Chapter 7 Chemical Reactions and Quantities Types of





- Slides: 21

Chapter 7 Chemical Reactions and Quantities Types of Reactions Oxidation-Reduction Reactions

Type of Reactions Chemical reactions are classified into four general types l Synthesis l Decomposition l Single Replacement l Double Replacement l Combustion

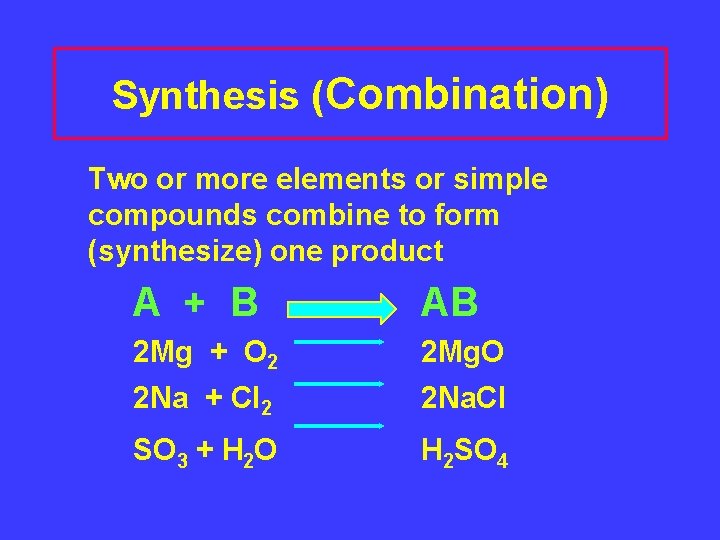
Synthesis (Combination) Two or more elements or simple compounds combine to form (synthesize) one product A + B AB 2 Mg + O 2 2 Mg. O 2 Na + Cl 2 2 Na. Cl SO 3 + H 2 O H 2 SO 4

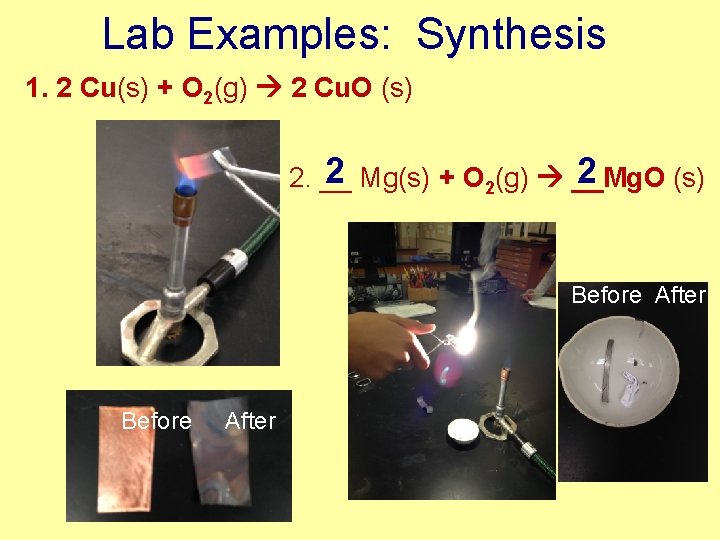
Lab Examples: Synthesis 1. 2 Cu(s) + O 2(g) 2 Cu. O (s) 2 Mg(s) + O 2(g) __Mg. O 2 2. __ (s) Before After

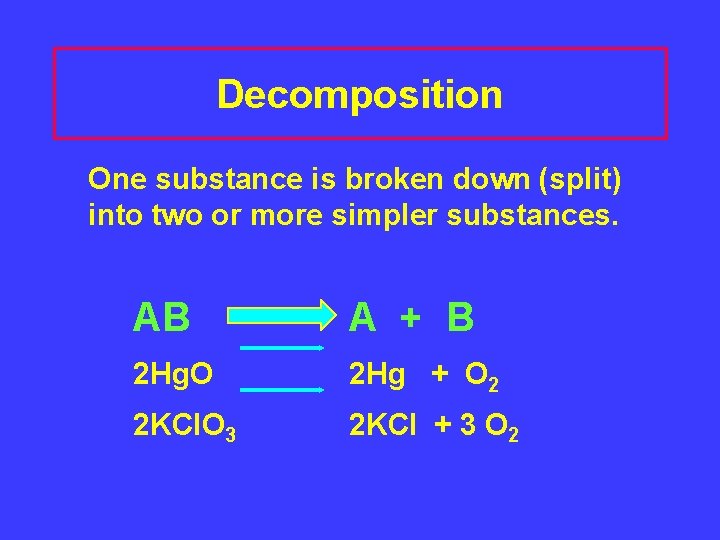
Decomposition One substance is broken down (split) into two or more simpler substances. AB A + B 2 Hg. O 2 Hg + O 2 2 KCl. O 3 2 KCl + 3 O 2

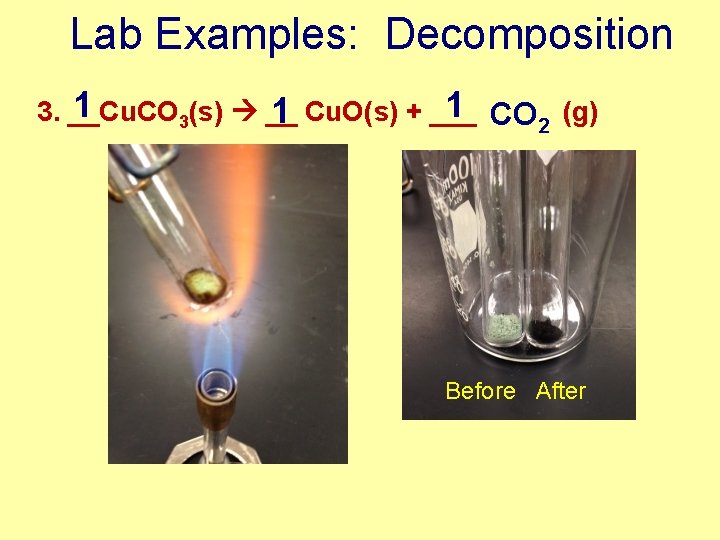
Lab Examples: Decomposition 1 1 CO 2 (g) 3. __Cu. CO 1 Cu. O(s) + ___ 3(s) __ Before After

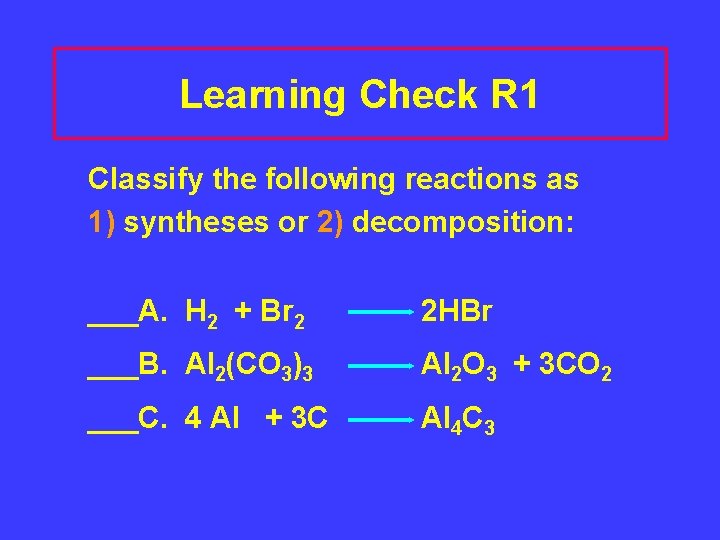
Learning Check R 1 Classify the following reactions as 1) syntheses or 2) decomposition: ___A. H 2 + Br 2 2 HBr ___B. Al 2(CO 3)3 Al 2 O 3 + 3 CO 2 ___C. 4 Al + 3 C Al 4 C 3

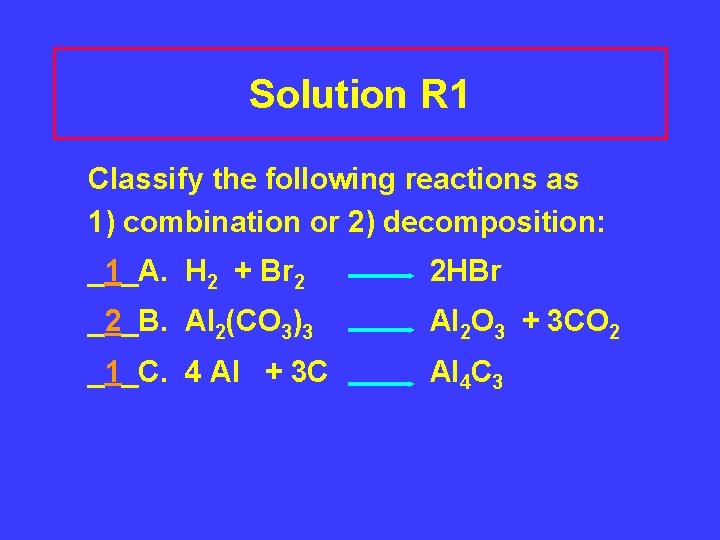
Solution R 1 Classify the following reactions as 1) combination or 2) decomposition: _1_A. H 2 + Br 2 2 HBr _2_B. Al 2(CO 3)3 Al 2 O 3 + 3 CO 2 _1_C. 4 Al + 3 C Al 4 C 3

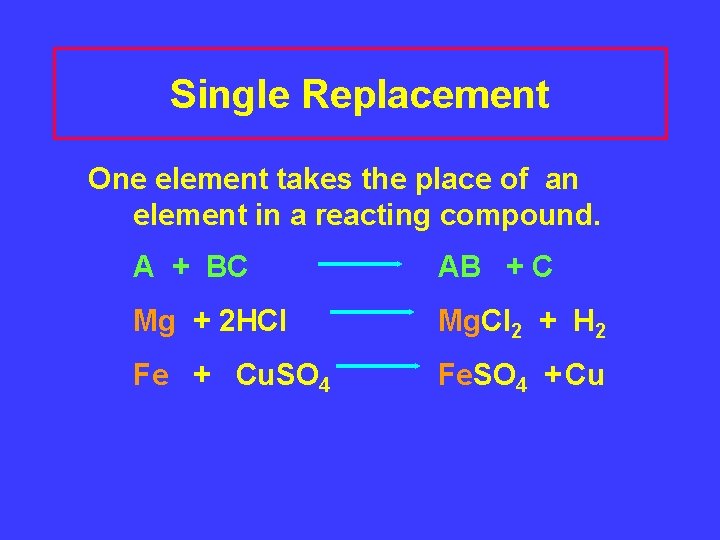
Single Replacement One element takes the place of an element in a reacting compound. A + BC AB + C Mg + 2 HCl Mg. Cl 2 + H 2 Fe + Cu. SO 4 Fe. SO 4 + Cu

Lab: Single Replacement 1 H 2 (g) Zn 2 HCl (aq) 1_______ Zn. Cl 2 (aq) + __ 4. 1 ___(s) + __ Before During After

Lab: Single Replacement Fe. SO 4 (aq) +1 Cu 5. 1____(s) + __ ___(s) Fe 1 Cu. SO 4 (aq) 1_______ Before During After

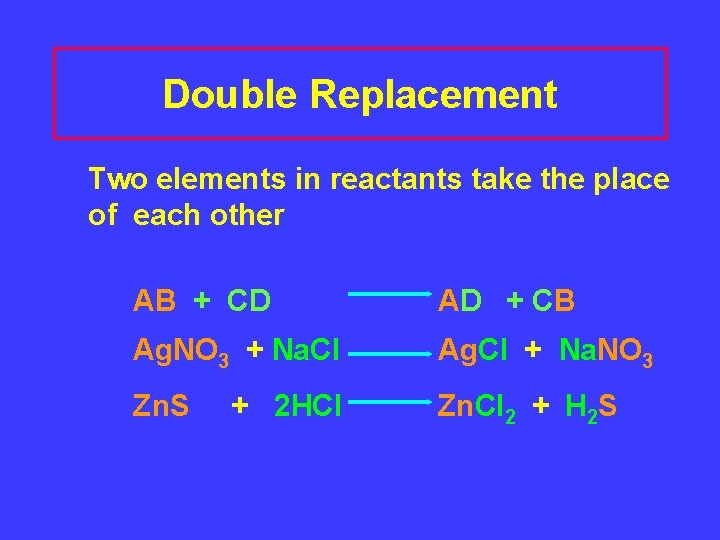
Double Replacement Two elements in reactants take the place of each other AB + CD AD + CB Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 Zn. S Zn. Cl 2 + H 2 S + 2 HCl

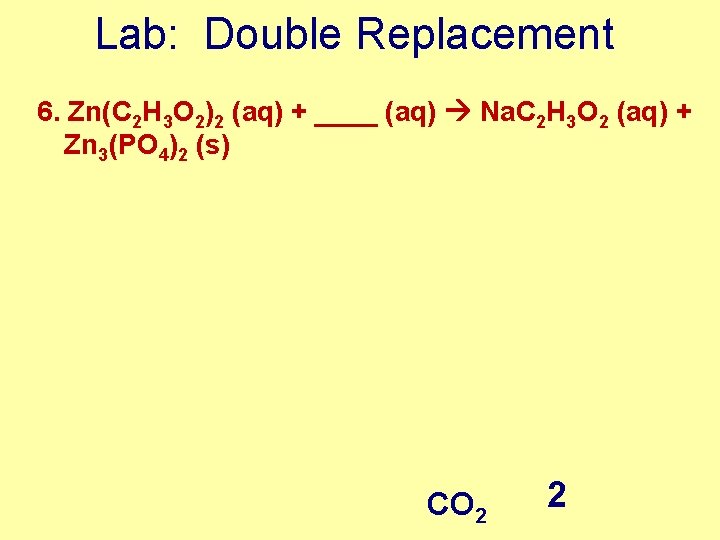
Lab: Double Replacement 6. Zn(C 2 H 3 O 2)2 (aq) + ____ (aq) Na. C 2 H 3 O 2 (aq) + Zn 3(PO 4)2 (s) CO 2 2

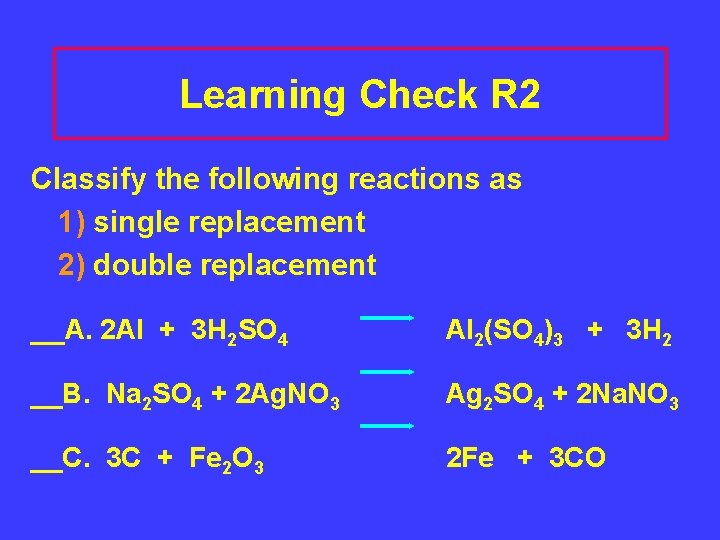
Learning Check R 2 Classify the following reactions as 1) single replacement 2) double replacement __A. 2 Al + 3 H 2 SO 4 Al 2(SO 4)3 + 3 H 2 __B. Na 2 SO 4 + 2 Ag. NO 3 Ag 2 SO 4 + 2 Na. NO 3 __C. 3 C + Fe 2 O 3 2 Fe + 3 CO

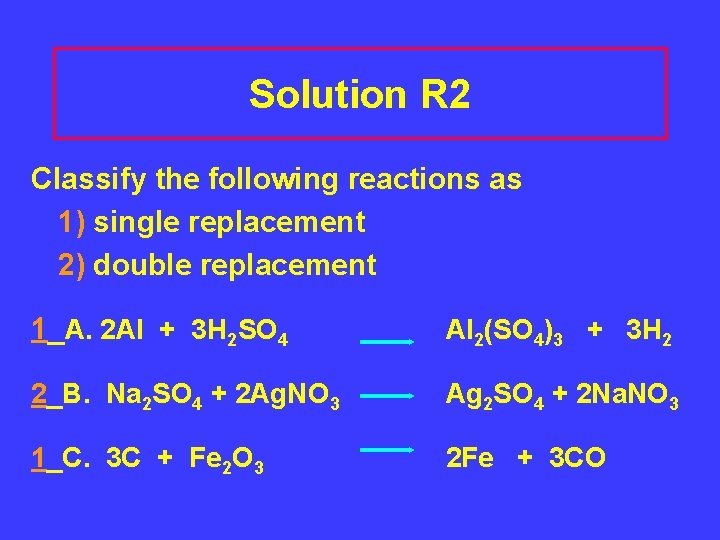
Solution R 2 Classify the following reactions as 1) single replacement 2) double replacement 1_A. 2 Al + 3 H 2 SO 4 Al 2(SO 4)3 + 3 H 2 2_B. Na 2 SO 4 + 2 Ag. NO 3 Ag 2 SO 4 + 2 Na. NO 3 1_C. 3 C + Fe 2 O 3 2 Fe + 3 CO

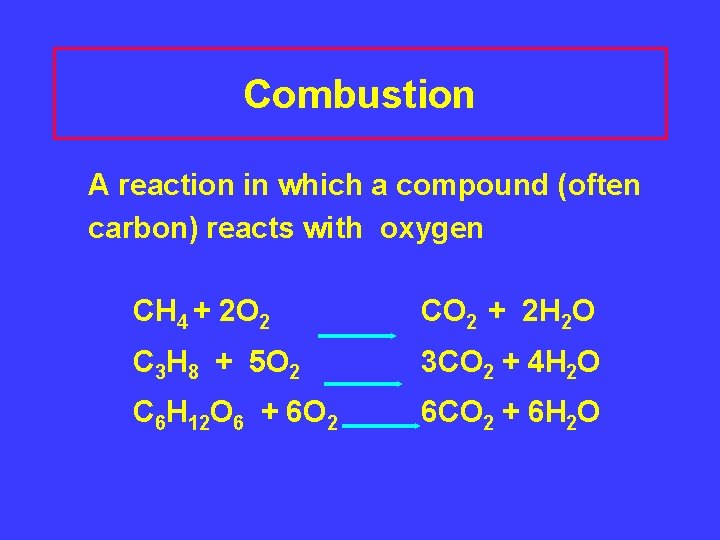
Combustion A reaction in which a compound (often carbon) reacts with oxygen CH 4 + 2 O 2 CO 2 + 2 H 2 O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

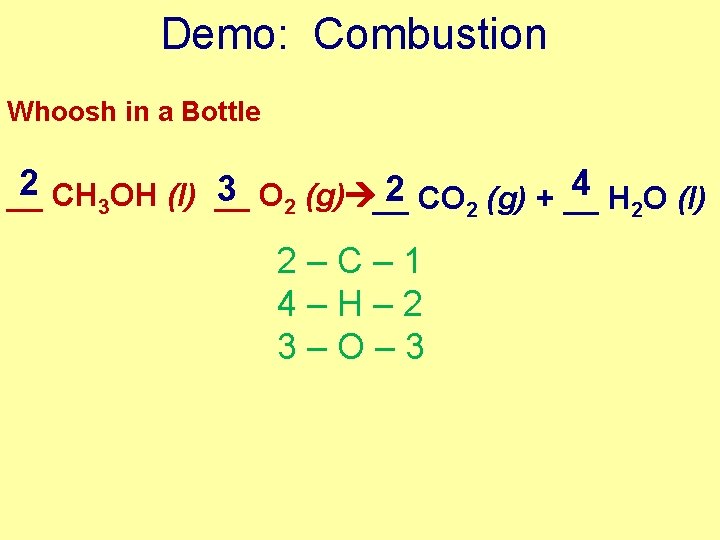
Demo: Combustion Whoosh in a Bottle 2 CH OH (l) __ 4 3 2 __ O (g) __ CO 2 (g) + __ H 2 O (l) 3 2 2–C– 1 4–H– 2 3–O– 3

Demo: Combustion

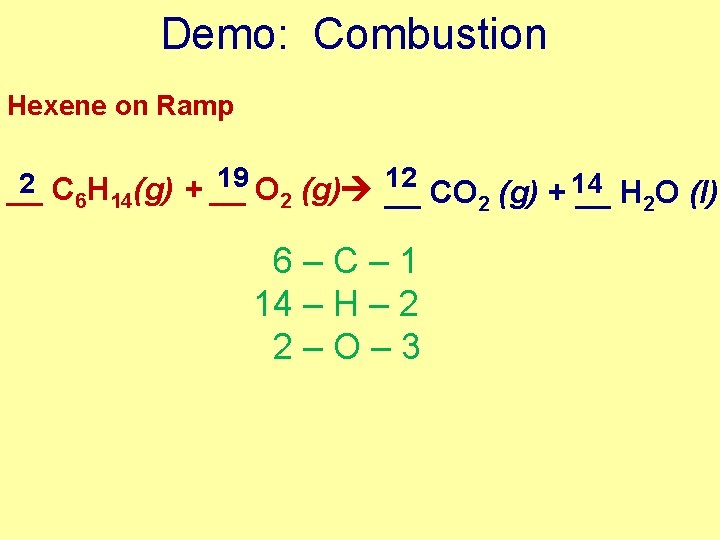
Demo: Combustion Hexene on Ramp 19 12 2 __ C 6 H 14(g) + __ O 2 (g) __ CO 2 (g) + 14 __ H 2 O (l) 6–C– 1 14 – H – 2 2–O– 3

Demo: Combustion

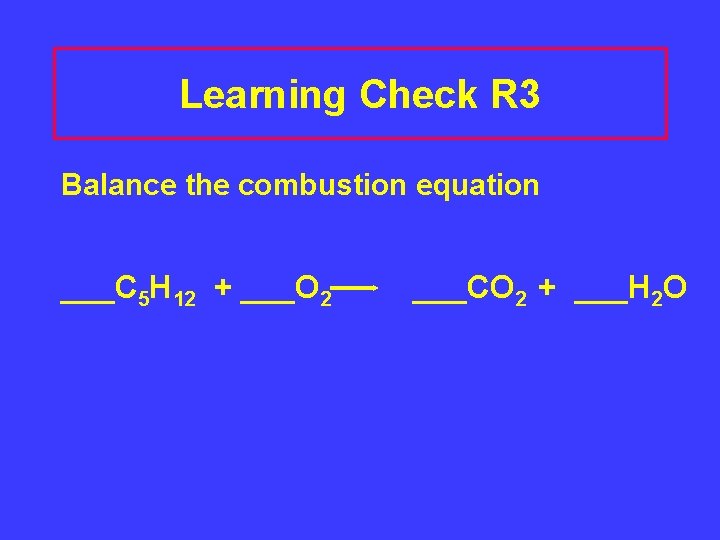
Learning Check R 3 Balance the combustion equation ___C 5 H 12 + ___O 2 ___CO 2 + ___H 2 O