Chapter 7 Chemical Formulas and Compounds Nomenclature Systematic

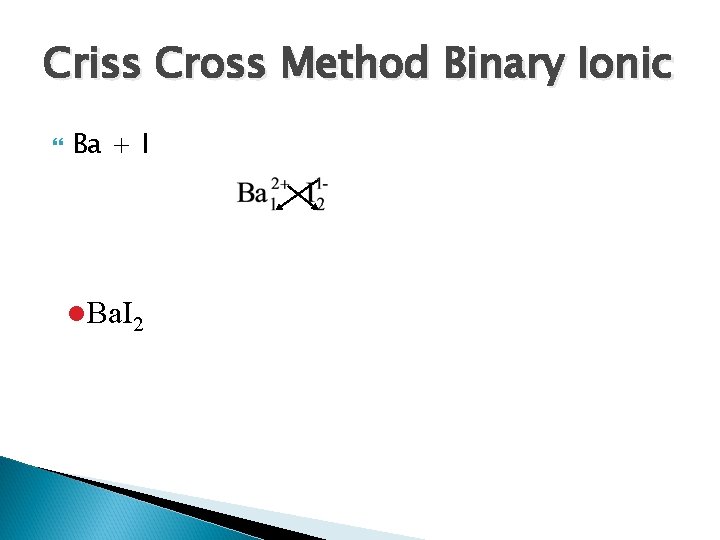





























- Slides: 57

Chapter 7 Chemical Formulas and Compounds Nomenclature ◦ Systematic way of writing and naming compounds. ◦ Purpose So we don’t have to memorize all the common names.

Formula Writing for Ionic Compounds Binary Ionic Compounds ◦ Ionic Bond between two atoms. ◦ Total charge of all compounds must equal zero! ◦ Total (+) = Total (-) Identify the charges of both + and – ions. ◦ If charges equal no subscripts needed. ◦ If charges are not equal, cross the charges without (+/-) signs as subscripts. ◦ If the subscripts can be reduced, reduce to the lowest whole number ratio.
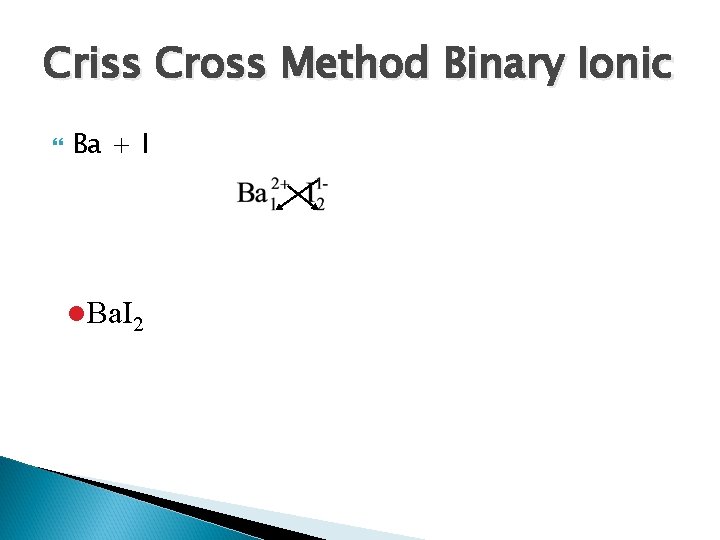
Criss Cross Method Binary Ionic Ba + I l. Ba. I 2

Practice Formulas Write the formula unit for the following Binary Ionic Compounds: ◦ ◦ ◦ (Zn + O) (Fe 3+ + Cl) (Ba + N) (Sn 4+ + S) (Cu 1+ + P)

Writing Ternary Ionic Compounds Polyatomic Ions ◦ Combinations of 2 or more non-metals that form common ions. ◦ Most are negatively charged except NH 4 1+ ◦ Must be placed in ( ) if more than 2 are needed. Ternary Ionic Compounds ◦ Ionic bond between an element and a polyatomic ion.

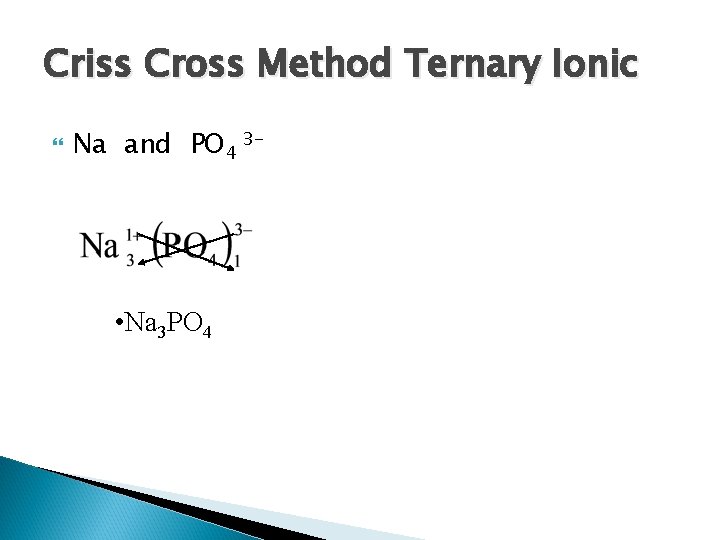
Criss Cross Method Ternary Ionic Na and PO 4 3 - • Na 3 PO 4

Practice Writing Ternary Ionic Write the formula unit for the following Ternary Ionic Compounds: ◦ ◦ ◦ Zn + OHFe 3+ + Cl. O 3 1 Ba + NO 3 1 Sn 2+ + SO 3 2 NH 4 1+ + P

Naming Ionic Compounds Binary Ionic Compounds ◦ Metals, retain the name of the element. Multiple charged metals must use either stock or classical naming system. (STOCK is preferred) ◦ Non-metals, root of name + ide ending. Chlorine = Chloride Nitrogen = Nitride Sulfur = Sulfide Oxygen = Oxide ◦ Example Mg. S Magnesium Sulfide Phosphorus = Phosphide Carbon = Carbide Selenium = Selenide

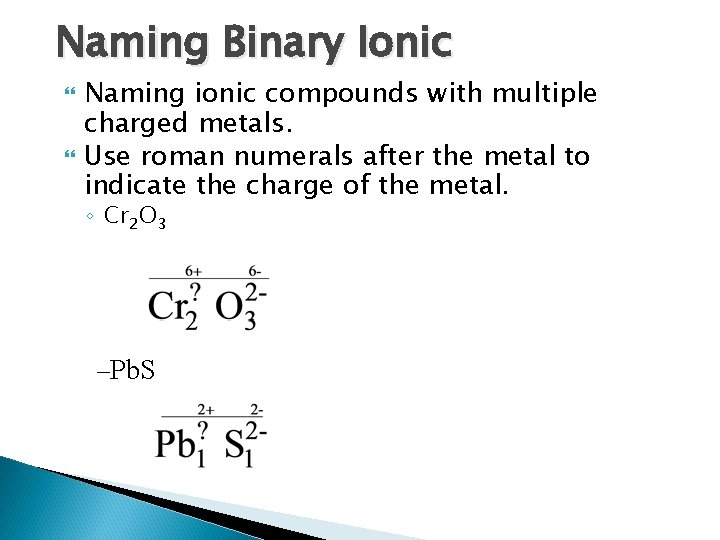
Naming Binary Ionic Naming ionic compounds with multiple charged metals. Use roman numerals after the metal to indicate the charge of the metal. ◦ Cr 2 O 3 –Pb. S

Practice Naming Binary Ionic Na. Cl Fe. Cl 2 Ca. F 2 KI Al 2 O 3 Sn. O

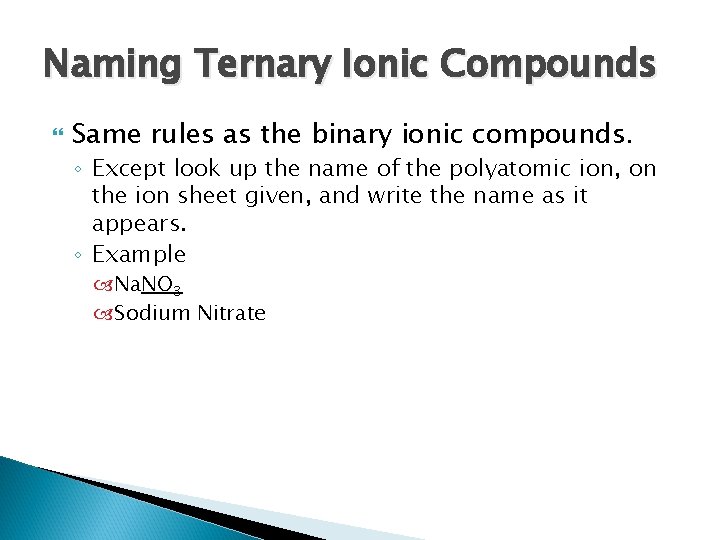
Naming Ternary Ionic Compounds Same rules as the binary ionic compounds. ◦ Except look up the name of the polyatomic ion, on the ion sheet given, and write the name as it appears. ◦ Example Na. NO 3 Sodium Nitrate

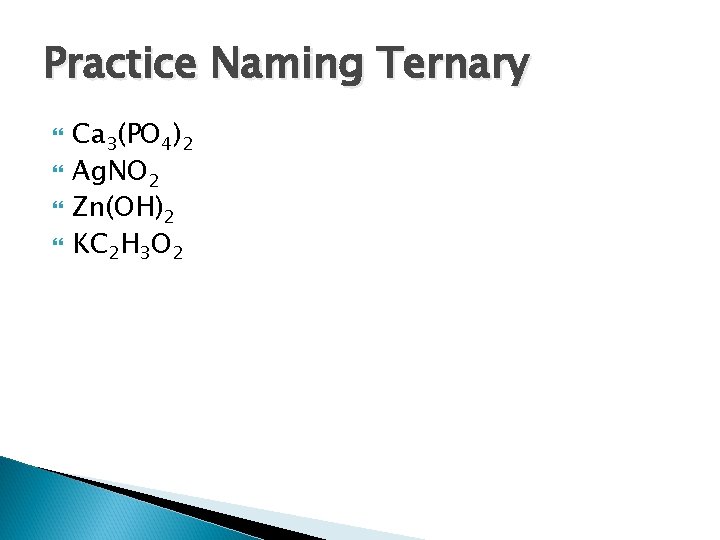
Practice Naming Ternary Ca 3(PO 4)2 Ag. NO 2 Zn(OH)2 KC 2 H 3 O 2

Summary of Writing Ionic Compounds Identify the ending of the compound. ◦ -ide, check to see if the first word is NOT ammonium or the last is NOT Hydroxide or Cyanide. If not, then it’s a binary ionic compound. If yes, then it’s a ternary ionic compound. ◦ -ate, or –Ite Ternary Ionic Compound.

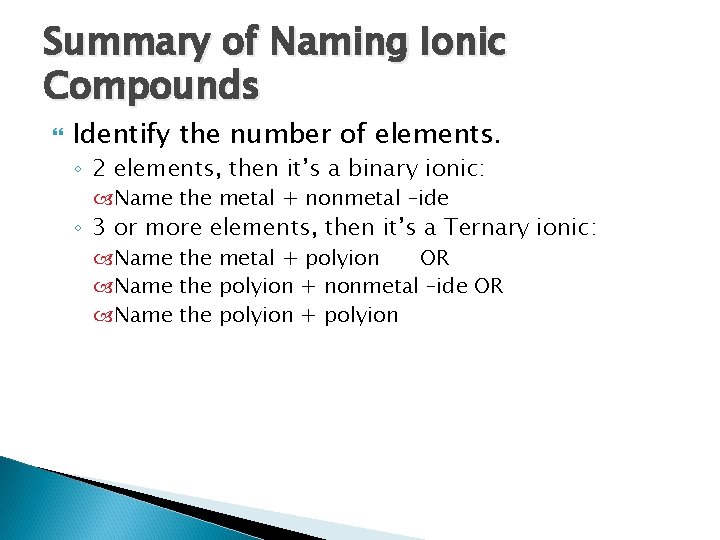
Summary of Naming Ionic Compounds Identify the number of elements. ◦ 2 elements, then it’s a binary ionic: Name the metal + nonmetal –ide ◦ 3 or more elements, then it’s a Ternary ionic: Name the metal + polyion OR Name the polyion + nonmetal –ide OR Name the polyion + polyion

Practice Write formulas for the following compounds: 1) 2) 3) 4) Silver Sulfide Magnesium Nitrate Copper(II) Nitride Chromium(III) Sulfite Name the following compounds: 1) 2) 3) 4) K 2 SO 4 Fe(NO 3)3 Ca 3 P 2 Sn. O 2

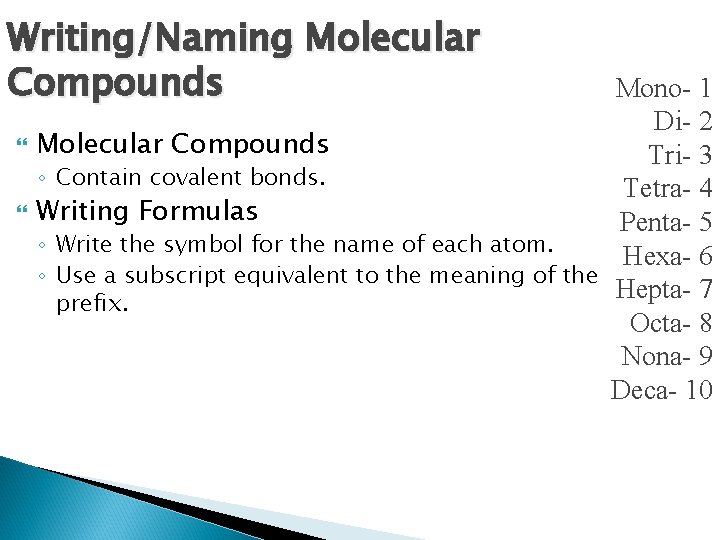
Writing/Naming Molecular Compounds Mono- 1 Di- 2 Molecular Compounds Tri- 3 ◦ Contain covalent bonds. Tetra- 4 Writing Formulas Penta- 5 ◦ Write the symbol for the name of each atom. Hexa- 6 ◦ Use a subscript equivalent to the meaning of the Hepta- 7 prefix. Octa- 8 Nona- 9 Deca- 10

Practice Writing Formulas Write the formulas for the following compounds: Dinitrogen Pentoxide Phosphorus Trichloride Sulfur Trioxide Carbon Tetrachloride

Naming Molecular Compounds First word ◦ Prefix + Name of element, only uses a prefix if there are 2 or more atoms. Second word ◦ MUST HAVE a prefix followed by name ending in – ide.

Practice Naming Write the name for the following formulas: ◦ ◦ ◦ P 4 O 10 N 2 O 5 CF 4 N 2 O 3 SO 2

Binary Acids / Oxyacids All acids contain the element H. H is always written 1 st in any formula representing acids. Binary Acids ◦ Contain Hydrogen + a non-metal. Oxyacids ◦ Contain Hydrogen + a polyatomic ion

Binary Acids Naming: ◦ Must begin with the prefix Hydro◦ Must end in the suffix –ic. ◦ Place the nonmetal between the prefix and suffix. Example ◦ HCl Hydrochloric Acid

Binary Acids Writing: ◦ Always place the H first. ◦ Identify the nonmetal in the acid. ◦ Cross the charges on the H 1+ and the nonmetal as subscripts. Example ◦ Hydrosulfuric Acid H +1 S H 2 S 2 -

Binary Acids Practice Write the name for each of the following acids: HI H 2 S H 3 N Write the formula for the following acids: Hydrofluoric acid Hydrophosphoric acid Hydroselenic acid

Oxyacids Naming: ◦ Identify the polyatomic ion after the H. ◦ Write the name of the non-metal in the polyatomic ion. ◦ If the polyatomic ion ends in: -ate change to –ic -ite change to –ous Example ◦ H 2 SO 4 Sulfate Sulfuric Acid ◦ H 2 SO 3 Sulfite Sulfurous Acid

Oxyacids Writing: ◦ Identify the root of the polyatomic ion. ◦ If the acid ends in: - ic use the –ate ending - ous use the –ite ending ◦ Place the H in front of the polyatomic ion and cross the charges if needed. Example ◦ Phosphoric Acid Phosphoric = Phosphate (PO 4) H 3 PO 4 3 -

Oxyacids Practice Write the name for the following acids: HNO 3 H 2 CO 3 HC 2 H 3 O 2 Write the formula for the following acids: ◦ Nitrous acid ◦ Perchloric acid ◦ Phosphorous acid

Hydrates Ionic compounds that contain absorbed water. Example Mg. SO 4 5 H 2 O Magnesium Sulfate Pentahydrate

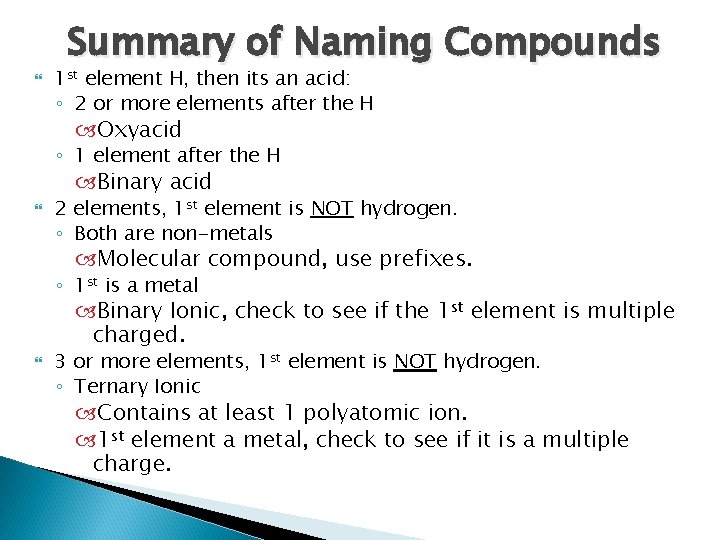
Summary of Naming Compounds 1 st element H, then its an acid: ◦ 2 or more elements after the H Oxyacid ◦ 1 element after the H Binary acid 2 elements, 1 st element is NOT hydrogen. ◦ Both are non-metals Molecular compound, use prefixes. ◦ 1 st is a metal Binary Ionic, check to see if the 1 st element is multiple charged. 3 or more elements, 1 st element is NOT hydrogen. ◦ Ternary Ionic Contains at least 1 polyatomic ion. 1 st element a metal, check to see if it is a multiple charge.

Summary of Writing Formulas Most formulas use only 2 words. Check the 2 nd word ending first! Ending is –ic or –ous ◦ Compound is an acid. ◦ Has –ic ending or –ous without a Hydro- prefix Oxyacid Contains a Hydrogen + Polyatomic Ion. ◦ Has –ic ending with a Hydro- prefix Binary Acid Contains a Hydrogen + Non-metal.

Writing Formulas Continued Ending is –ide. ◦ If it ends in Hydroxide or Cyanide. Ternary Ionic, check charges and cross. Reminder!! More than 1 polyatomic ion, use ( ). ◦ If it isn’t Hydroxide or Cyanide. Binary Compound Check if prefixes are used on the 2 nd word. Prefix then Binary molecular, just write subscripts = to prefixes. No Prefix Binary Ionic, check charges and cross if needed. ◦ If it ends in –ite or –ate. Ternary Ionic, check charges and cross. More than 1 polyatomic ion needed, use ( ).

Chapter 7. 2 Oxidation Numbers To show the general distribution of electrons among the bonded atoms in a molecule or polyatomic ion, oxidation states are assigned to the atoms in the compound or ion. Similar to ionic charges, but they don’t have an exact physical meaning. Useful when explaining chemical reactions and balanced equations.

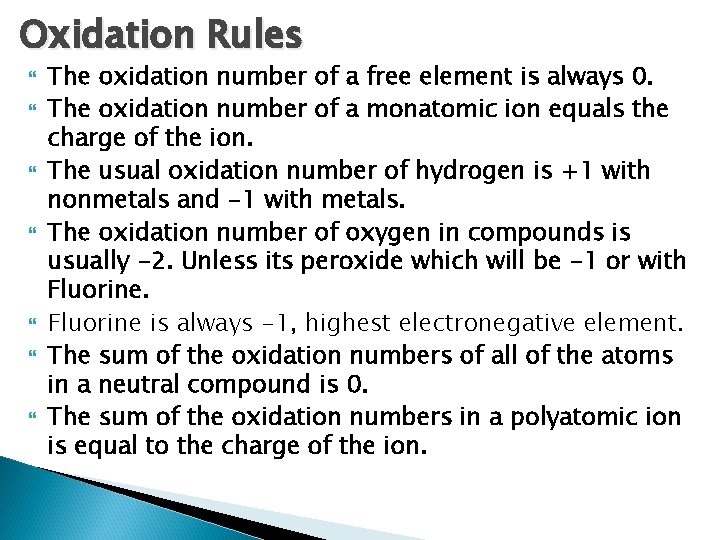
Oxidation Rules The oxidation number of a free element is always 0. The oxidation number of a monatomic ion equals the charge of the ion. The usual oxidation number of hydrogen is +1 with nonmetals and -1 with metals. The oxidation number of oxygen in compounds is usually -2. Unless its peroxide which will be -1 or with Fluorine is always -1, highest electronegative element. The sum of the oxidation numbers of all of the atoms in a neutral compound is 0. The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion.

Rules for Oxidation Numbers Page 232 list 8 rules you should follow. Note that all elements in the pure state have oxidation numbers equal to zero. Identify the oxidation states the practice problems page 234.

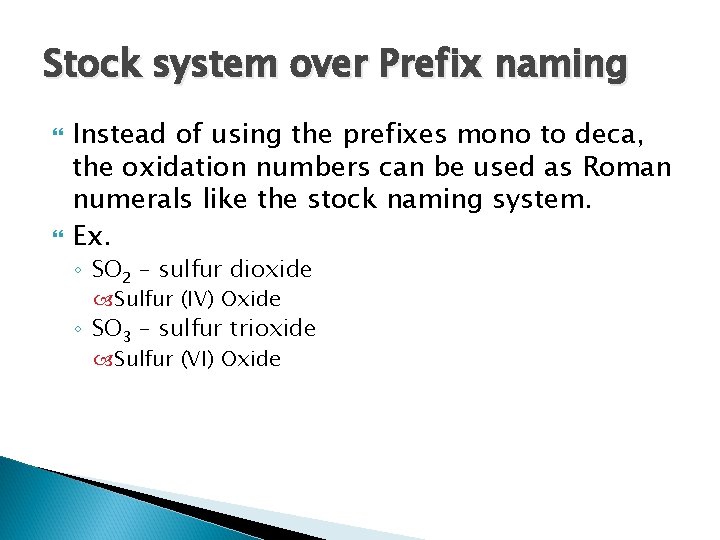
Stock system over Prefix naming Instead of using the prefixes mono to deca, the oxidation numbers can be used as Roman numerals like the stock naming system. Ex. ◦ SO 2 – sulfur dioxide Sulfur (IV) Oxide ◦ SO 3 – sulfur trioxide Sulfur (VI) Oxide

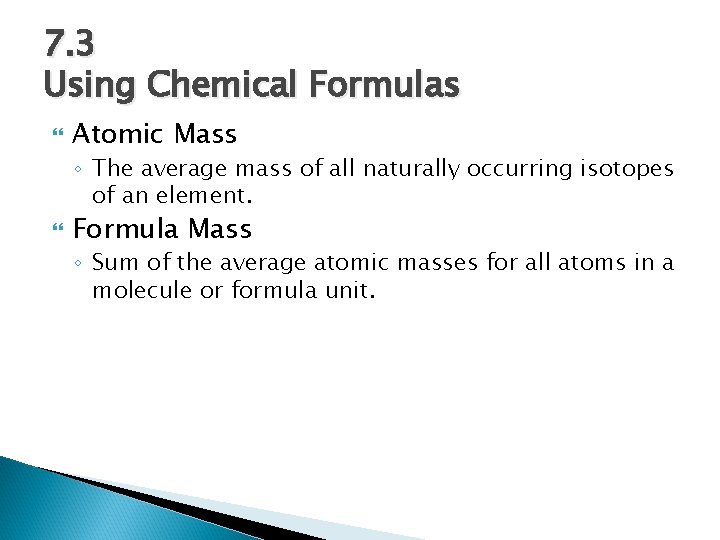
7. 3 Using Chemical Formulas Atomic Mass ◦ The average mass of all naturally occurring isotopes of an element. Formula Mass ◦ Sum of the average atomic masses for all atoms in a molecule or formula unit.

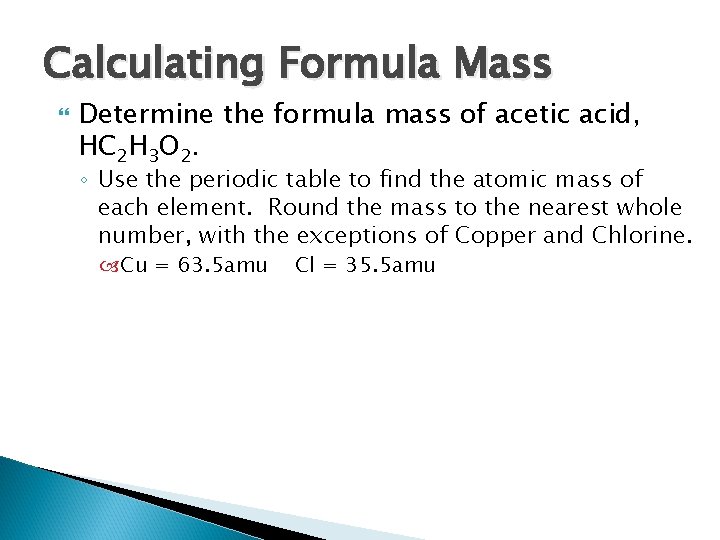
Calculating Formula Mass Determine the formula mass of acetic acid, HC 2 H 3 O 2. ◦ Use the periodic table to find the atomic mass of each element. Round the mass to the nearest whole number, with the exceptions of Copper and Chlorine. Cu = 63. 5 amu Cl = 35. 5 amu

Practice: Formula Mass Determine the formula mass for the following: 1. 2. 3. H 2 SO 4 (NH 4)2 CO 3 Ca. Cl. O 3

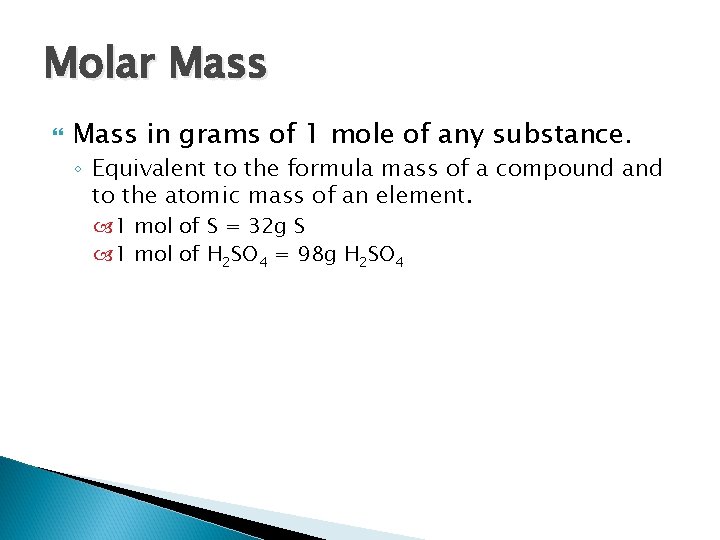
Molar Mass in grams of 1 mole of any substance. ◦ Equivalent to the formula mass of a compound and to the atomic mass of an element. 1 mol of S = 32 g S 1 mol of H 2 SO 4 = 98 g H 2 SO 4

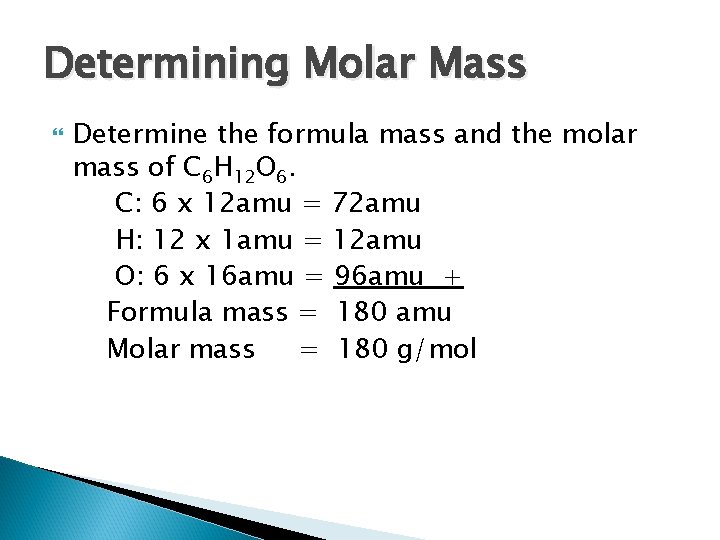
Determining Molar Mass Determine the formula mass and the molar mass of C 6 H 12 O 6. C: 6 x 12 amu = 72 amu H: 12 x 1 amu = 12 amu O: 6 x 16 amu = 96 amu + Formula mass = 180 amu Molar mass = 180 g/mol

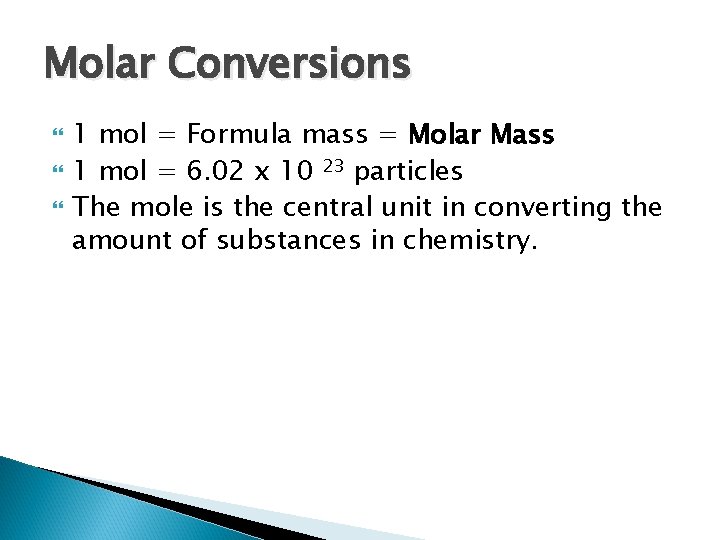
Molar Conversions 1 mol = Formula mass = Molar Mass 1 mol = 6. 02 x 10 23 particles The mole is the central unit in converting the amount of substances in chemistry.

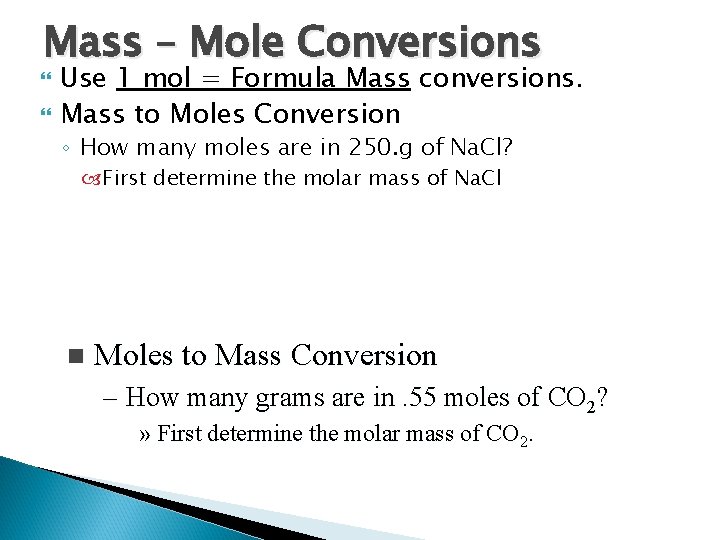
Mass – Mole Conversions Use 1 mol = Formula Mass conversions. Mass to Moles Conversion ◦ How many moles are in 250. g of Na. Cl? First determine the molar mass of Na. Cl n Moles to Mass Conversion – How many grams are in. 55 moles of CO 2? » First determine the molar mass of CO 2.

Mole – Particle Conversion Use 1 mol = 6. 02 x 10 23 particles Particle to Mole Conversion ◦ How many moles are equivalent to 550. molecules of SO 3? n Mole to Particle Conversion – How many atoms are in. 525 moles of Ca?

Molar Conversions 1 mol = Formula mass(g) 1 mol = 6. 02 x 1023 particles ◦ Particles: Atoms Molecules Formula Units(f. u. ) Determine the number of particles in 45. 0 g of Ca(NO 3)2.

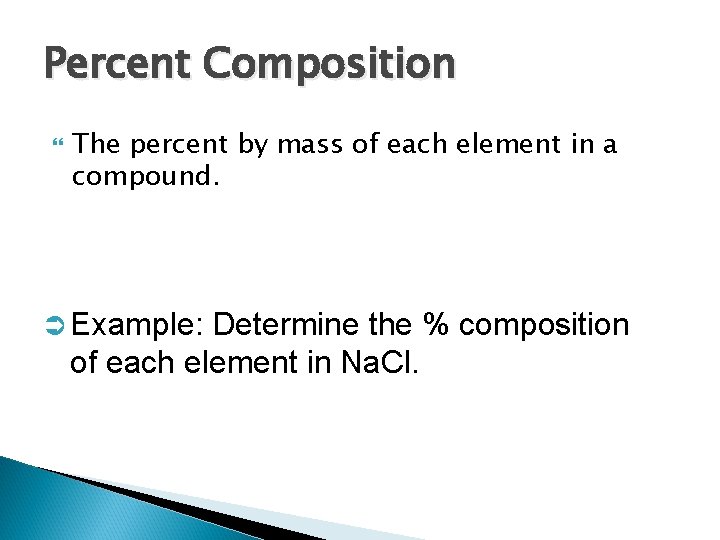
Percent Composition The percent by mass of each element in a compound. Ü Example: Determine the % composition of each element in Na. Cl.

Practice % Composition Determine the % composition of each element in Na 2 SO 4.

Hydrates – compounds that contain water. ◦ Example : Mg. SO 4 5 H 2 O ◦ The ( )really is equivalent to (+). ◦ Determine the formula mass of this compound.

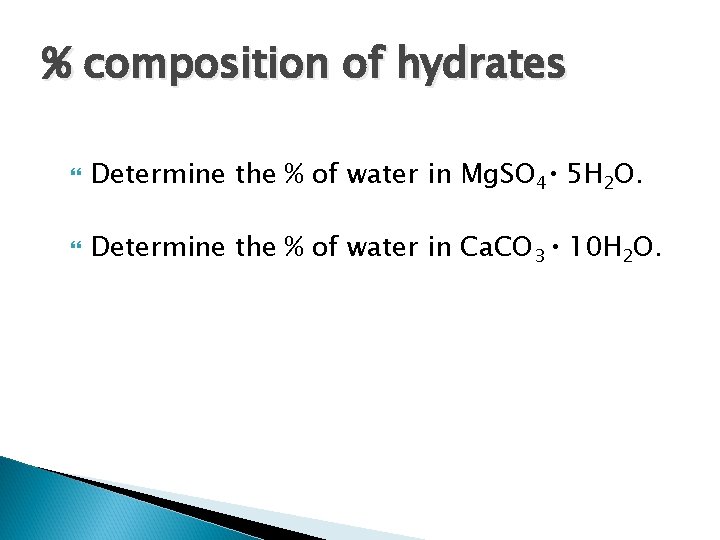
% composition of hydrates Determine the % of water in Mg. SO 4 5 H 2 O. Determine the % of water in Ca. CO 3 10 H 2 O.

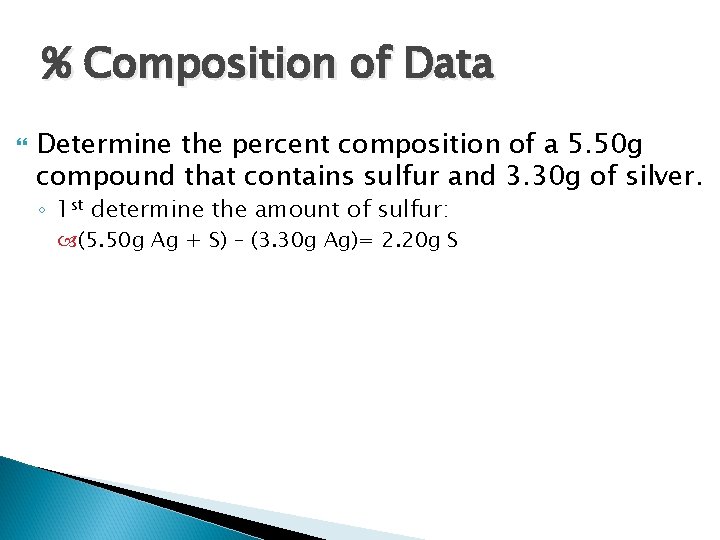
% Composition of Data Determine the percent composition of a 5. 50 g compound that contains sulfur and 3. 30 g of silver. ◦ 1 st determine the amount of sulfur: (5. 50 g Ag + S) – (3. 30 g Ag)= 2. 20 g S

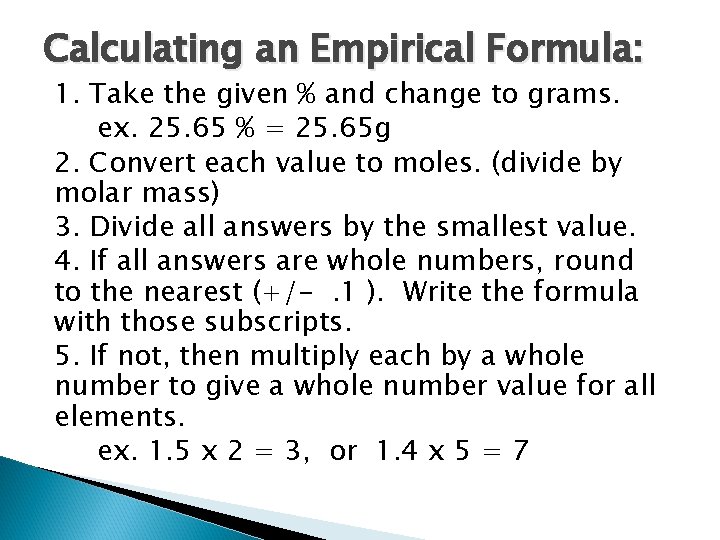
Using Percent composition. Determine the mass of an element in a given quantity of a compound. ◦ Multiply the % of the element in decimal form by the total mass of the compound. ◦ Example: Determine the mass of Ag in 120 g of Ag. Cl.

Practice % by mass 1. Determine the mass of Ca, in 400 g of Ca. CO 3. 2. Determine the mass of O, in 125 g of H 2 SO 4. 3. Determine the mass of K, in 50 g of KCl. O 3 4 H 2 O.

Determining Chemical formulas Empirical Formula (Simplest Formula)– Smallest whole number ratio of atoms in a chemical formula.

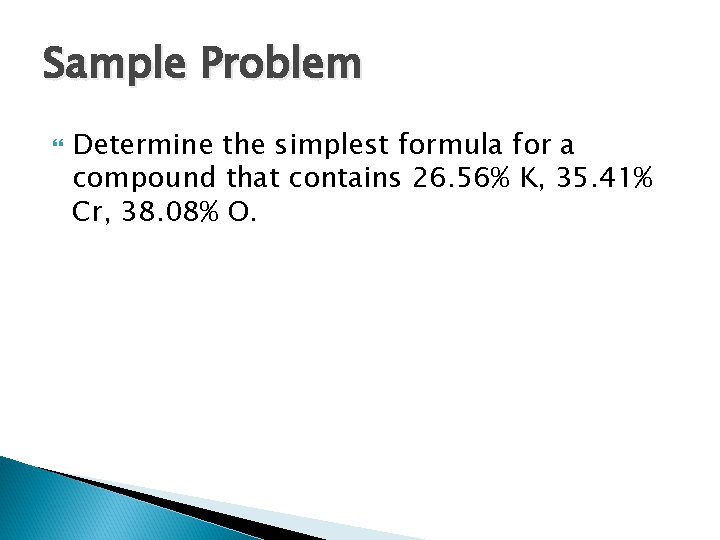
Calculating an Empirical Formula: 1. Take the given % and change to grams. ex. 25. 65 % = 25. 65 g 2. Convert each value to moles. (divide by molar mass) 3. Divide all answers by the smallest value. 4. If all answers are whole numbers, round to the nearest (+/-. 1 ). Write the formula with those subscripts. 5. If not, then multiply each by a whole number to give a whole number value for all elements. ex. 1. 5 x 2 = 3, or 1. 4 x 5 = 7

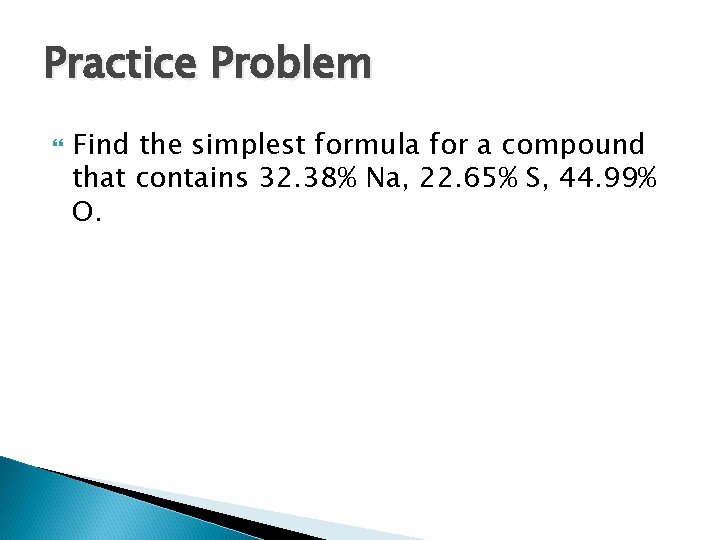
Sample Problem Determine the simplest formula for a compound that contains 26. 56% K, 35. 41% Cr, 38. 08% O.

Practice Problem Find the simplest formula for a compound that contains 32. 38% Na, 22. 65% S, 44. 99% O.

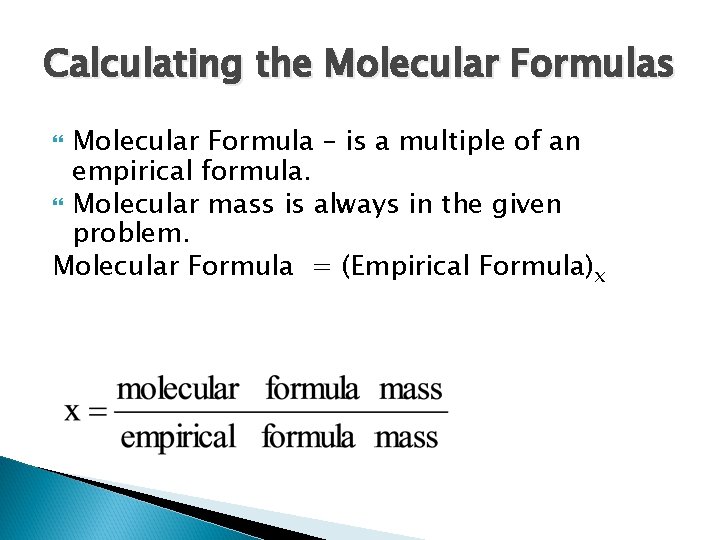
Calculating the Molecular Formulas Molecular Formula – is a multiple of an empirical formula. Molecular mass is always in the given problem. Molecular Formula = (Empirical Formula)x

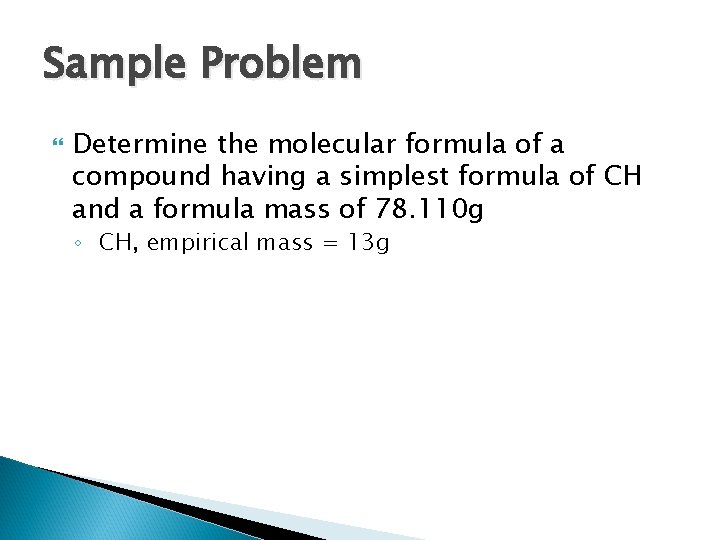
Sample Problem Determine the molecular formula of a compound having a simplest formula of CH and a formula mass of 78. 110 g ◦ CH, empirical mass = 13 g

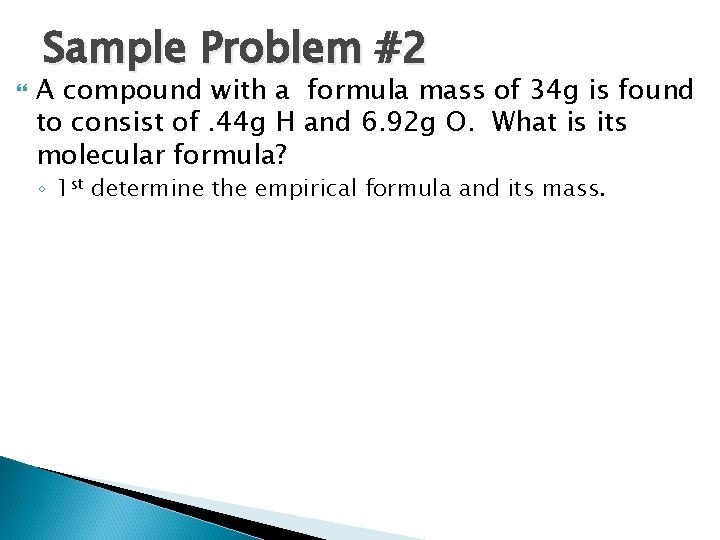
Sample Problem #2 A compound with a formula mass of 34 g is found to consist of. 44 g H and 6. 92 g O. What is its molecular formula? ◦ 1 st determine the empirical formula and its mass.