Chapter 7 Chemical Formulas Equations What goes in








![How did you get on? ? Here are the answers: [1] CH 4 + How did you get on? ? Here are the answers: [1] CH 4 +](https://slidetodoc.com/presentation_image_h/0ab8962a212a867004b1dddde71d80a0/image-20.jpg)

- Slides: 21

Chapter 7 Chemical Formulas & Equations What goes in must come out!

Objectives: l l l Determine how to read and understand a balanced chemical equation. Examine some reactions that release energy and others that absorb energy. Explain the Law of Conservation of Mass.

Why It’s Important? l l Chemical Reactions warm your home, cook your meals, digest your food, and power cars and trucks. Allows chemists to save time in performing a chemical reaction.

Vocabulary : l l l Chemical reaction Reactant Product Chemical equation Endothermic Reaction Exothermic Reaction

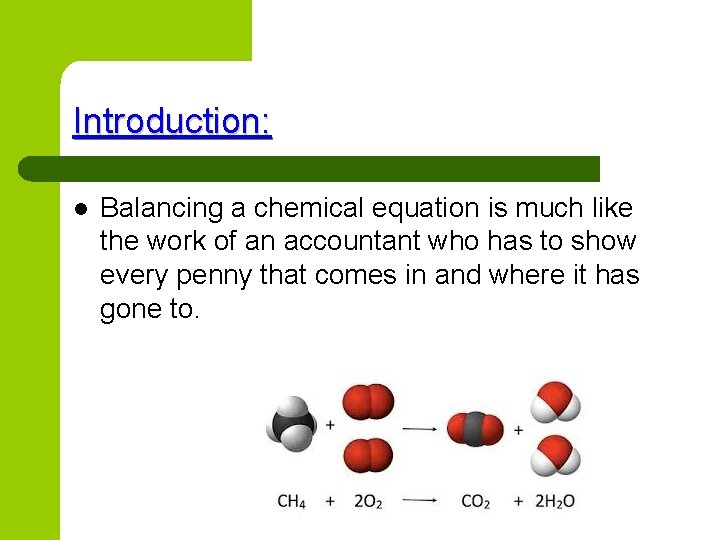
Introduction: l Balancing a chemical equation is much like the work of an accountant who has to show every penny that comes in and where it has gone to.

Review: Chemical Reaction l A process of Chemical Change l Write your observations. (Demo)

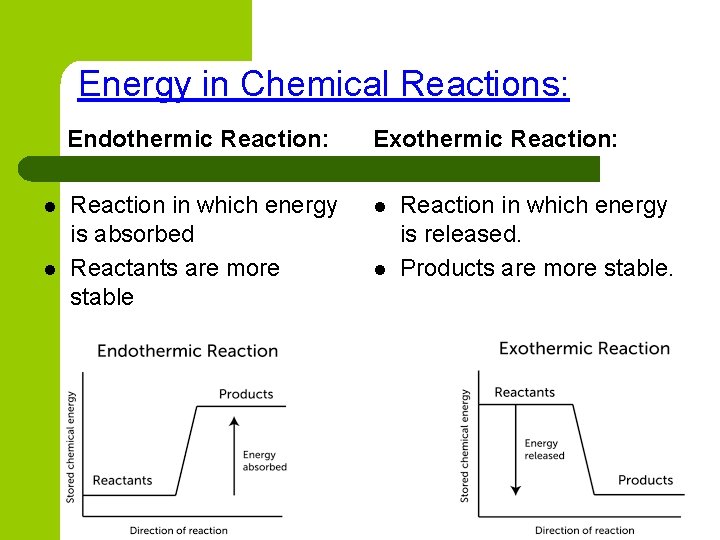
Energy in Chemical Reactions: l l Endothermic Reaction: Exothermic Reaction: Reaction in which energy is absorbed Reactants are more stable l l Reaction in which energy is released. Products are more stable.

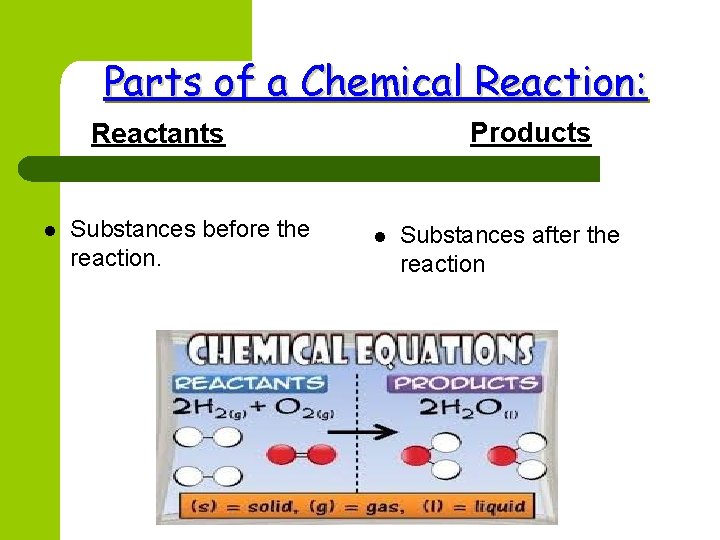
Parts of a Chemical Reaction: Products Reactants l Substances before the reaction. l Substances after the reaction

Define Chemical Equation: l Consists of reactants, products, physical state and the number of substances on the reaction. Ex. Vinegar + Baking Soda bubbles + gas

Using Chemical Names: Acetic Acid( vinegar) + Sodium hydrogen carbonate (baking soda) Sodium Acetate + Water + Carbon dioxide (Gas)

Using Chemical Formulas: CH 3 COOH (acetic acid) + Na. HCO 3 (Sodium Hydrogen Carbonate) CH 3 COONA (Sodium Acetate) + H 2 O (water) + CO 2 (carbon dioxide)

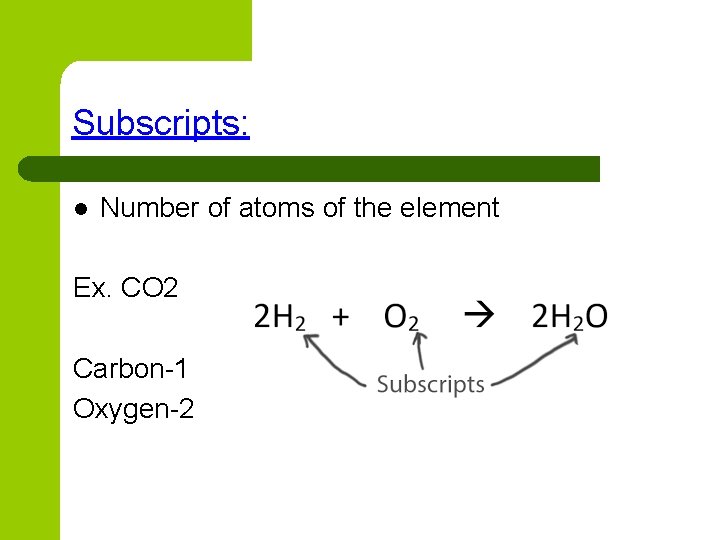
Subscripts: l Number of atoms of the element Ex. CO 2 Carbon-1 Oxygen-2

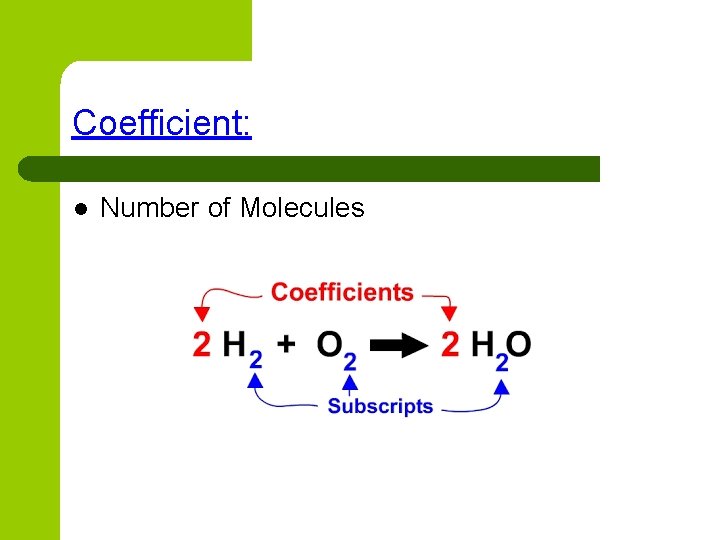
Coefficient: l Number of Molecules

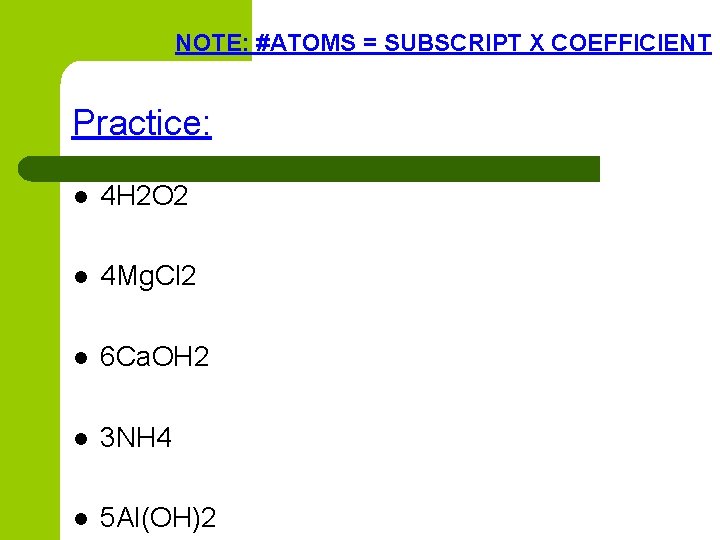
NOTE: #ATOMS = SUBSCRIPT X COEFFICIENT Practice: l 4 H 2 O 2 l 4 Mg. Cl 2 l 6 Ca. OH 2 l 3 NH 4 l 5 Al(OH)2

Why Balance a Chemical Equation? Because of the principle of the Conservation of Matter, an equation must be balanced. * It must have the same number of atoms of the kind on both sides. same Lavoisier, 1788

Law of Conservation of Mass You need to remember this law! l The Law of Conservation of Mass states: that mass is neither created nor destroyed in any chemical reaction. * Therefore balancing of equations requires the same number of atoms on both sides of a chemical reaction. l The number of atoms in the Reactants must equal the Number of atoms in the Products

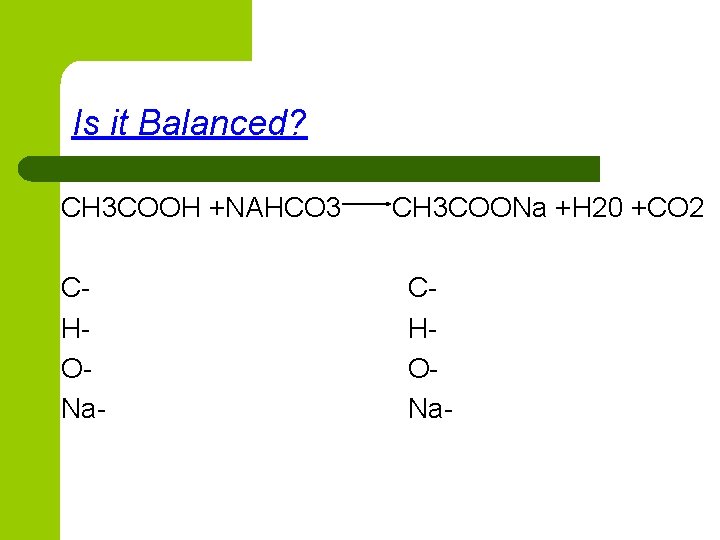
Is it Balanced? CH 3 COOH +NAHCO 3 CHONa- CH 3 COONa +H 20 +CO 2 CHONa-

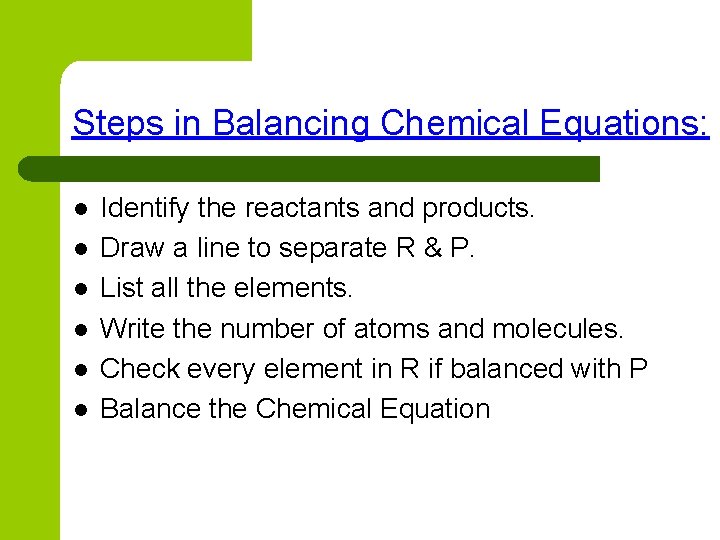
Steps in Balancing Chemical Equations: l l l Identify the reactants and products. Draw a line to separate R & P. List all the elements. Write the number of atoms and molecules. Check every element in R if balanced with P Balance the Chemical Equation

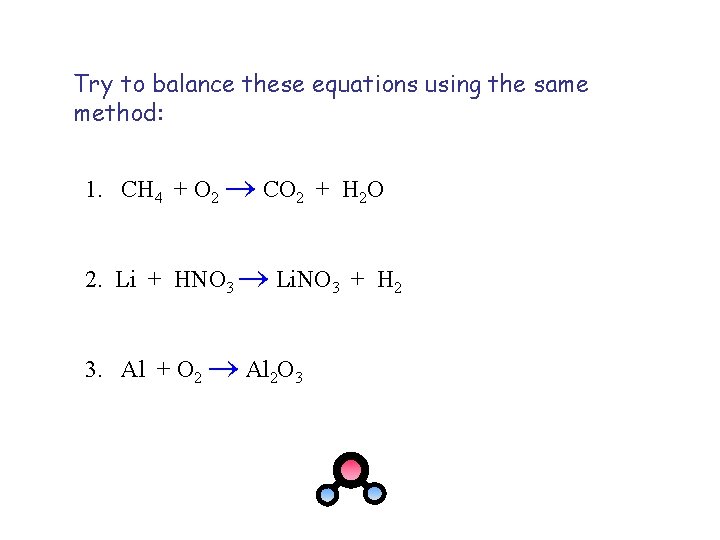
Try to balance these equations using the same method: 1. CH 4 + O 2 CO 2 + H 2 O 2. Li + HNO 3 Li. NO 3 + H 2 3. Al + O 2 Al 2 O 3
![How did you get on Here are the answers 1 CH 4 How did you get on? ? Here are the answers: [1] CH 4 +](https://slidetodoc.com/presentation_image_h/0ab8962a212a867004b1dddde71d80a0/image-20.jpg)
How did you get on? ? Here are the answers: [1] CH 4 + 2 O 2 CO 2 + 2 H 2 O [2] 2 Li + 2 HNO 3 2 Li. NO 3 + H 2 [3] 4 Al + 3 O 2 2 Al 2 O 3

Closure: Reflection: • In 2 -3 sentences explain why balancing equation is important?