Chapter 7 Chemical Formulas and Chemical Compounds Section

















- Slides: 22

Chapter 7: Chemical Formulas and Chemical Compounds Section 1: Chemical Names and Formulas (Emily Brown) Section 2: Oxidation Numbers (Anu Ninan) Section 3: Using Chemical Formulas (Johana Barxha) Section 4: Determining Chemical Formulas (Justine Baird)

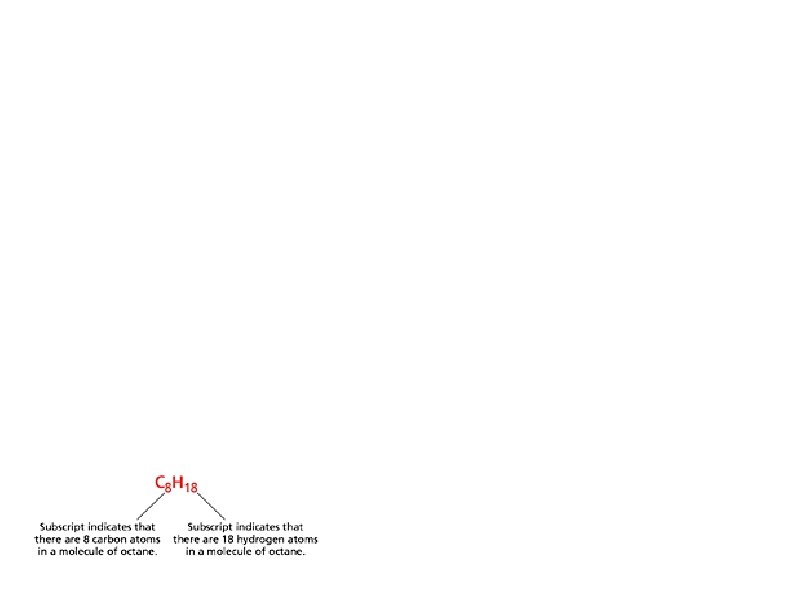
7. 1: Chemical Names and Formulas Vocabulary Hydrocarbons—molecular compounds composed solely of carbon and hydrogen Monatomic ions—ions formed from a single atom Binary compounds—compounds composed of two different elements Nomenclature—naming system Oxyanions—polyatomic ions that contain oxygen Binary acids—acids that consist of two elements, usually hydrogen and one of the halogens (fluorine, chlorine, bromine, iodine) Oxyacids—acids that contain hydrogen, oxygen, and a third element (usually a nonmetal) Hydrochloric acid—a water solution of the molecular compound hydrogen chloride Salt—an ionic compound composed of a cation and the anion from an acid

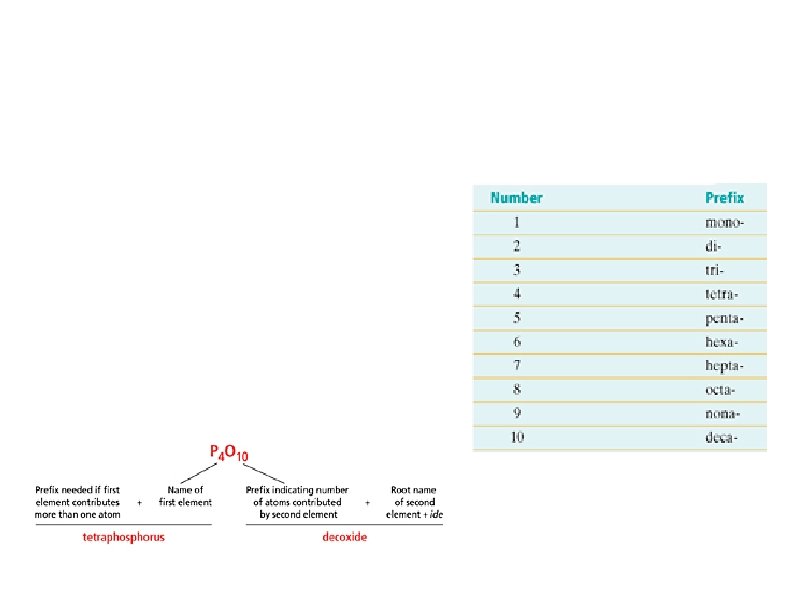
7. 1 The Naming System These are the prefixes used in the stock system for naming binary molecular compounds: 1. The element with the smaller group number always goes first, except if both elements have the same group number (in which the greatest period number goes first) 2. The second element combines a prefix giving the number of atom contributed, the root name, and an -ide suffix

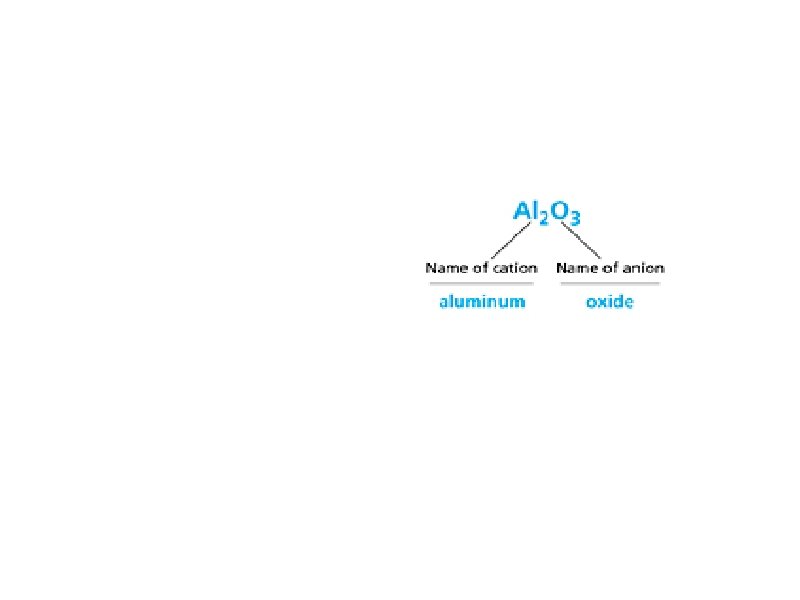
7. 1 Naming Cont. The following is the naming system for binary ionic compounds: a. In this method, the elements are sorted according to cations (+) and anions (-). These can be determined by an atom's charge b. The positive element always goes first, followed by the anion. c. The cation retains its original name, but the anion drops its ending and adds an -ide suffix

7. 2 Oxidation Numbers VOCABULARY: a. Oxidation numbers: the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion a. Oxidation states: another name for oxidation numbers

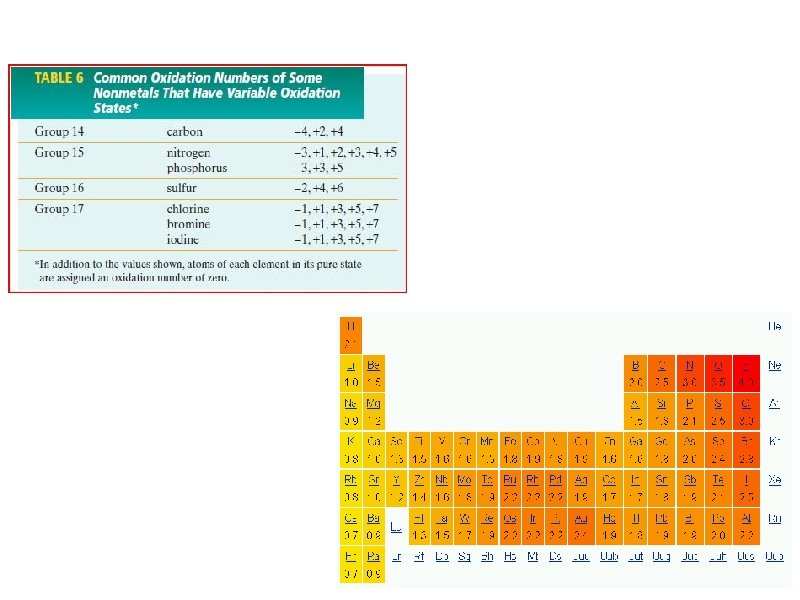
7. 2 Steps Involved In Assigning Oxidation Numbers The main key is that the sum of the oxidation numbers of the atoms equals 0 a. In a pure element, the oxidation numbers of all the atoms in 0. (ex. pure Na, each sodium atom's oxidation number is 0, and in P 4 each phosphorus atom has an oxidation number of 0) • In a binary molecular compound: o the more electronegative element is given the number equal to its negative charge it would have as an anion o the less electronegative element is given the number equal to its positive charge it would have as an anion • Fluorine, F, is ALWAYS given the oxidation number of -1, because it is the most electronegative

7. 2 Steps Involved In Assigning Oxidation Numbers a. Oxygen has an oxidation number of -2 in almost all compounds. Exceptions: when it is in peroxides or in a compound with fluorine b. The algebraic sum of the oxidation numbers is always equal to 0, as long as the compound is neutral c. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion d. Oxidation numbers can also be assigned to ions e. monoatomic ions have oxidation numbers equal to the charge of the ion (ex. Ca 2+ has an oxidation number of +2) f. Hydrogen, H, has an oxidation number of +1 in compound where the other atoms are more electronegative, but the oxidation number is -1 in compounds where there are metals

7. 2 Oxidation numbers of certain nonmetals

7. 2 Steps Involved In Assigning Oxidation Numbers UF 6 Cl. O 3 - H 2 SO 4 N 2 O 5 CS 2 P 4 O 10

7. 2 Stock System and Oxidation. Numbers Ionic Compound have names based on prefixes as well as names based on the Stock System Example Pb. O 2 can be written as: Lead dioxide prefix system Lead (IV) oxide Stock System because oxygen has a charge of -2 and there are to oxygen so it become (-2)(2)=-4 and lead must make the chemical formular balance to 0, so -4+x=0 x=4

7. 2 Practice with naming using the Stock System As 2 S 3 NCl 3 SO 3 Mo 2 O 3 NO PCl 5

7. 3: Formula Masses a. The formula mass of any molecule, formula unit, or ion is the sum of the average atomic masses of all atoms represented in its formula. For example, the mass of a water molecule is found by adding the masses of the three atoms in the molecule. average mass of H: 1. 01 amu average mass of O: 16. 00 amu 2 H atoms x 1. 01 amu = 02. 02 amu H atom 1 O atom x 16. 00 amu = 16. 0 amu O atom When added, the sum equals the average mass, about 18. 02 amu.

7. 3: Molar Masses a. The molar mass is calculated by summing the masses of the elements in a mole of the molecules that make up the compound. For example, the mass of a water molecule is found by adding the masses of the three atoms in the molecule. 2 mol H x 1. 01 g H = 02. 02 g H H atom 1 mol O x 16. 00 g O = 16. 0 g O O atom When added, the sum equals the molar mass of H 2 O, about 18. 02 g/mol. A compound's molar mass is numerically equal to its formula mass.

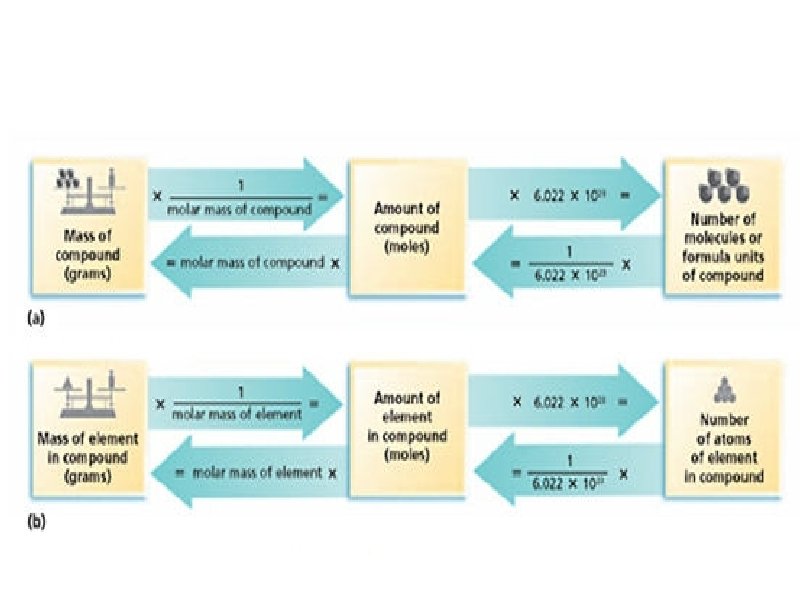
Molar Mass as a Conversion Factor

7. 3: Percentage Composition a. The percent composition is the percentage by mass of each element in a compound. It is calculated by dividing the mass of the element of the sample in a compound by the total mass of the sample. Then, multiply this value by 100. mass of element in sample of compound x 100 = % element in mass of sample of compound mass of element in 1 mol of compound x 100 = % element in molar mass of compound

7. 3: Percentage Composition Find the percent composition of copper(I) sulfide, Cu 2 S.

7. 3: Percentage Composition Find the percent composition of Ba(NO 3)2.

7. 4: Determining Chemical Formulas Empirical Formula- Is the smallest is the smallest whole number ratio of the elements in a compound. a. For ionic compounds the empirical formula is usually the formula unit but. . . • For molecular compounds the empirical formula does not always represent the number of atoms in the molecule. Practice: What is the empirical formula for N 6 H 4 S 8 O 4 P 2 H 5

7. 4 Finding the Empirical Formula Steps: 1. If given the % composition (if given in grams skip to step 2) figure out the amount of each substance in the sample by putting the % into grams (remember 32% of a substance is 32 grams). Question: if a substance has 24% carbon and 76% oxygen what is the empirical formula? 1. 24%= 24 grams 84%= 84 grams 2. Then divide the amount of each element by it's molar mass (found on the periodic table). 2. 24 g/12 g = 2 mole 76 g/16 g = 4. 75 mole

7. 4 Finding the Empirical Formula Con. 3. Next divide the numbers from both elements ( which are in moles now) by the smallest number. 4. Finally round the results to a whole number and write them as a subscript of the appropriate element 3. 2 mol of carbon/ 2= 1 4. 75 mol of oxygen/2 = 2. 4 4. Co 2

7. 4 Finding the Molecular Formula The Molecular Formula, or the actual formula of a molecular compound can be found using the empirical formula ad the equation x(empirical formula)= the molecular formula. To solve for the molecular formula you. . . 1. Dividing the molecular What is the molecular formula mass (given) by the a compound with the emperical empirical formula mass ( formula HO and a formula mass of sum of the element's molar 34 amu? mass) 1. 34/ 17 = 2 2. Then multiply the 2. 2(HO) = H 2 O 2 empirical formula by that number rounded to a whole number.

7. 4 Practice What is the empirical formula for a compound that is 64% Sulfur, 20% Carbon and 16 % Oxygen? If the molecular formula mass for the above empirical formula is 312 grams what is the molecular formula? What is the difference between a molecular and an empirical formula?