Chapter 7 Chemical Quantities 7 3 Molar Mass

- Slides: 12

• Chapter 7 Chemical Quantities 7. 3 Molar Mass 1

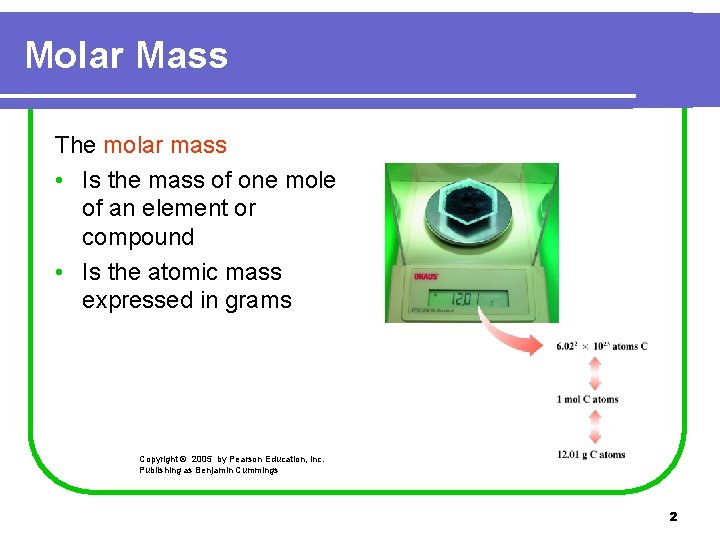
Molar Mass The molar mass • Is the mass of one mole of an element or compound • Is the atomic mass expressed in grams Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 2

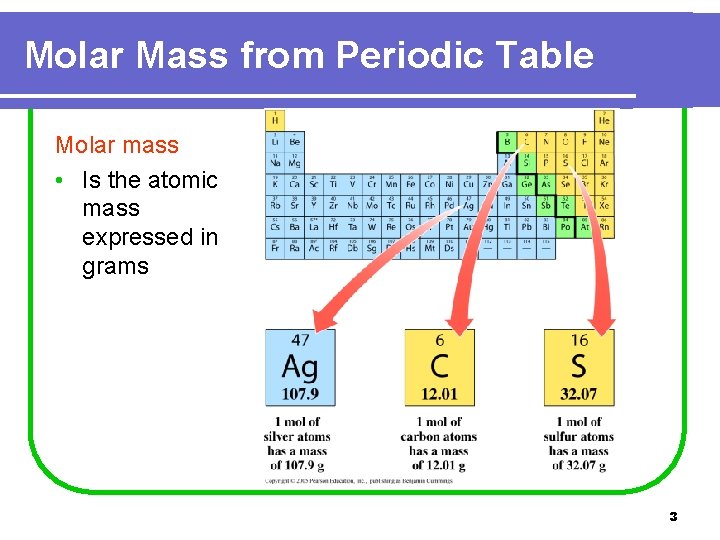
Molar Mass from Periodic Table Molar mass • Is the atomic mass expressed in grams 3

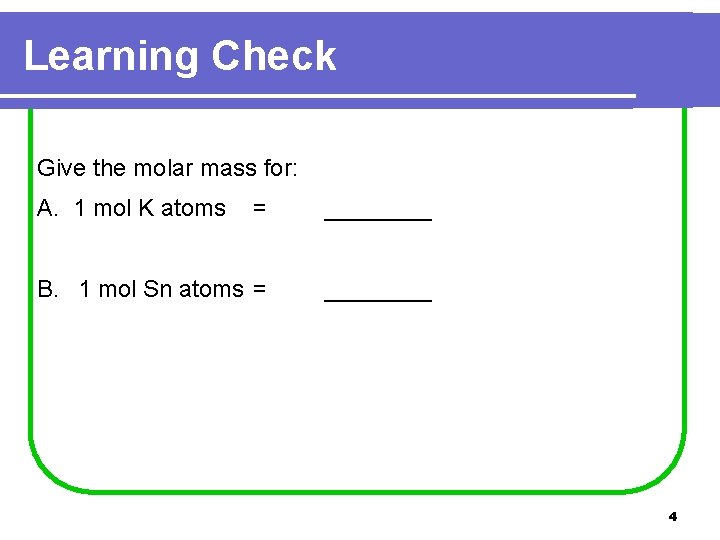
Learning Check Give the molar mass for: A. 1 mol K atoms = ____ B. 1 mol Sn atoms = ____ 4

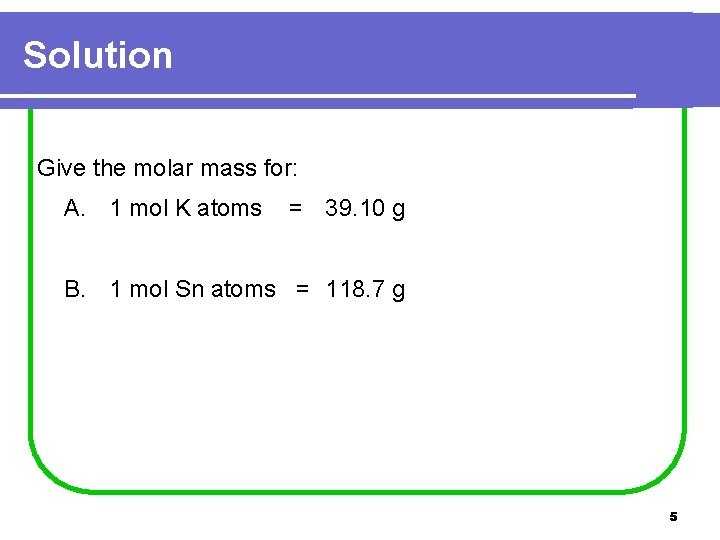
Solution Give the molar mass for: A. 1 mol K atoms = 39. 10 g B. 1 mol Sn atoms = 118. 7 g 5

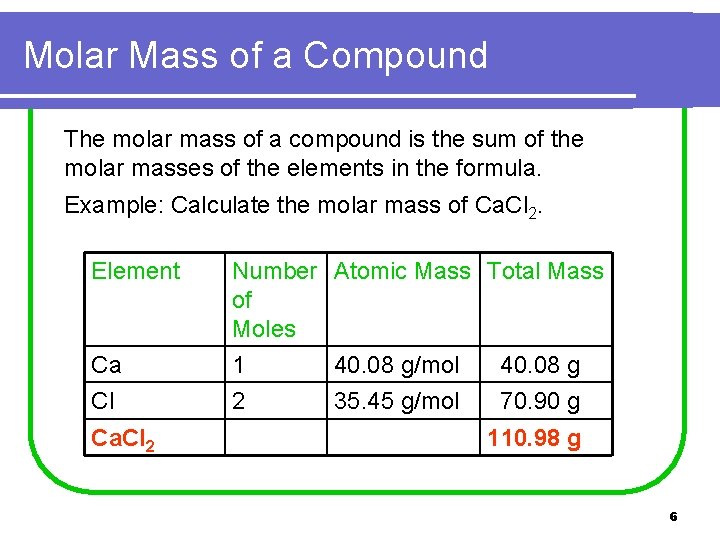
Molar Mass of a Compound The molar mass of a compound is the sum of the molar masses of the elements in the formula. Example: Calculate the molar mass of Ca. Cl 2. Element Ca Number Atomic Mass Total Mass of Moles 1 40. 08 g/mol 40. 08 g Cl 2 Ca. Cl 2 35. 45 g/mol 70. 90 g 110. 98 g 6

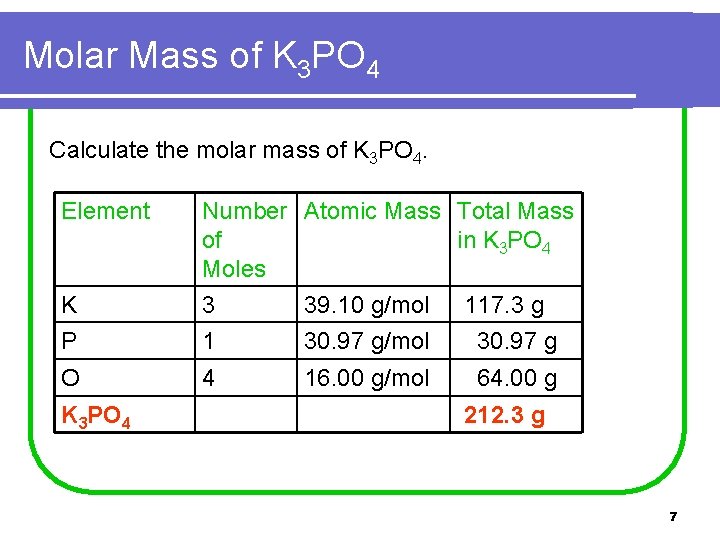
Molar Mass of K 3 PO 4 Calculate the molar mass of K 3 PO 4. Element K Number Atomic Mass Total Mass of in K 3 PO 4 Moles 3 39. 10 g/mol 117. 3 g P 1 30. 97 g/mol 30. 97 g O 4 16. 00 g/mol 64. 00 g K 3 PO 4 212. 3 g 7

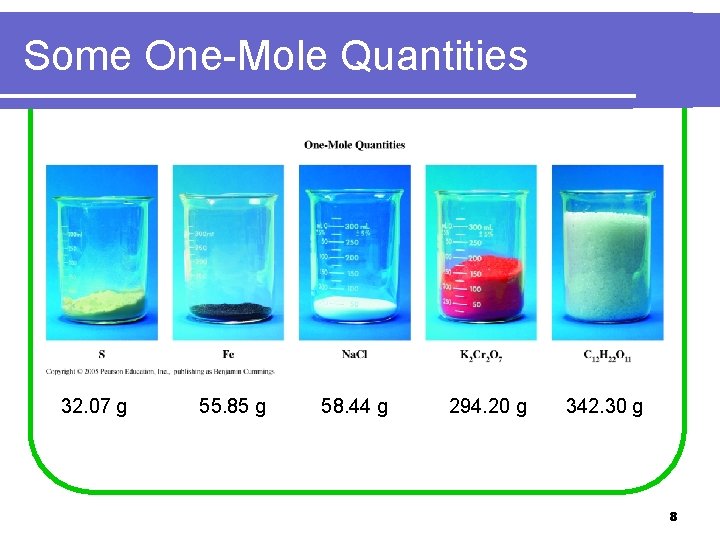
Some One-Mole Quantities 32. 07 g 55. 85 g 58. 44 g 294. 20 g 342. 30 g 8

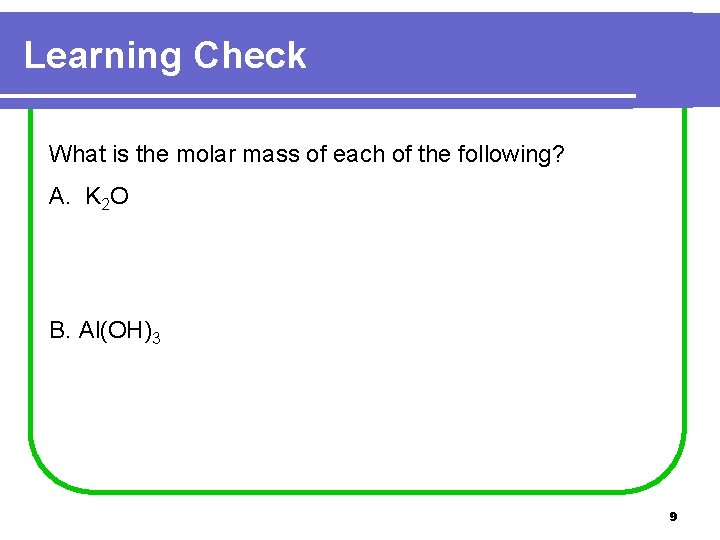
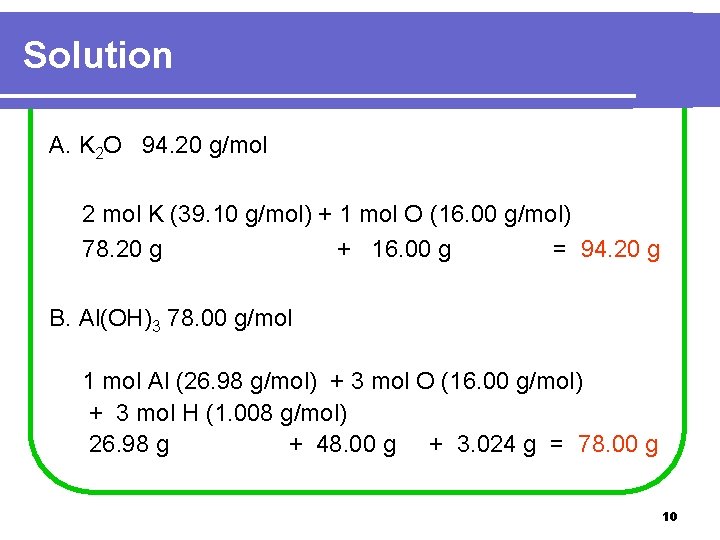
Learning Check What is the molar mass of each of the following? A. K 2 O B. Al(OH)3 9

Solution A. K 2 O 94. 20 g/mol 2 mol K (39. 10 g/mol) + 1 mol O (16. 00 g/mol) 78. 20 g + 16. 00 g = 94. 20 g B. Al(OH)3 78. 00 g/mol 1 mol Al (26. 98 g/mol) + 3 mol O (16. 00 g/mol) + 3 mol H (1. 008 g/mol) 26. 98 g + 48. 00 g + 3. 024 g = 78. 00 g 10

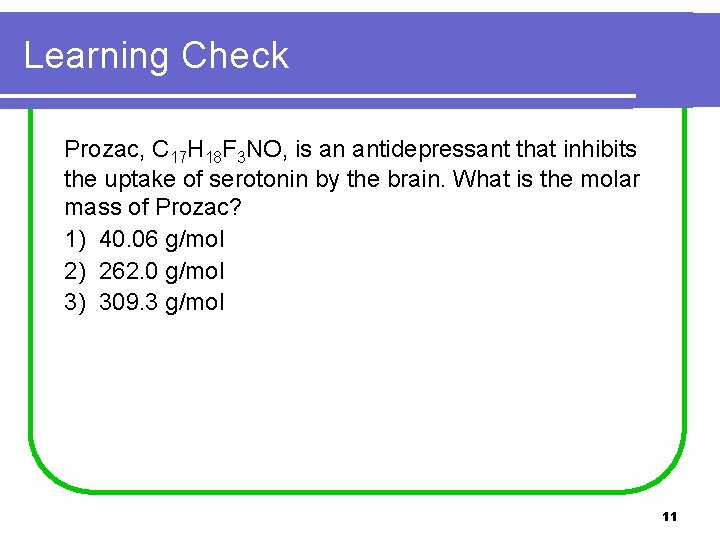
Learning Check Prozac, C 17 H 18 F 3 NO, is an antidepressant that inhibits the uptake of serotonin by the brain. What is the molar mass of Prozac? 1) 40. 06 g/mol 2) 262. 0 g/mol 3) 309. 3 g/mol 11

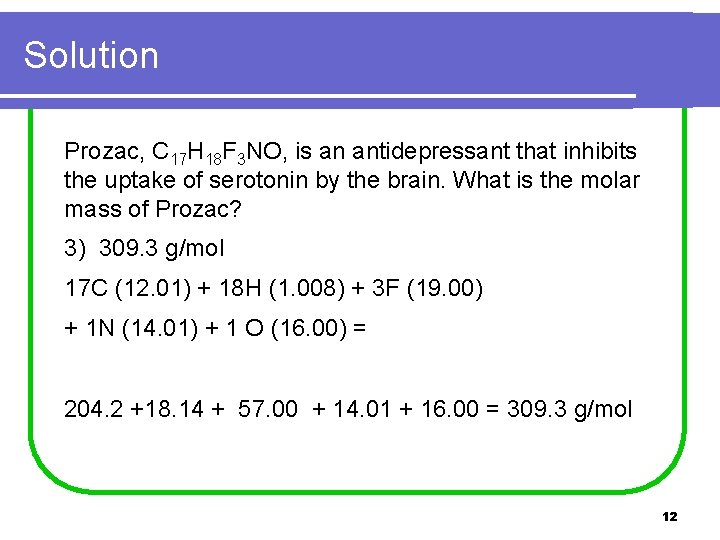
Solution Prozac, C 17 H 18 F 3 NO, is an antidepressant that inhibits the uptake of serotonin by the brain. What is the molar mass of Prozac? 3) 309. 3 g/mol 17 C (12. 01) + 18 H (1. 008) + 3 F (19. 00) + 1 N (14. 01) + 1 O (16. 00) = 204. 2 +18. 14 + 57. 00 + 14. 01 + 16. 00 = 309. 3 g/mol 12