Chapter 7 Compounds and Their Bonds Ionic Compounds

Chapter 7: Compounds and Their Bonds Ionic Compounds Naming Ionic Formulas
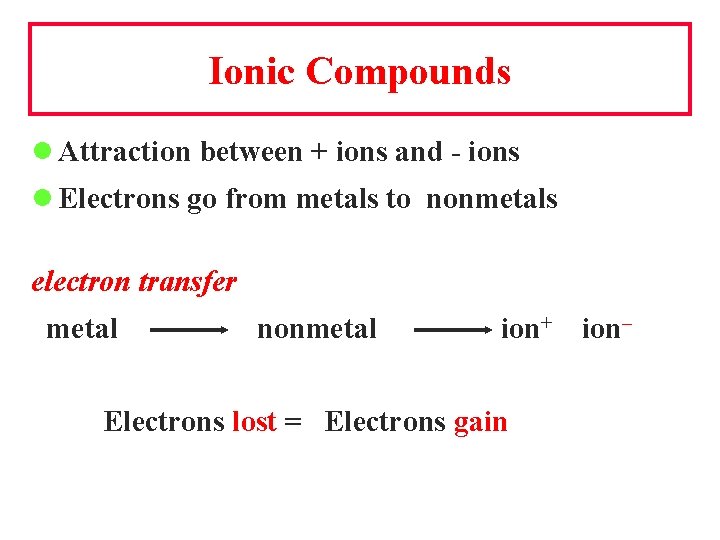
Ionic Compounds l Attraction between + ions and - ions l Electrons go from metals to nonmetals electron transfer metal nonmetal ion+ Electrons lost = Electrons gain ion–
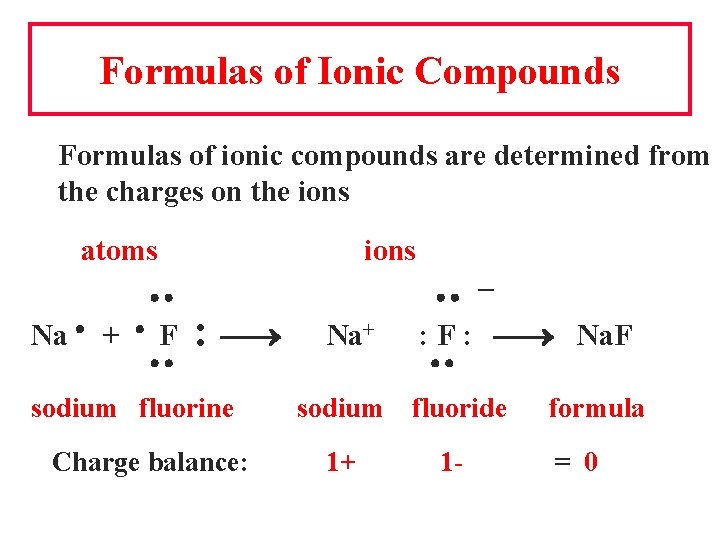
Formulas of Ionic Compounds Formulas of ionic compounds are determined from the charges on the ions atoms ions Na + F : sodium fluorine Charge balance: Na+ – : F : Na. F sodium fluoride 1+ 1 - formula = 0
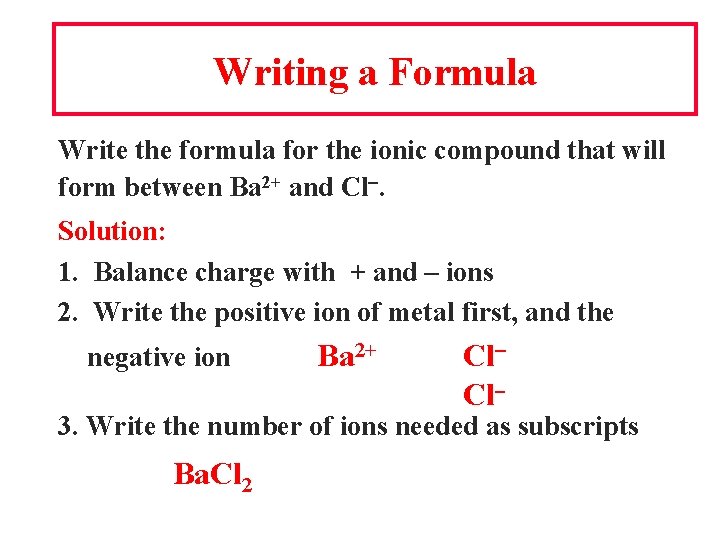
Writing a Formula Write the formula for the ionic compound that will form between Ba 2+ and Cl. Solution: 1. Balance charge with + and – ions 2. Write the positive ion of metal first, and the negative ion Ba 2+ Cl Cl 3. Write the number of ions needed as subscripts Ba. Cl 2
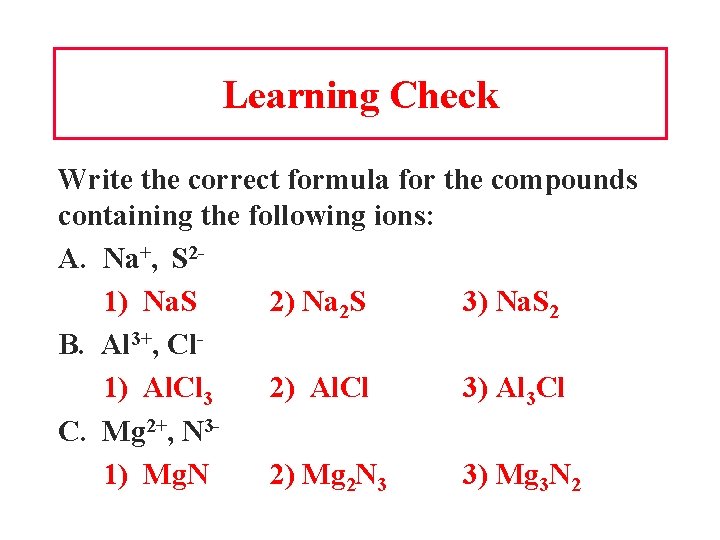
Learning Check Write the correct formula for the compounds containing the following ions: A. Na+, S 21) Na. S 2) Na 2 S 3) Na. S 2 B. Al 3+, Cl 1) Al. Cl 3 2) Al. Cl 3) Al 3 Cl C. Mg 2+, N 31) Mg. N 2) Mg 2 N 3 3) Mg 3 N 2
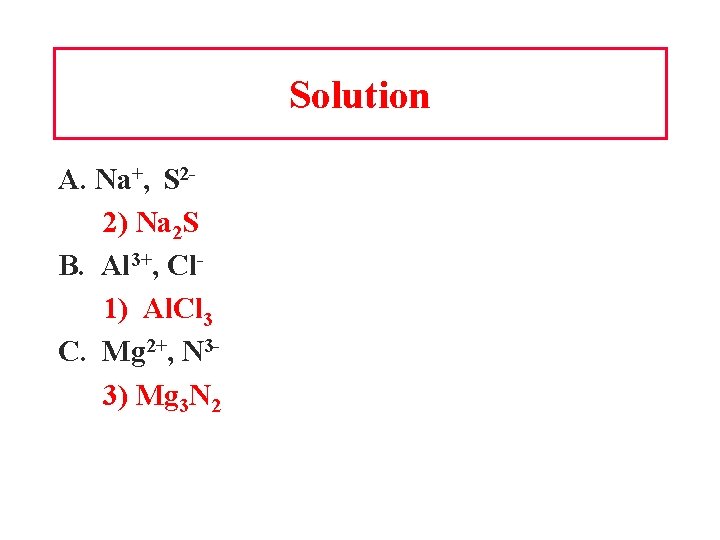
Solution A. Na+, S 22) Na 2 S B. Al 3+, Cl 1) Al. Cl 3 C. Mg 2+, N 33) Mg 3 N 2
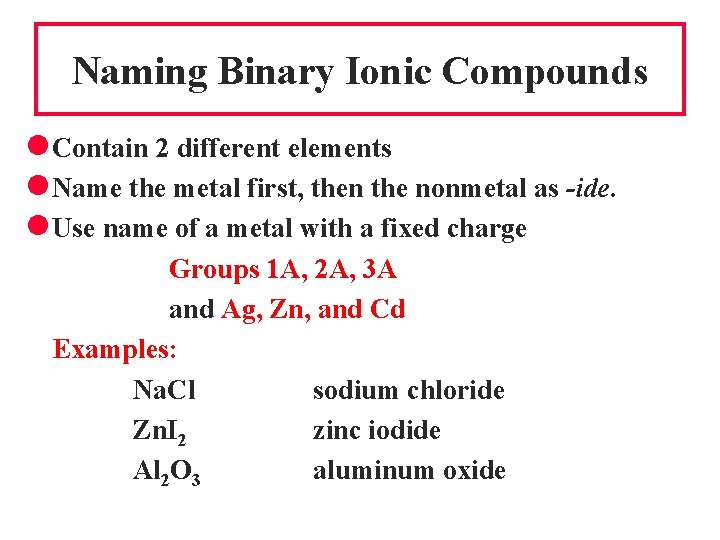
Naming Binary Ionic Compounds l Contain 2 different elements l Name the metal first, then the nonmetal as -ide. l Use name of a metal with a fixed charge Groups 1 A, 2 A, 3 A and Ag, Zn, and Cd Examples: Na. Cl sodium chloride Zn. I 2 zinc iodide Al 2 O 3 aluminum oxide
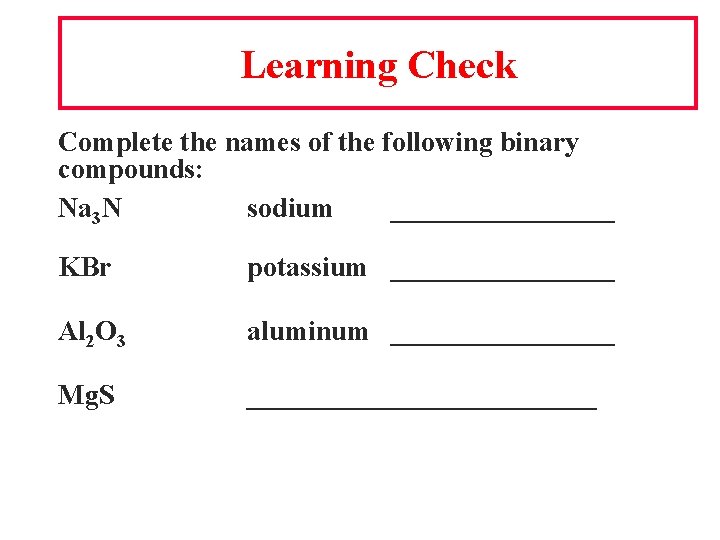
Learning Check Complete the names of the following binary compounds: Na 3 N sodium ________ KBr potassium ________ Al 2 O 3 aluminum ________ Mg. S _____________
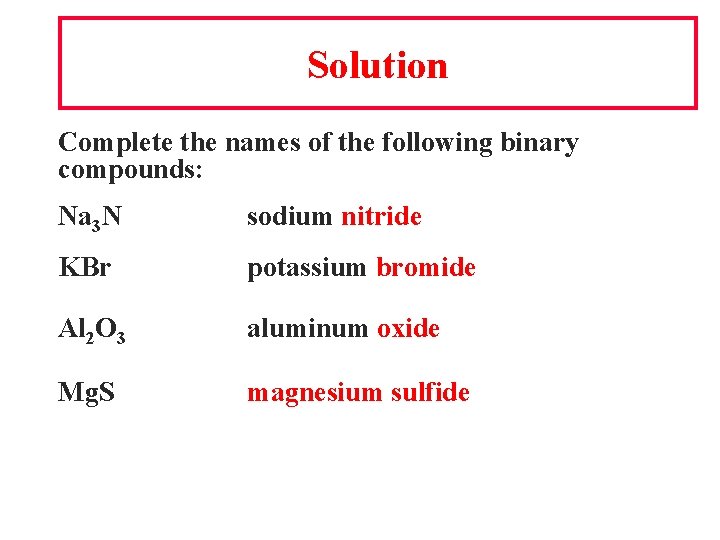
Solution Complete the names of the following binary compounds: Na 3 N sodium nitride KBr potassium bromide Al 2 O 3 aluminum oxide Mg. S magnesium sulfide
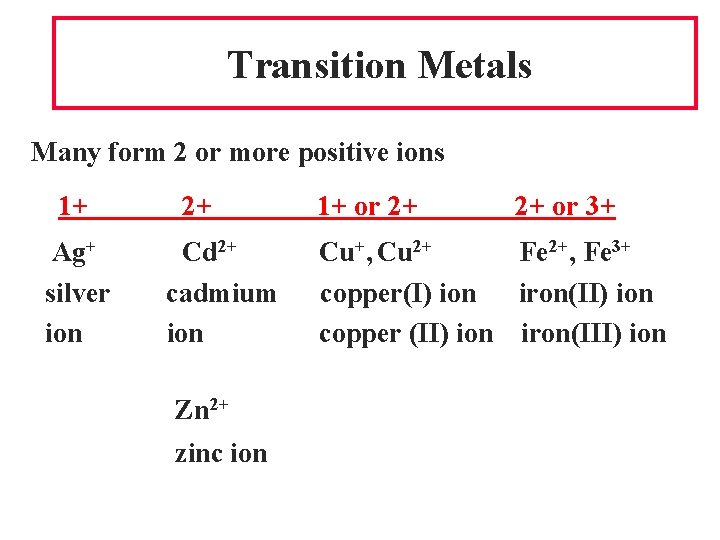
Transition Metals Many form 2 or more positive ions 1+ Ag+ silver ion 2+ Cd 2+ cadmium ion Zn 2+ zinc ion 1+ or 2+ 2+ or 3+ Cu+, Cu 2+ Fe 2+, Fe 3+ copper(I) ion iron(II) ion copper (II) ion iron(III) ion
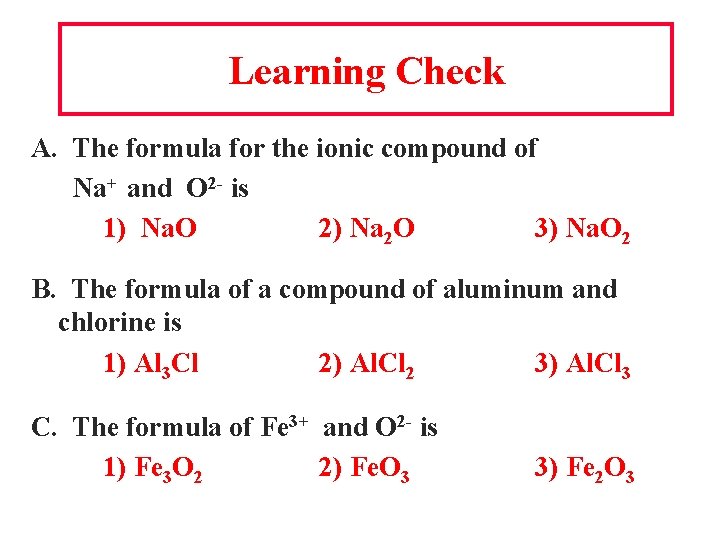
Learning Check A. The formula for the ionic compound of Na+ and O 2 - is 1) Na. O 2) Na 2 O 3) Na. O 2 B. The formula of a compound of aluminum and chlorine is 1) Al 3 Cl 2) Al. Cl 2 3) Al. Cl 3 C. The formula of Fe 3+ and O 2 - is 1) Fe 3 O 2 2) Fe. O 3 3) Fe 2 O 3
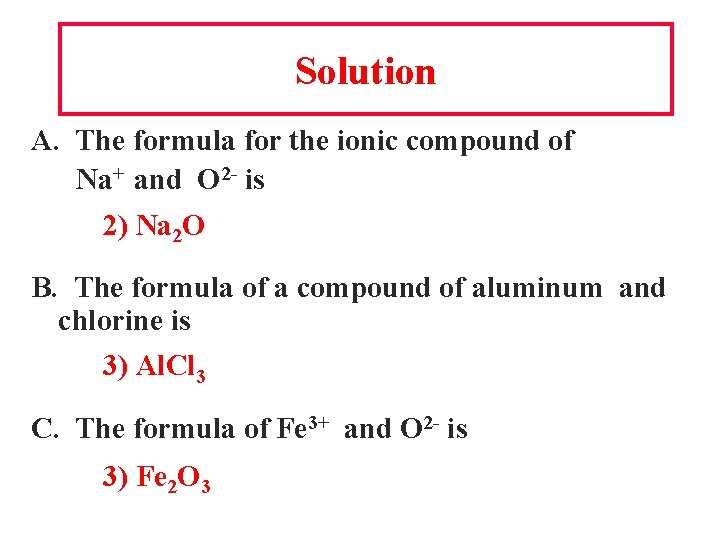
Solution A. The formula for the ionic compound of Na+ and O 2 - is 2) Na 2 O B. The formula of a compound of aluminum and chlorine is 3) Al. Cl 3 C. The formula of Fe 3+ and O 2 - is 3) Fe 2 O 3
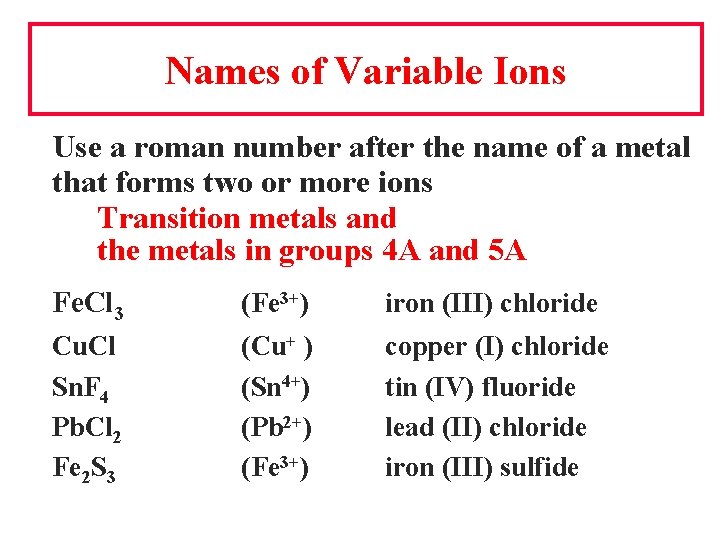
Names of Variable Ions Use a roman number after the name of a metal that forms two or more ions Transition metals and the metals in groups 4 A and 5 A Fe. Cl 3 (Fe 3+) iron (III) chloride Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Cu+ ) (Sn 4+) (Pb 2+) (Fe 3+) copper (I) chloride tin (IV) fluoride lead (II) chloride iron (III) sulfide
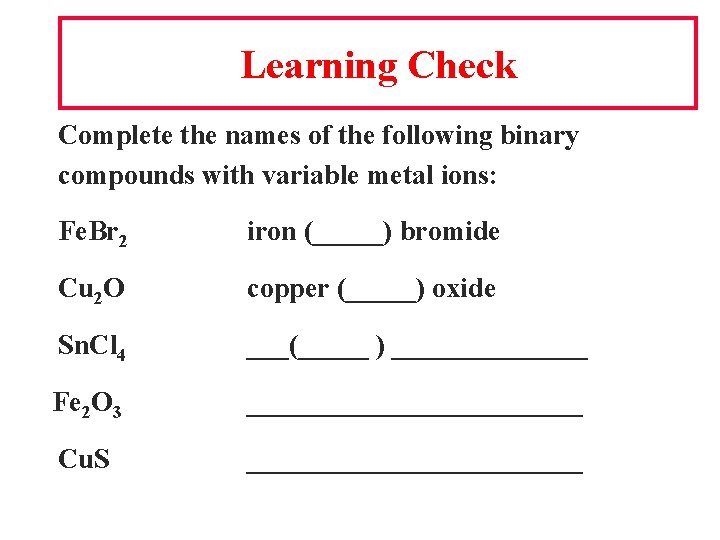
Learning Check Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron (_____) bromide Cu 2 O copper (_____) oxide Sn. Cl 4 ___(_____ ) _______ Fe 2 O 3 ____________ Cu. S ____________
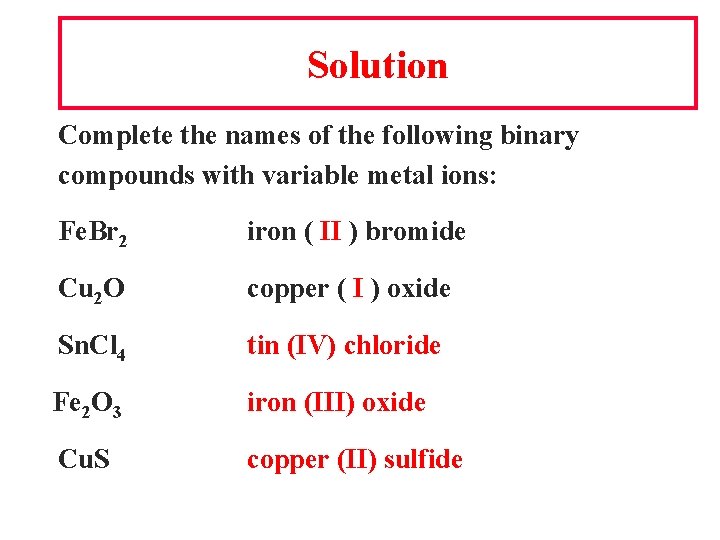
Solution Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron ( II ) bromide Cu 2 O copper ( I ) oxide Sn. Cl 4 tin (IV) chloride Fe 2 O 3 iron (III) oxide Cu. S copper (II) sulfide
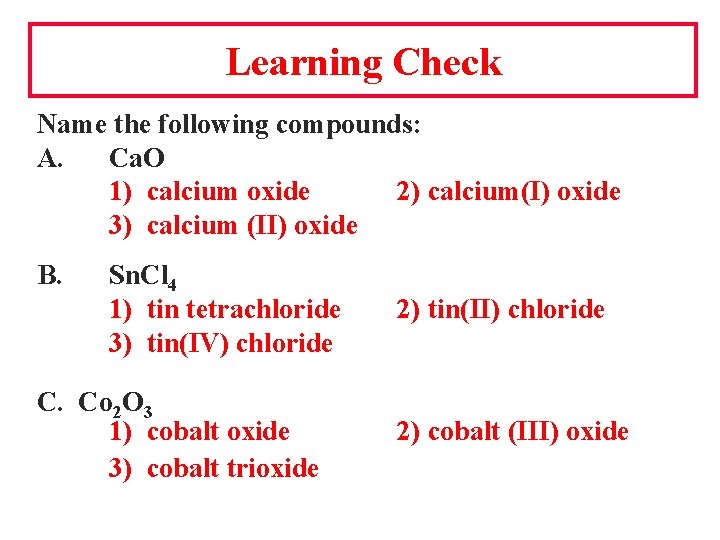
Learning Check Name the following compounds: A. Ca. O 1) calcium oxide 2) calcium(I) oxide 3) calcium (II) oxide B. Sn. Cl 4 1) tin tetrachloride 3) tin(IV) chloride C. Co 2 O 3 1) cobalt oxide 3) cobalt trioxide 2) tin(II) chloride 2) cobalt (III) oxide
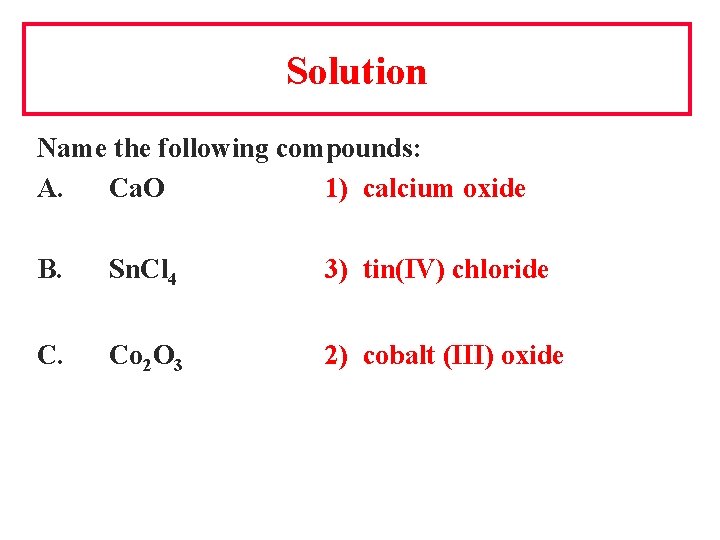
Solution Name the following compounds: A. Ca. O 1) calcium oxide B. Sn. Cl 4 3) tin(IV) chloride C. Co 2 O 3 2) cobalt (III) oxide
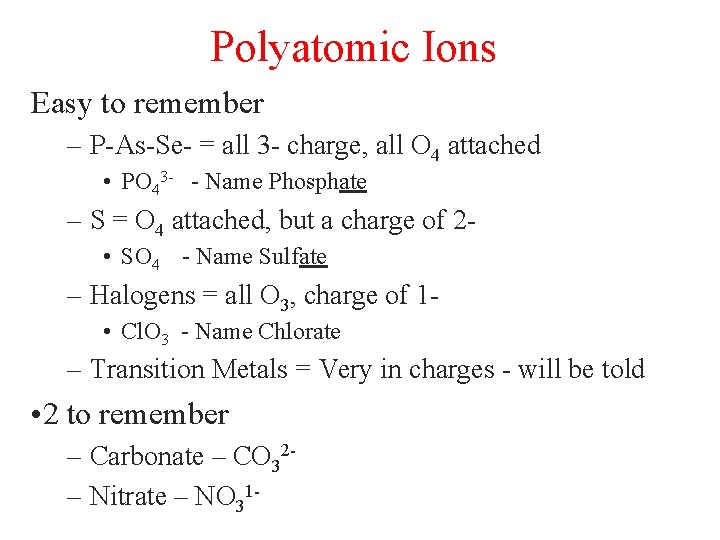
Polyatomic Ions Easy to remember – P-As-Se- = all 3 - charge, all O 4 attached • PO 43 - - Name Phosphate – S = O 4 attached, but a charge of 2 • SO 4 - Name Sulfate – Halogens = all O 3, charge of 1 • Cl. O 3 - Name Chlorate – Transition Metals = Very in charges - will be told • 2 to remember – Carbonate – CO 32– Nitrate – NO 31 -
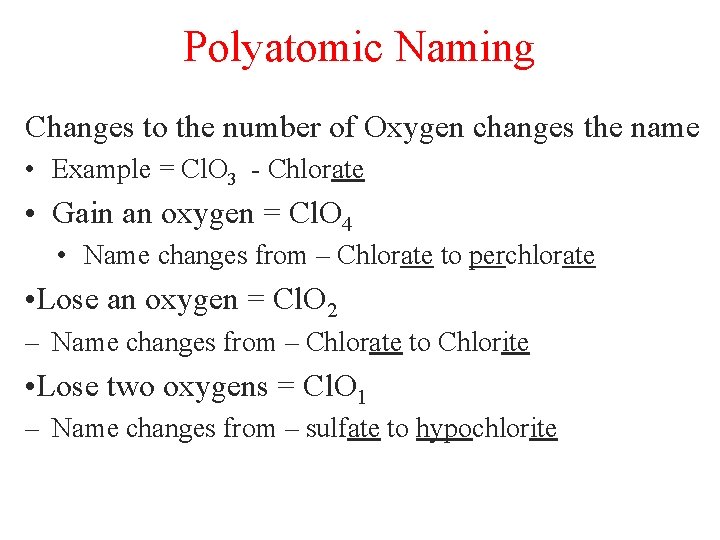
Polyatomic Naming Changes to the number of Oxygen changes the name • Example = Cl. O 3 - Chlorate • Gain an oxygen = Cl. O 4 • Name changes from – Chlorate to perchlorate • Lose an oxygen = Cl. O 2 – Name changes from – Chlorate to Chlorite • Lose two oxygens = Cl. O 1 – Name changes from – sulfate to hypochlorite
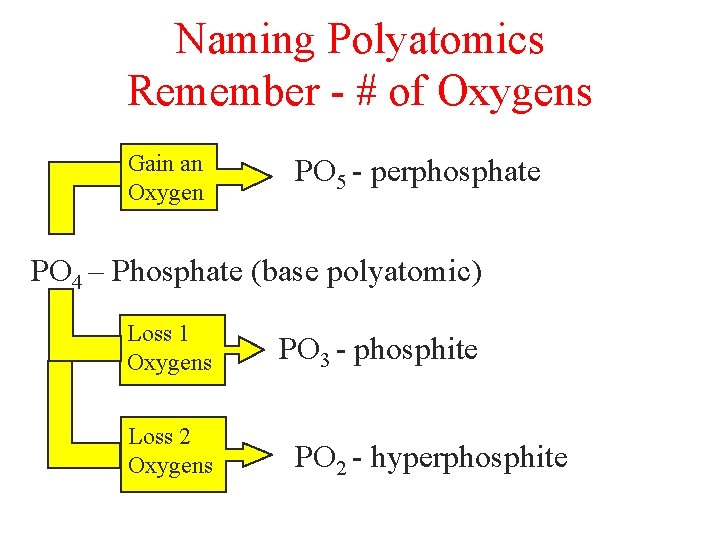
Naming Polyatomics Remember - # of Oxygens Gain an Oxygen PO 5 - perphosphate PO 4 – Phosphate (base polyatomic) Loss 1 Oxygens Loss 2 Oxygens PO 3 - phosphite PO 2 - hyperphosphite
- Slides: 20