 4 Activity and activity coefficient

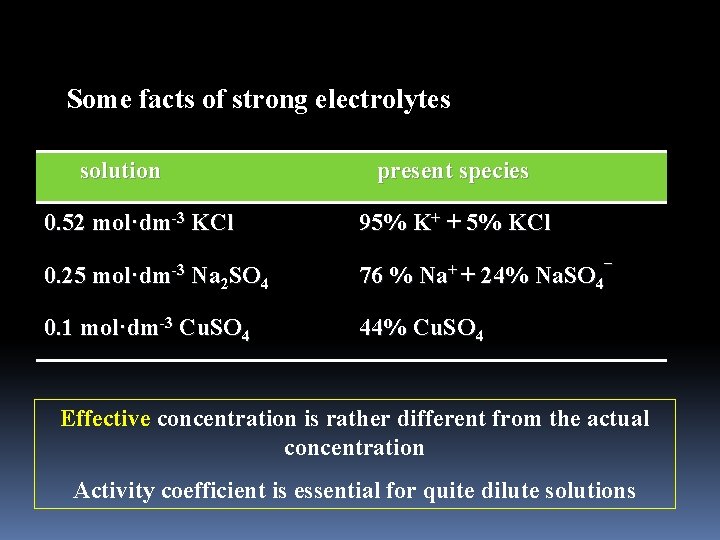
Some facts of strong electrolytes solution present species 0. 52 mol·dm-3 KCl 95% K+ + 5% KCl 0. 25 mol·dm-3 Na 2 SO 4 76 % Na+ + 24% Na. SO 4¯ 0. 1 mol·dm-3 Cu. SO 4 44% Cu. SO 4 Effective concentration is rather different from the actual concentration Activity coefficient is essential for quite dilute solutions

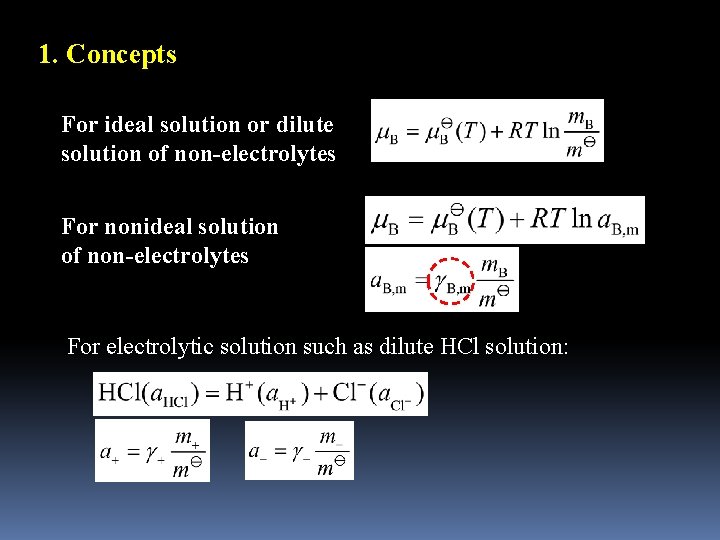
1. Concepts For ideal solution or dilute solution of non-electrolytes For nonideal solution of non-electrolytes For electrolytic solution such as dilute HCl solution:

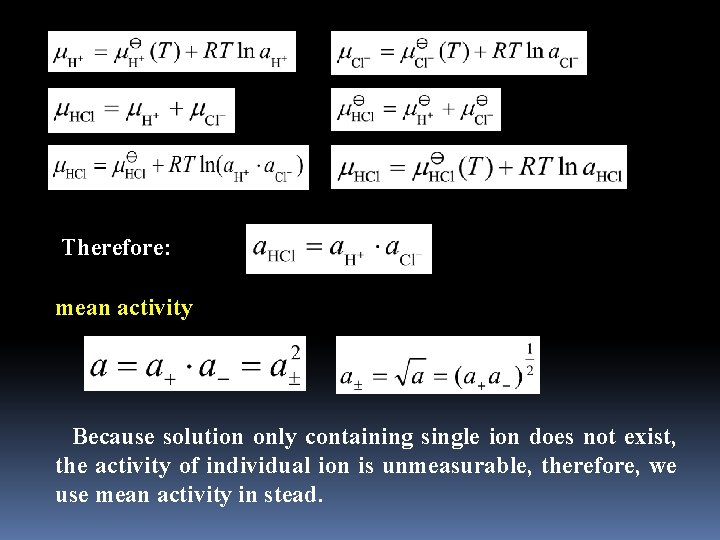
Therefore: mean activity Because solution only containing single ion does not exist, the activity of individual ion is unmeasurable, therefore, we use mean activity in stead.

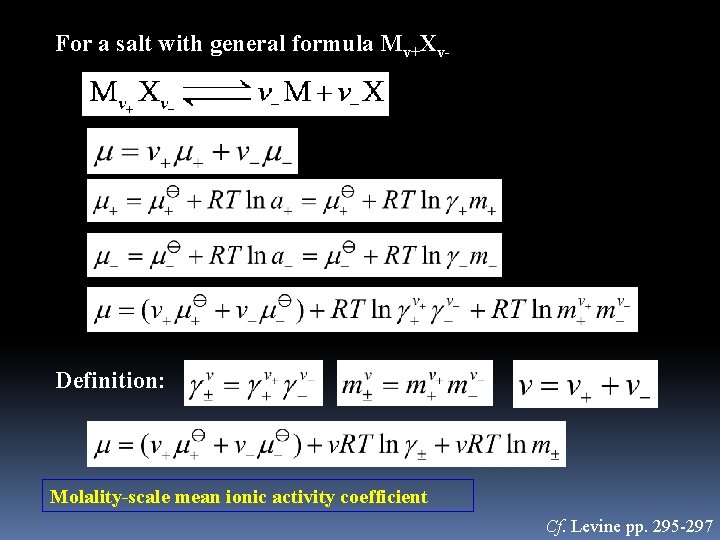
For a salt with general formula Mv+Xv- Definition: Molality-scale mean ionic activity coefficient Cf. Levine pp. 295 -297

mean ionic molality mean ionic activity coefficient mean ionic activity Mean ionic molality can be expressed in term of the molality of the solution, mean ionic activity coefficient can be measured experimentally, and then mean ionic activity can be determined.

Exercises: 1) Write the expression for the activity (a) of Mg 3(PO 4)2 in terms of its molality and mean ionic activity coefficient. 2) The mean ionic activity coefficient of an 0. 005 mol·kg-1 K 2 SO 4 aqueous solution is measured to be 0. 781. Calculate the mean ionic activity of the solution.

2. Methods for determination of mean ionic activity coefficient (1) (2) (3) (4) (5)

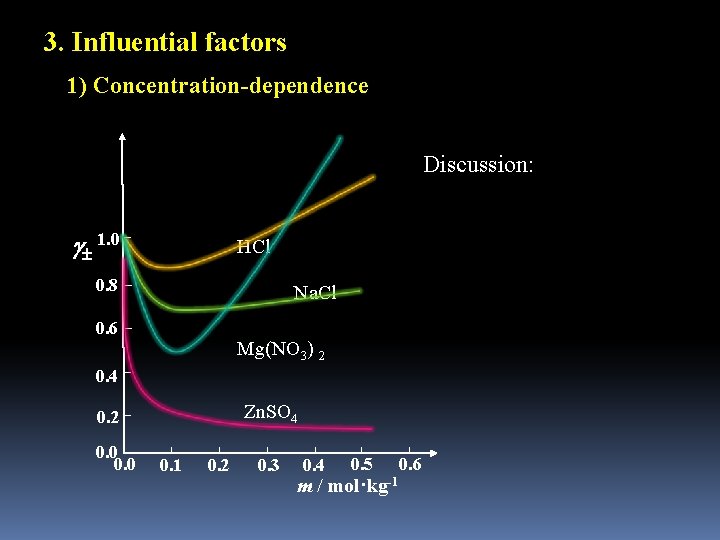
3. Influential factors 1) Concentration-dependence Discussion: 1. 0 HCl 0. 8 Na. Cl 0. 6 Mg(NO 3) 2 0. 4 Zn. SO 4 0. 2 0. 0 0. 1 0. 2 0. 3 0. 4 0. 5 m / mol·kg-1 0. 6

m 0. 001 0. 965 0. 01 0. 905 0. 1 0. 797 0. 5 0. 754 1 0. 803 5 2. 70 10 20 486 Activity coefficient of Li. Br in water at 25 o. C and 1 atm Cf. Levine p. 299

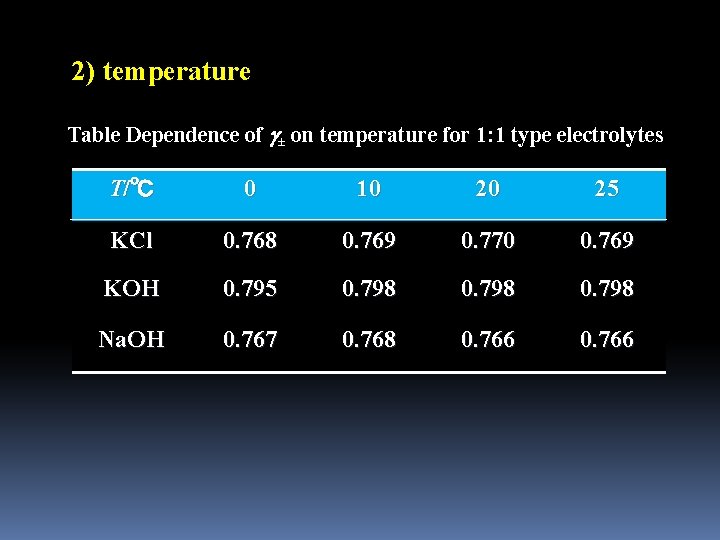
2) temperature Table Dependence of ± on temperature for 1: 1 type electrolytes T/℃ 0 10 20 25 KCl 0. 768 0. 769 0. 770 0. 769 KOH 0. 795 0. 798 Na. OH 0. 767 0. 768 0. 766

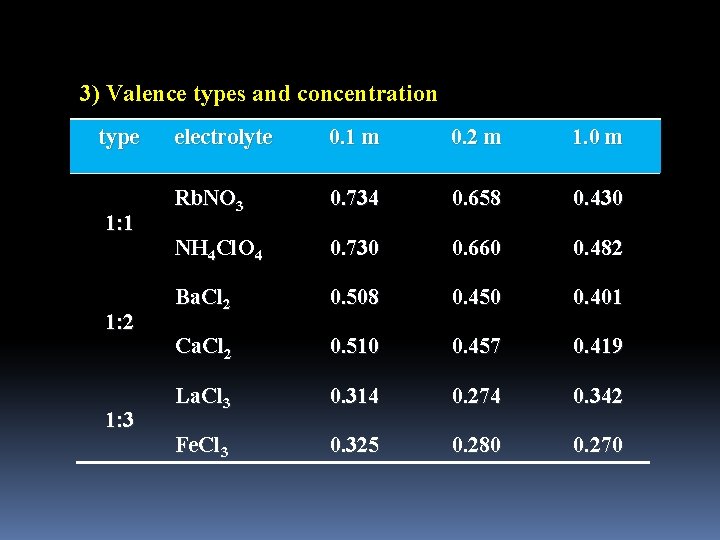
3) Valence types and concentration type 1: 1 1: 2 1: 3 electrolyte 0. 1 m 0. 2 m 1. 0 m Rb. NO 3 0. 734 0. 658 0. 430 NH 4 Cl. O 4 0. 730 0. 660 0. 482 Ba. Cl 2 0. 508 0. 450 0. 401 Ca. Cl 2 0. 510 0. 457 0. 419 La. Cl 3 0. 314 0. 274 0. 342 Fe. Cl 3 0. 325 0. 280 0. 270

4) ionic strength Lewis, who noted that the nonideality observed in electrolytic solutions primarily stems from the total concentration of charges present rather than from the chemical nature of the individual ionic species, introduced ionic strength in 1921. It is merely an hypothesis! Valid when c < 0. 01 m

Self reading: Ira N. Levine, Physical Chemistry, 5 th Ed. , Mc. Graw-Hill, 2002. pp. 294 -300 Section 10. 6 solutions of electrolytes Section 10. 7 determination of electrolyte activity coefficients