CHAPTER 7 CHEMICAL FORMULAS AND COMPOUNDS TEST REVIEW









- Slides: 14

CHAPTER 7: CHEMICAL FORMULAS AND COMPOUNDS TEST REVIEW SHEET

1. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate___ 1. Number of atoms or ions of each elements that are combined in the compound. 2. What is the formula for sulfur dichloride? 2. SCl 2 3. How many atoms of fluorine are present in a molecule of carbon tetraflouride, CF 4? 3. 4 4. What is the formula for the compound formed between calcium ions and chloride ions? 4. Ca. Cl 2

5. What is the formula for barium hydroxide? 5. Ba(OH)2 6. Name the compound Ni(Cl. O 3)2. 6. Nickel (II) Chlorate 7. Name the compound KCl. O 3 7. Potassium Chlorate 8. Name the compound Si. O 2 (Si is on the right side of the metal/nonmetal line 8. Silicon Dioxide

9. What is the oxidation number of hydrogen in H 2 O? 9. +1 10. Name the compound SO 3 10. Sulfur Trioxide 11. What is the formula for the compound formed by lead (II) ions and chromate ions? 11. Pb. Cr. O 4 12. What is the formula for aluminum sulfate? 12. Al 2(SO 4)3

13. Name the compound N 2 O 3 13. Dinitrogen Trioxide 14. What is the formula silicon dioxide? 14. Si. O 2 15. Name the compound Zn 3(PO 4)2 15. Zinc Phosphate 16. What is the formula for dinitrogen trioxide? 16. N 2 O 3

17. What is the formula for diphosphorus pentoxide? 17. P 2 O 5 18. What is the formula for hydrochloric acid? 18. HCl 19. The oxidation number of fluorine is? 19. -1 20. What is the oxidation number of a pure element or unbounded element ex. Na 20. 0

21. In a compound, the algebraic sum of the oxidation numbers of all atoms equals 21. The charge of the compound 22. Name the compound Al 2 S 3 22. Aluminum Sulfide 23. Name the compound Fe(NO 3)2 23. Iron (II) Nitrate 24. Name the compound CF 4 24. Carbon Tetrafluoride

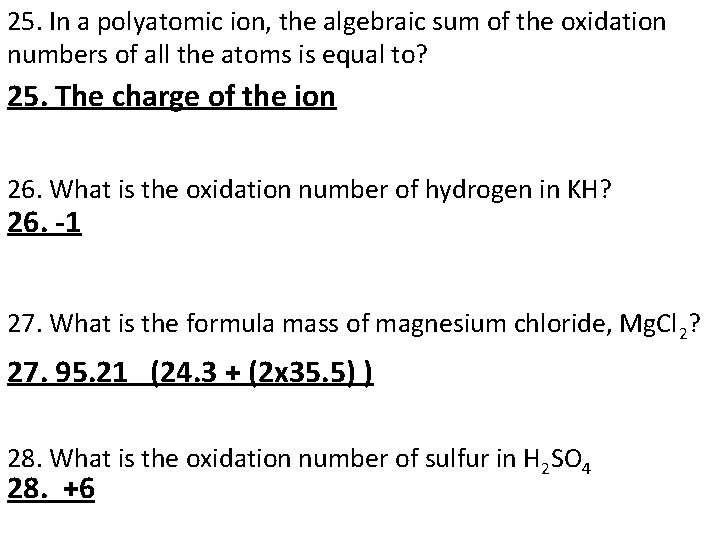
25. In a polyatomic ion, the algebraic sum of the oxidation numbers of all the atoms is equal to? 25. The charge of the ion 26. What is the oxidation number of hydrogen in KH? 26. -1 27. What is the formula mass of magnesium chloride, Mg. Cl 2? 27. 95. 21 (24. 3 + (2 x 35. 5) ) 28. What is the oxidation number of sulfur in H 2 SO 4 28. +6

29. Name the compound SO 2 29. Sulfur Dioxide 30. The formula for carbon dioxide, CO 2, can represent 30. One molecule of carbon dioxide, 1 mol of carbon dioxide molecules , The combination of 1 atom of carbon and 2 atoms of oxygen 31. Name the compound CCl 4 31. Carbon Tetrachloride 32. Name the compound CO 2 32. Carbon Dioxide

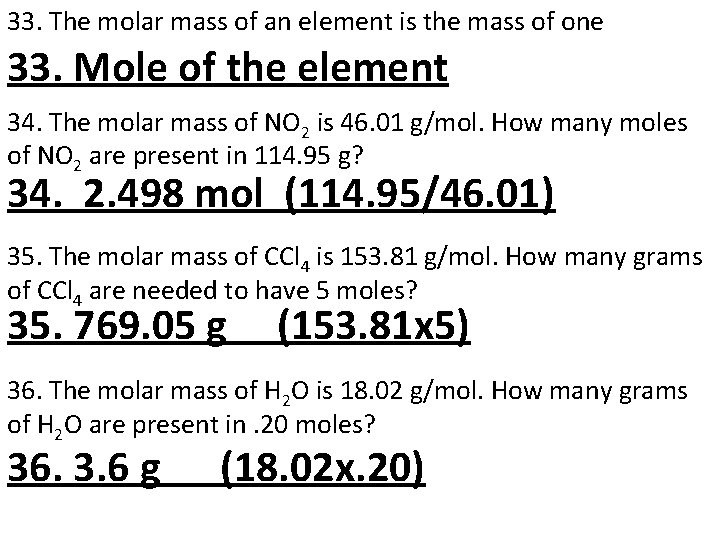
33. The molar mass of an element is the mass of one 33. Mole of the element 34. The molar mass of NO 2 is 46. 01 g/mol. How many moles of NO 2 are present in 114. 95 g? 34. 2. 498 mol (114. 95/46. 01) 35. The molar mass of CCl 4 is 153. 81 g/mol. How many grams of CCl 4 are needed to have 5 moles? 35. 769. 05 g (153. 81 x 5) 36. The molar mass of H 2 O is 18. 02 g/mol. How many grams of H 2 O are present in. 20 moles? 36. 3. 6 g (18. 02 x. 20)

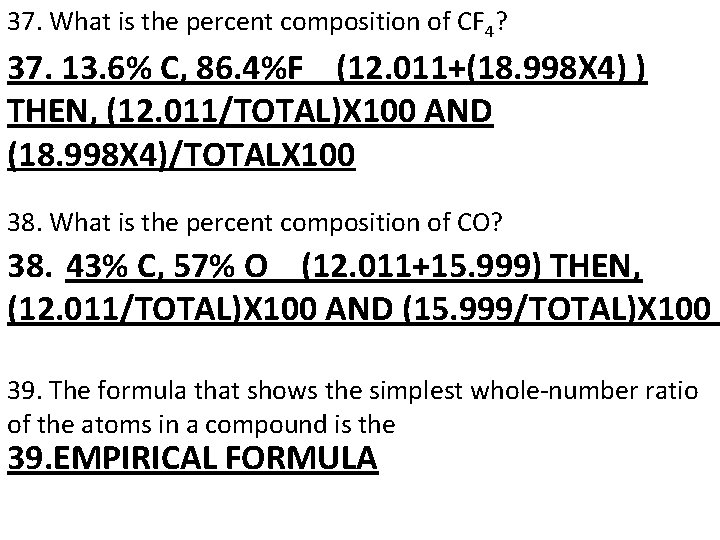
37. What is the percent composition of CF 4? 37. 13. 6% C, 86. 4%F (12. 011+(18. 998 X 4) ) THEN, (12. 011/TOTAL)X 100 AND (18. 998 X 4)/TOTALX 100 38. What is the percent composition of CO? 38. 43% C, 57% O (12. 011+15. 999) THEN, (12. 011/TOTAL)X 100 AND (15. 999/TOTAL)X 100 39. The formula that shows the simplest whole number ratio of the atoms in a compound is the 39. EMPIRICAL FORMULA

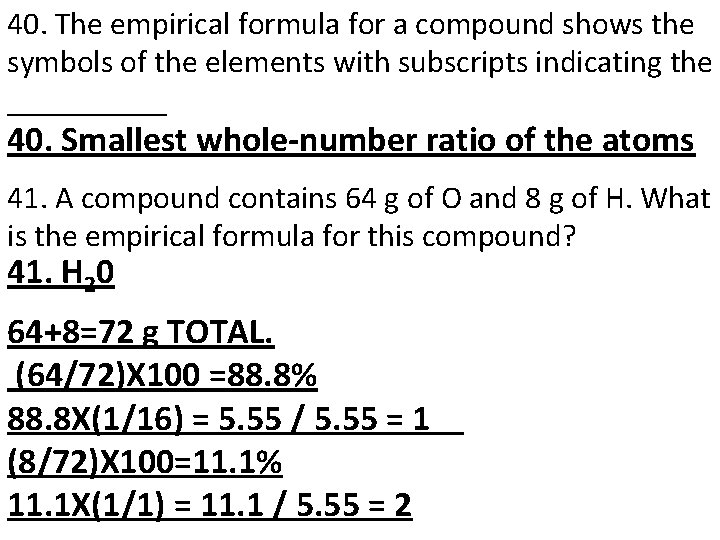
40. The empirical formula for a compound shows the symbols of the elements with subscripts indicating the _____ 40. Smallest whole-number ratio of the atoms 41. A compound contains 64 g of O and 8 g of H. What is the empirical formula for this compound? 41. H 20 64+8=72 g TOTAL. (64/72)X 100 =88. 8% 88. 8 X(1/16) = 5. 55 / 5. 55 = 1 (8/72)X 100=11. 1% 11. 1 X(1/1) = 11. 1 / 5. 55 = 2

42. An oxide of Chromium is found to have the following % composition: 68. 4% Cr and 31. 6% O. What is the compound’s empirical formula? 42. Cr 2 O 3 68. 4 x(1/51. 99) = 1. 32 / 1. 32 = 1 x 2 = 2 31. 6 x(1/15. 99)=1. 98 / 1. 32 = 1. 5 x 2 = 3 43. A compound has an empirical formula of NH 2 and a formula mass of 32. 06 amu. What is the compound’s molecular formula? 43. N 2 H 4 (14. 007+(1. 0079 X 2) ) =16. 0228 THEN 32. 06/16. 0228 = 2 SO 2 X NH 2

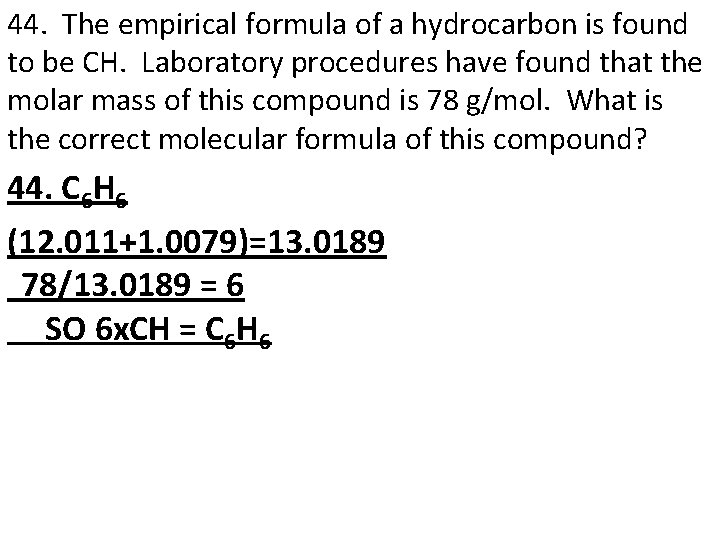
44. The empirical formula of a hydrocarbon is found to be CH. Laboratory procedures have found that the molar mass of this compound is 78 g/mol. What is the correct molecular formula of this compound? 44. C 6 H 6 (12. 011+1. 0079)=13. 0189 78/13. 0189 = 6 SO 6 x. CH = C 6 H 6