Chapter 7 Electrochemistry 7 13 Corrosion and protection








![Tension corrosion Nails in a solution with phenolphthalein and K 3[Fe(CN)6] Anodic region Cathodic Tension corrosion Nails in a solution with phenolphthalein and K 3[Fe(CN)6] Anodic region Cathodic](https://slidetodoc.com/presentation_image_h2/77847426ab7c4858b53fedefd4ffea11/image-12.jpg)






- Slides: 29

Chapter 7 Electrochemistry § 7. 13 Corrosion and protection of metals


1. General introduction 1) Corrosion: Destruction of materials due to the chemical, electrochemical and physical attack of the media. Railway bridge in Boston White marble of Jinshui Bridge, Beijing Stone Sculpture before the Capitol, Washington D. C.

Warship of Pacific Fleet, Russia Brass sculpture before the Capitol, Washington D. C. Brooklyn Bridge, New York One-fifth of the iron and steel produced annually in the world is used to replace rusted metal. Since corroded metal often loses its structural integrity and attractiveness, corrosion of metal probably results in disaster and has great impact on national economics and safety.

2) Why does metal undergo corrosion? Naturally occurring copper sheet Naturally occurring copper single crystals, Museum of Natural Sci. , Washington, D. C. USA Naturally occurring gold Only few elements exist in free element state in natural circumstance. Corrosion of metal, i. e. conversion of element to stable compound, is thermodynamically favored.

2. Classification of corrosion 1) Based on materials: Corrosion of metals; Corrosion of non-metals (wood, plastic, concrete, stone, etc. ) 2) Based on Media: natural corrosion; industrial corrosion (with surface solution containing acid, base, H 2 S, etc. ) 3) Based on mechanism: chemical corrosion (2 Fe + O 2 = 2 Fe. O); electrochemical corrosion; biochemical corrosion

4) Based on uniformity: general corrosion; local corrosion 5) Other kinds: tension corrosion; contact corrosion; friction corrosion; external current corrosion; Concussion corrosion

3. local corrosion Local corrosion is initiated due to the ununiformity of metal and / or solution. 1) The ununiformity of metal: 2) The ununiformity of metal surface 3) The ununiformity of solution

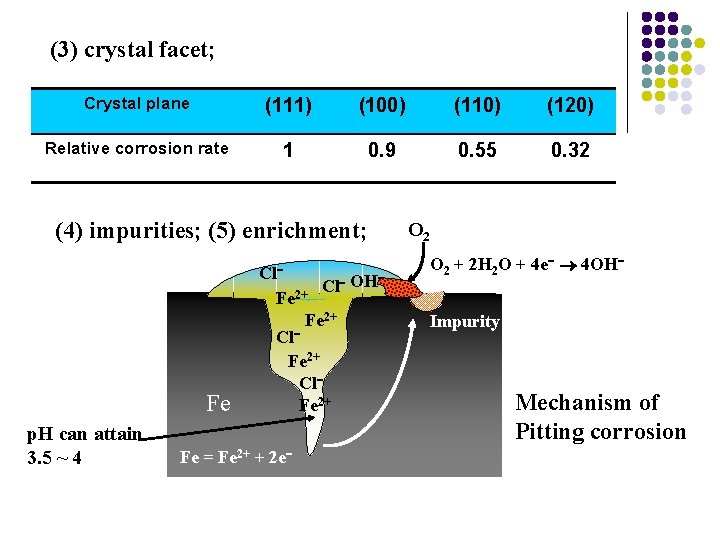
1) The ununiformity of metal: (1) multiphase texture; (2) crystal boundary; (3) crystal facet; (4) impurities; (5) enrichment; (6) tension and deformity

(1) multiphase texture (2) crystal boundary; Trans-crystal corrosion Corrosion of crystal boundary transgranular corrosion intergranular corrosion

(3) crystal facet; Crystal plane (111) (100) (110) (120) Relative corrosion rate 1 0. 9 0. 55 0. 32 (4) impurities; (5) enrichment; Fe p. H can attain 3. 5 ~ 4 Cl OH Cl Fe 2+ Fe = Fe 2+ + 2 e O 2 + 2 H 2 O + 4 e 4 OH Impurity Mechanism of Pitting corrosion
![Tension corrosion Nails in a solution with phenolphthalein and K 3FeCN6 Anodic region Cathodic Tension corrosion Nails in a solution with phenolphthalein and K 3[Fe(CN)6] Anodic region Cathodic](https://slidetodoc.com/presentation_image_h2/77847426ab7c4858b53fedefd4ffea11/image-12.jpg)
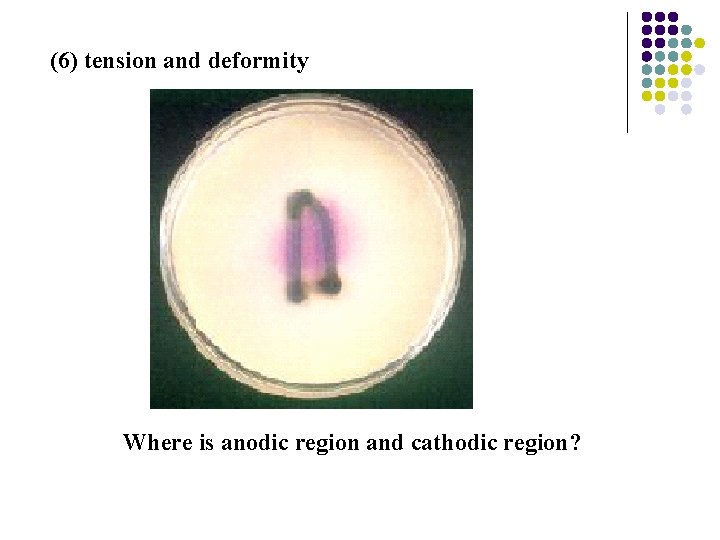
Tension corrosion Nails in a solution with phenolphthalein and K 3[Fe(CN)6] Anodic region Cathodic region Reactions: Anodic reaction: Fe 2++2 e¯ Cathodic reaction: 2 H 2 O + 2 e¯ H 2+2 OH ¯

(6) tension and deformity Where is anodic region and cathodic region?

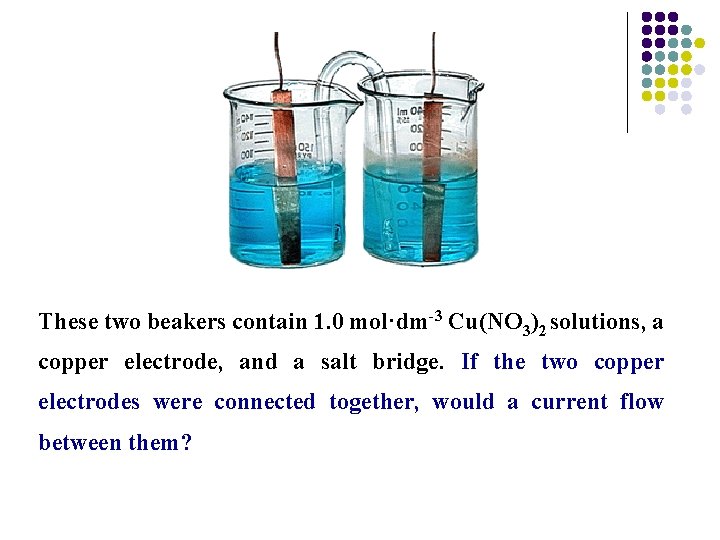
These two beakers contain 1. 0 mol·dm-3 Cu(NO 3)2 solutions, a copper electrode, and a salt bridge. If the two copper electrodes were connected together, would a current flow between them?

2) The ununiformity of metal surface (1) Smoothness of the surface; (2) Micropore in protective layer; (3) Corrosion products 3) The ununiformity of solution (1) Concentration difference of metal ions; (2) Concentration difference of media ions; (3) Accumulation of H+ in pit or cracks; (4) Concentration difference of dissolved oxygen

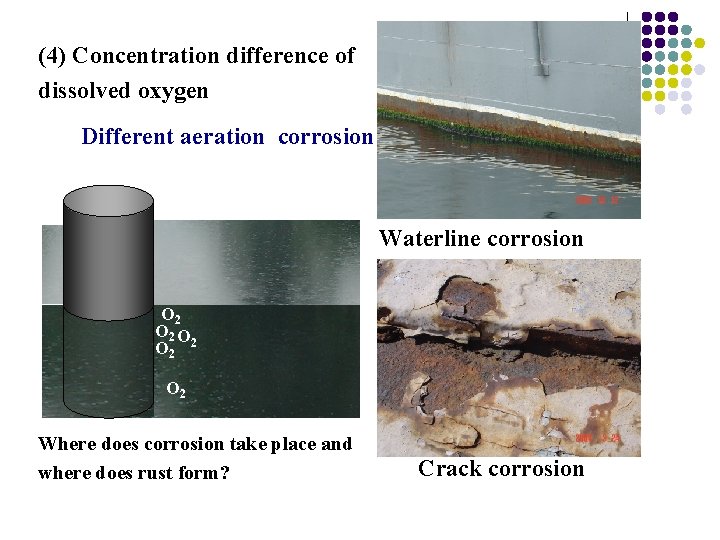
(4) Concentration difference of dissolved oxygen Different aeration corrosion Waterline corrosion O 2 O O 2 2 O 2 Where does corrosion take place and where does rust form? Crack corrosion

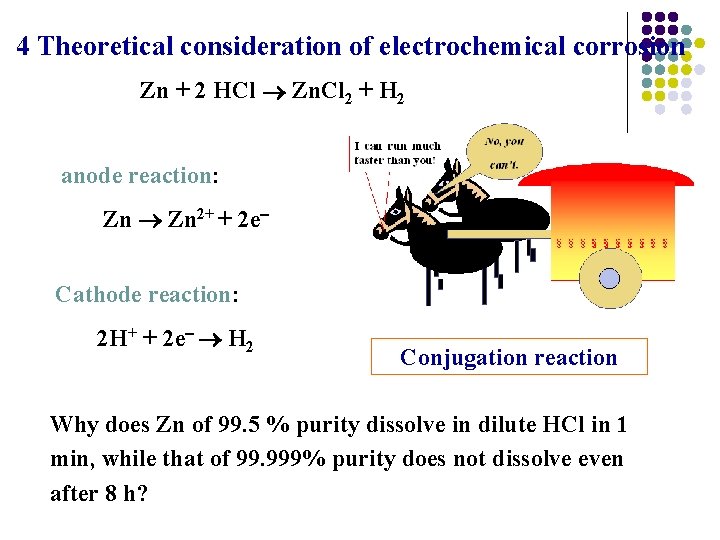
4 Theoretical consideration of electrochemical corrosion Zn + 2 HCl Zn. Cl 2 + H 2 anode reaction: Zn 2+ + 2 e Cathode reaction: 2 H+ + 2 e H 2 Conjugation reaction Why does Zn of 99. 5 % purity dissolve in dilute HCl in 1 min, while that of 99. 999% purity does not dissolve even after 8 h?

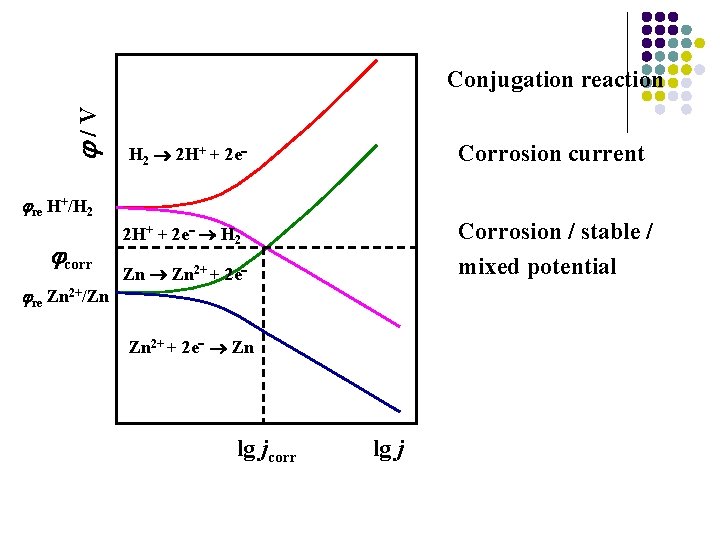
/V Conjugation reaction Corrosion current H 2 2 H+ + 2 e re H+/H 2 corr re Zn 2+/Zn Corrosion / stable / mixed potential 2 H+ + 2 e H 2 Zn 2+ + 2 e Zn lg jcorr lg j

/V re H+/H 2 corr re Zn 2+/Zn H 2 2 H+ + 2 e 2+ H 2 Zn + 2 e Zn 2+ + 2 e Zn lg jcorr lg j Positive shift of the metal or increase of the hydrogen evolution overpotential can both hinder the corrosion of the metal.

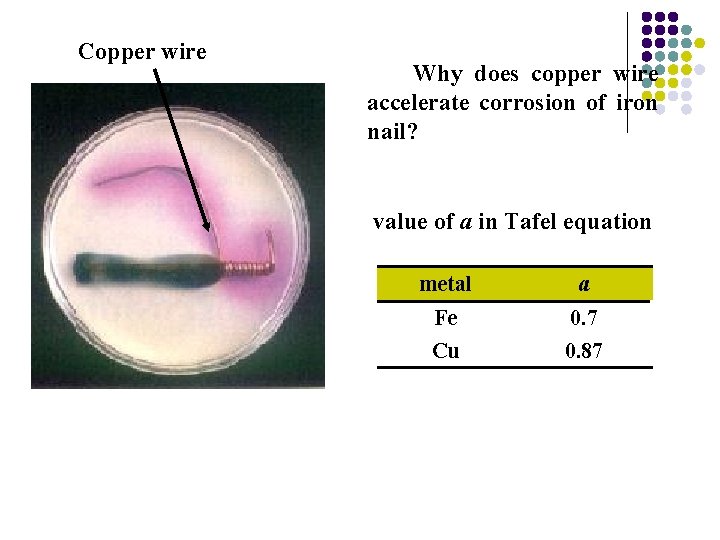
Copper wire Why does copper wire accelerate corrosion of iron nail? value of a in Tafel equation metal a Fe 0. 7 Cu 0. 87

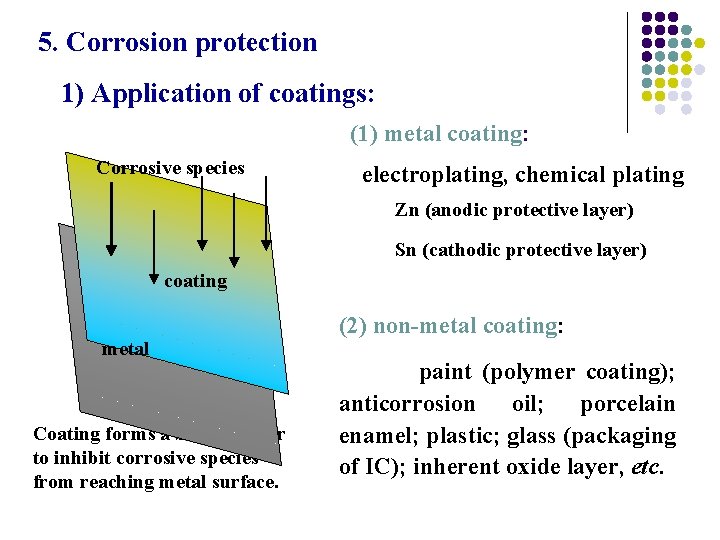
5. Corrosion protection 1) Application of coatings: (1) metal coating: Corrosive species electroplating, chemical plating Zn (anodic protective layer) Sn (cathodic protective layer) coating metal Coating forms a barrier layer to inhibit corrosive species from reaching metal surface. (2) non-metal coating: paint (polymer coating); anticorrosion oil; porcelain enamel; plastic; glass (packaging of IC); inherent oxide layer, etc.

Both thick inorganic coating and organic coating were applied to protect the cable and steel structure of Brooklyn Bridge, New York, USA.

When Al contacting with the air, a thin inherent layer of aluminum oxide forms on its surface. Being stable in the air, water and even some dilute acidic solution, this thin oxide layer inhibits further corrosion of the metal. With potential of naked aluminum of – 0. 6 V, the oxide-coated aluminum becomes more stable even than the common metals, such as iron, zinc, etc.

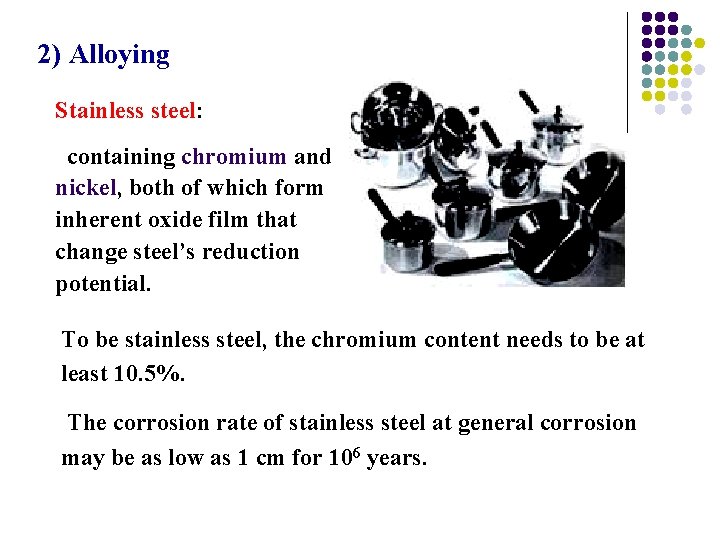
2) Alloying Stainless steel: containing chromium and nickel, both of which form inherent oxide film that change steel’s reduction potential. To be stainless steel, the chromium content needs to be at least 10. 5%. The corrosion rate of stainless steel at general corrosion may be as low as 1 cm for 106 years.

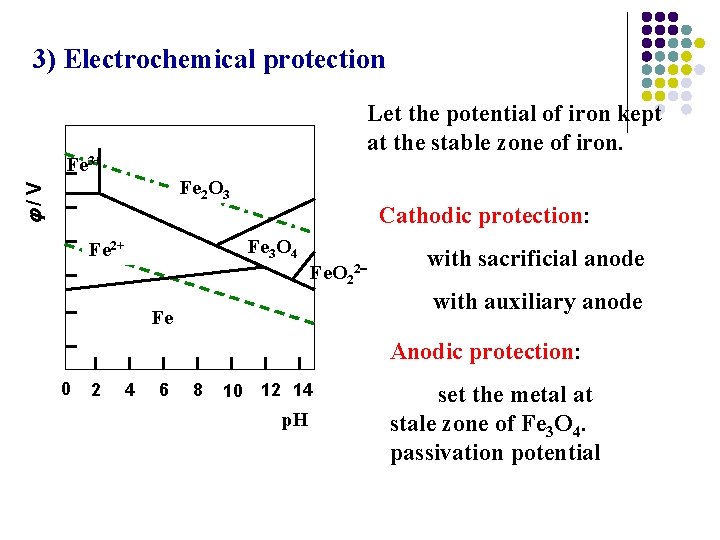
3) Electrochemical protection Let the potential of iron kept at the stable zone of iron. Fe 3+ /V Fe 2 O 3 Cathodic protection: Fe 3 O 4 Fe 2+ Fe. O 22 with sacrificial anode with auxiliary anode Fe Anodic protection: 0 2 4 6 8 10 12 14 p. H set the metal at stale zone of Fe 3 O 4. passivation potential

Cathodic protection: with sacrificial anode magnesium / aluminum / zinc alloys Cathodic protection: with auxiliary anode: Pipeline

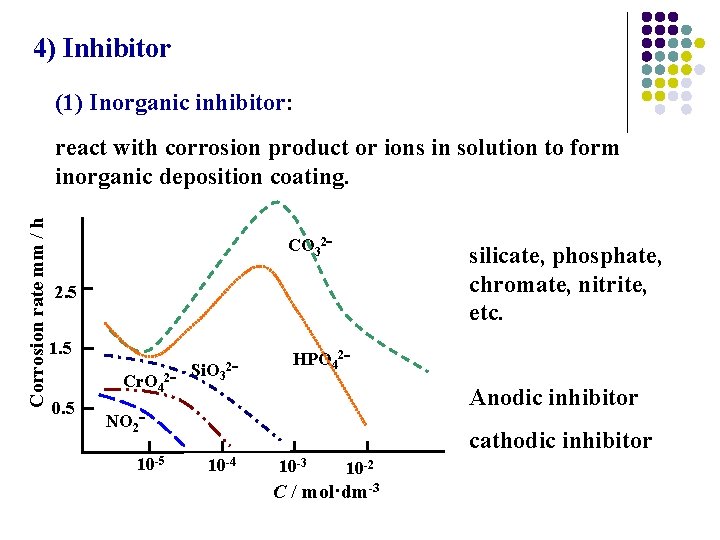
4) Inhibitor (1) Inorganic inhibitor: Corrosion rate mm / h react with corrosion product or ions in solution to form inorganic deposition coating. CO 32 silicate, phosphate, chromate, nitrite, etc. 2. 5 1. 5 Cr. O 42 0. 5 Si. O 3 2 HPO 42 Anodic inhibitor NO 2 10 -5 cathodic inhibitor 10 -4 10 -3 10 -2 C / mol·dm-3

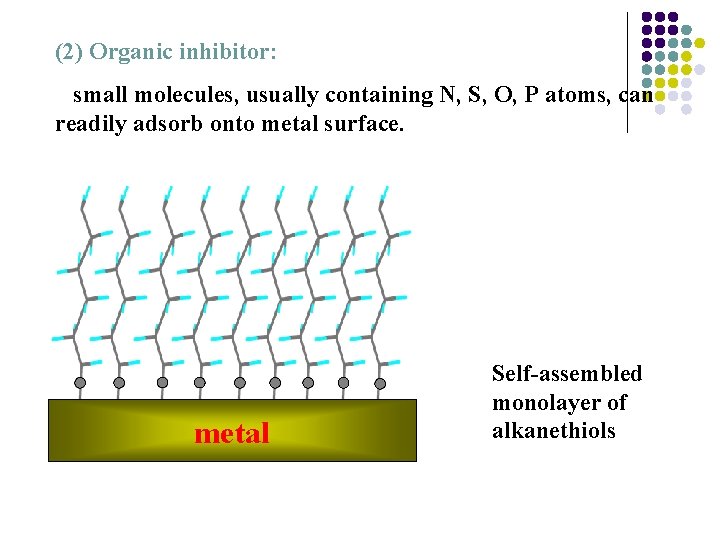
(2) Organic inhibitor: small molecules, usually containing N, S, O, P atoms, can readily adsorb onto metal surface. metal Self-assembled monolayer of alkanethiols

Discussion: 1) Cu does not react with dilute sulfuric acid, but why does the solution gradually turn blue upon exposure of the system to the air? 2) Why can Au dissolve in Na. CN solution when the air was purged. 3) Annihilation can reduce corrosion rate of metal, why?