CHAPTER 7 Chemical Formulas and Bonding 118 BONDING
CHAPTER 7 Chemical Formulas and Bonding

1/18 BONDING Why if there are only 100 or so elements in nature, do we have so many different types of materials in the world? Atoms combine together to form different materials! Bonding – the combination of two more atoms to form a compound Two main types of bonds Ionic Covalent or
IONIC BONDING Why do clothes sometimes stick together when they come out of the dryer? One has slight positive charge, one has slight negative charge OPPOSITES ATTRACT! Ionic Bond – occurs when oppositely charged ions are held together by electrical attraction caused by transferring electrons.
IONIC COMPOUNDS Ionic Compounds contain both metals and nonmetals Ionic compounds always form in set ratios For example: 1 Li atom to 1 F atom

IONIC COMPOUNDS Properties – High melting points Strong Bonds Brittle solids Many are dissolvable in water Dissolved or in liquid state – good electrical conductors Solids cannot conduct electricity **Ionic Compounds do not share the properties of the elements that they contain! Soft salt! silvery metal + highly poisonous gas react to form table
IONIC COMPOUNDS How do ions form? (REVIEW) Octet Rule! Atoms tend to lose, gain or share electrons in order to acquire a full set of valence electrons! In most cases, this is 8 valence electrons!! H and He are the exception – they are full with 2! When atoms complete their valence shell, they have the electron configuration of the closest noble gas.

1/19 Today I will determine the ionic compound that will form between elements. Warm Up – What is a compound?

IONIC COMPOUNDS Each unit of an ionic compound is called a FORMULA UNIT.
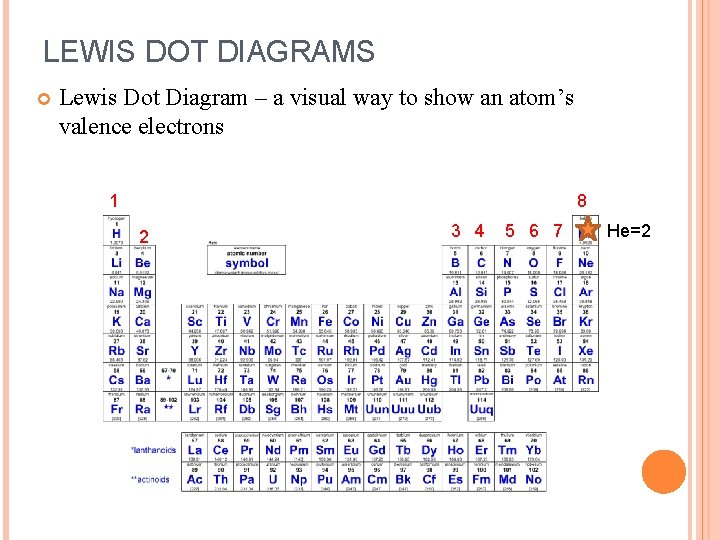
LEWIS DOT DIAGRAMS Lewis Dot Diagram – a visual way to show an atom’s valence electrons 1 8 2 3 4 5 6 7 He=2
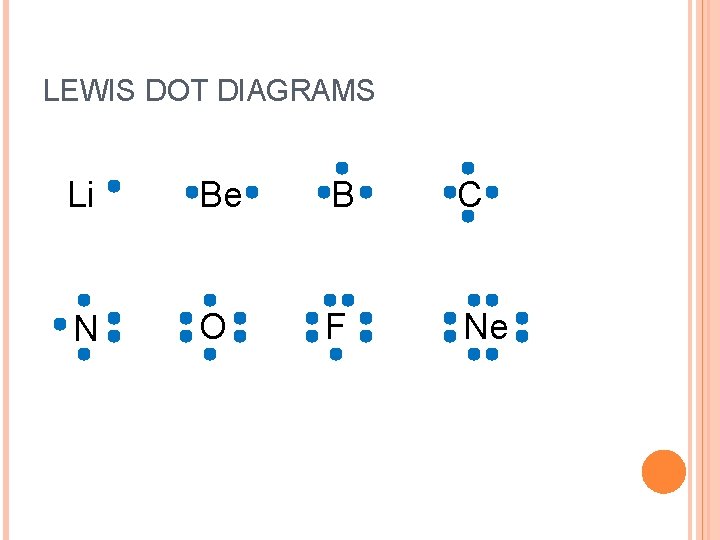
LEWIS DOT DIAGRAMS Li Be B C N O F Ne

LEWIS DOT DIAGRAMS We can use the Lewis Dot Diagrams to see how atoms bond!
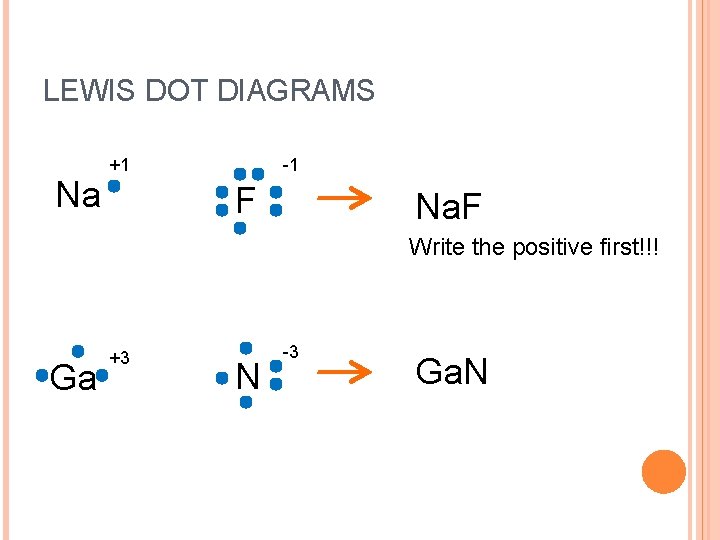
LEWIS DOT DIAGRAMS Na +1 -1 F Na. F Write the positive first!!! Ga +3 N -3 Ga. N
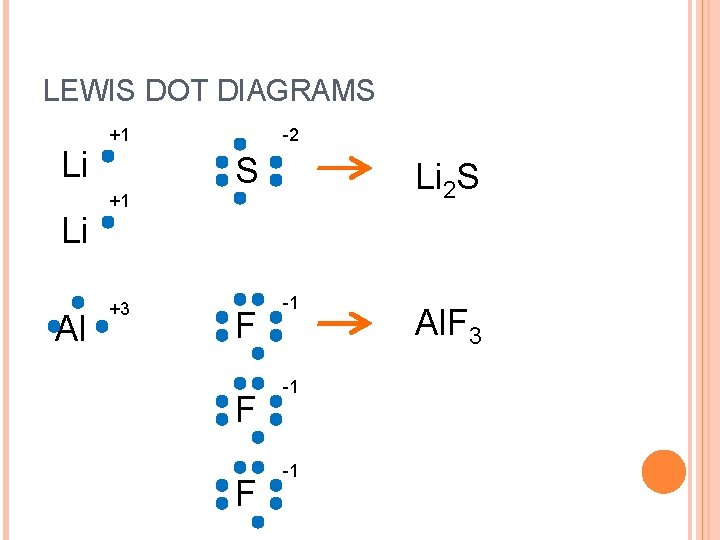
LEWIS DOT DIAGRAMS Li Li Al +1 -2 S Li 2 S +1 +3 F F F -1 -1 -1 Al. F 3
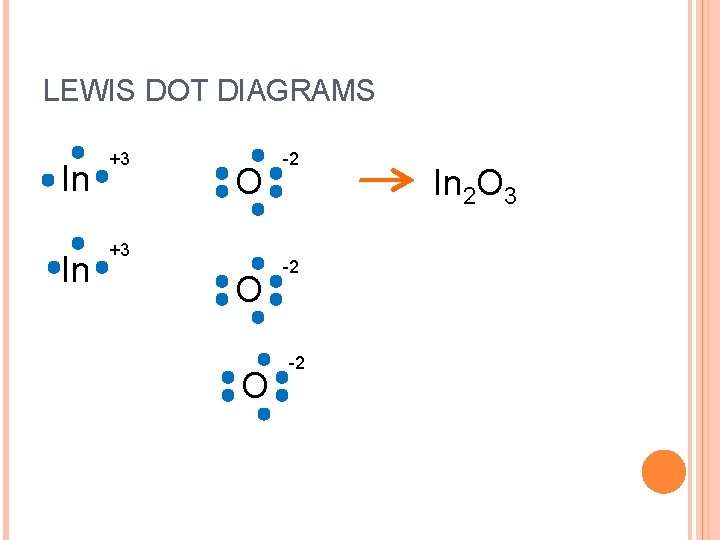
LEWIS DOT DIAGRAMS In In +3 O O -2 -2 -2 In 2 O 3
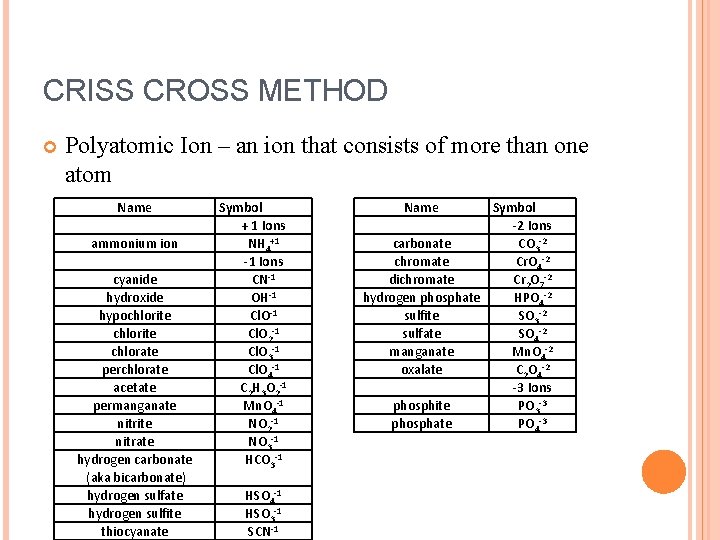
CRISS CROSS METHOD Polyatomic Ion – an ion that consists of more than one atom Name ammonium ion cyanide hydroxide hypochlorite chlorate perchlorate acetate permanganate nitrite nitrate hydrogen carbonate (aka bicarbonate) hydrogen sulfate hydrogen sulfite thiocyanate Symbol + 1 Ions NH 4+1 -1 Ions CN-1 OH-1 Cl. O 2 -1 Cl. O 3 -1 Cl. O 4 -1 C 2 H 3 O 2 -1 Mn. O 4 -1 NO 2 -1 NO 3 -1 HCO 3 -1 HSO 4 -1 HSO 3 -1 SCN-1 Name Symbol -2 Ions carbonate CO 3 -2 chromate Cr. O 4 -2 dichromate Cr 2 O 7 -2 hydrogen phosphate HPO 4 -2 sulfite SO 3 -2 sulfate SO 4 -2 manganate Mn. O 4 -2 oxalate C 2 O 4 -2 -3 Ions phosphite PO 3 -3 phosphate PO 4 -3

1/20 Today I will determine formulas using the criss cross method. Warm Up – 1. Using lewis dot diagrams, what compound would form between Ra and I?

CRISS CROSS METHOD There is an easier way to determine what ionic compounds will form between two elements that works when lewis dots diagrams won’t. Criss Cross Method!
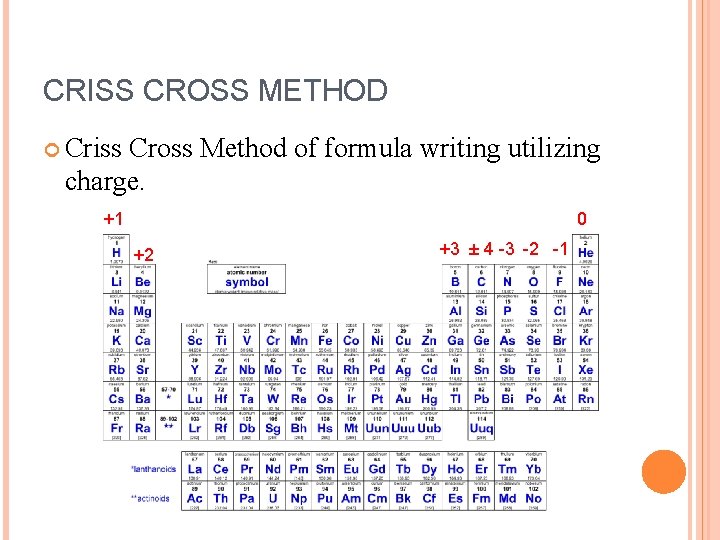
CRISS CROSS METHOD Criss Cross Method of formula writing utilizing charge. +1 0 +2 +3 ± 4 -3 -2 -1
CRISS CROSS METHOD What about the other elements?
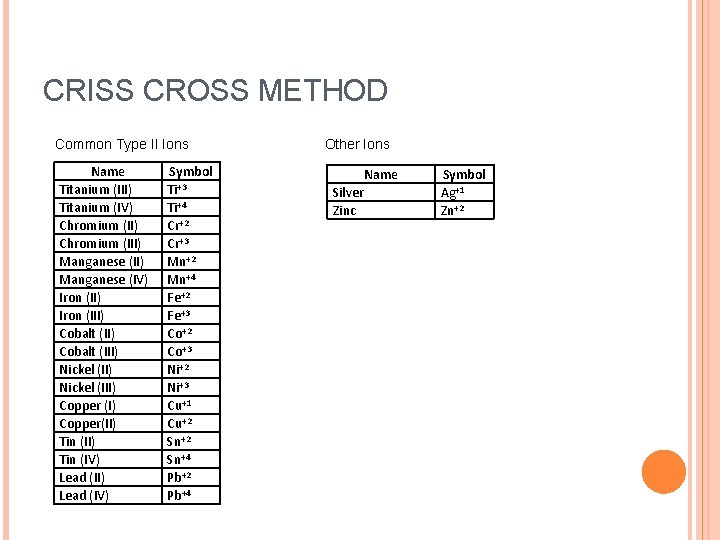
CRISS CROSS METHOD Common Type II Ions Name Titanium (III) Titanium (IV) Chromium (III) Manganese (IV) Iron (III) Cobalt (III) Nickel (III) Copper (I) Copper(II) Tin (IV) Lead (II) Lead (IV) Symbol Ti+3 Ti+4 Cr+2 Cr+3 Mn+2 Mn+4 Fe+2 Fe+3 Co+2 Co+3 Ni+2 Ni+3 Cu+1 Cu+2 Sn+4 Pb+2 Pb+4 Other Ions Name Silver Zinc Symbol Ag+1 Zn+2
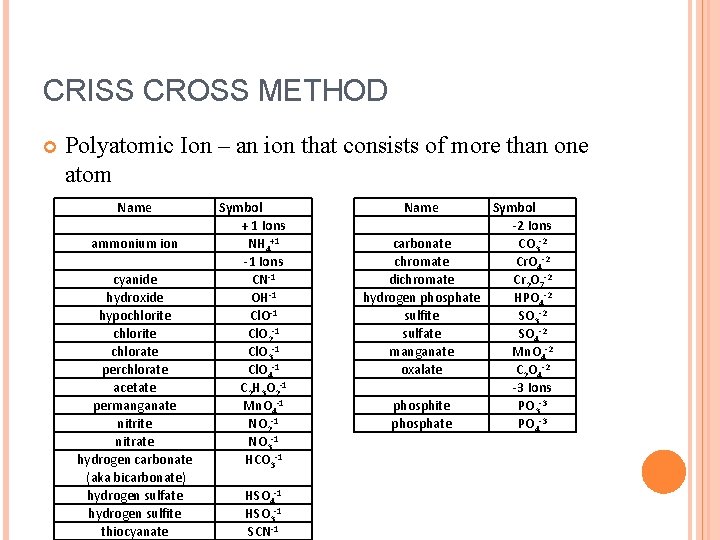
CRISS CROSS METHOD Polyatomic Ion – an ion that consists of more than one atom Name ammonium ion cyanide hydroxide hypochlorite chlorate perchlorate acetate permanganate nitrite nitrate hydrogen carbonate (aka bicarbonate) hydrogen sulfate hydrogen sulfite thiocyanate Symbol + 1 Ions NH 4+1 -1 Ions CN-1 OH-1 Cl. O 2 -1 Cl. O 3 -1 Cl. O 4 -1 C 2 H 3 O 2 -1 Mn. O 4 -1 NO 2 -1 NO 3 -1 HCO 3 -1 HSO 4 -1 HSO 3 -1 SCN-1 Name Symbol -2 Ions carbonate CO 3 -2 chromate Cr. O 4 -2 dichromate Cr 2 O 7 -2 hydrogen phosphate HPO 4 -2 sulfite SO 3 -2 sulfate SO 4 -2 manganate Mn. O 4 -2 oxalate C 2 O 4 -2 -3 Ions phosphite PO 3 -3 phosphate PO 4 -3
CRISS CROSS METHOD Criss Cross works with various types of compounds #1. Binary ionic compounds – an ionic compound that consists of only two types of elements Simple cation and a simple anion
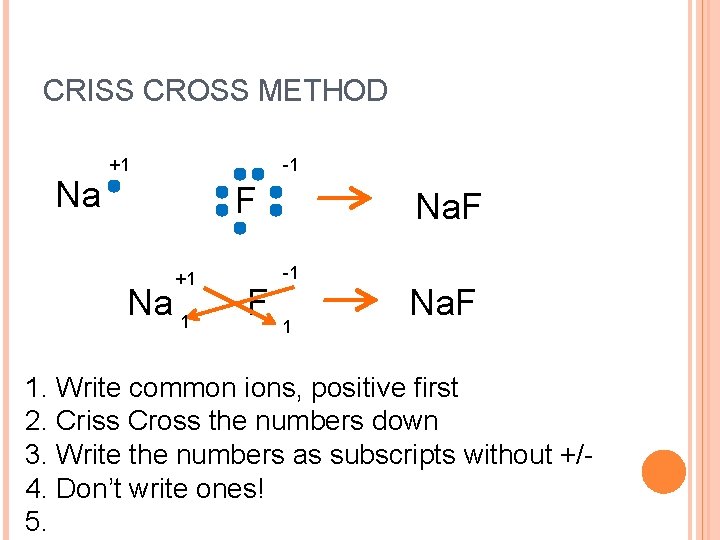
CRISS CROSS METHOD Na +1 -1 F +1 Na 1 F Na. F -1 1 Na. F 1. Write common ions, positive first 2. Criss Cross the numbers down 3. Write the numbers as subscripts without +/4. Don’t write ones! 5.
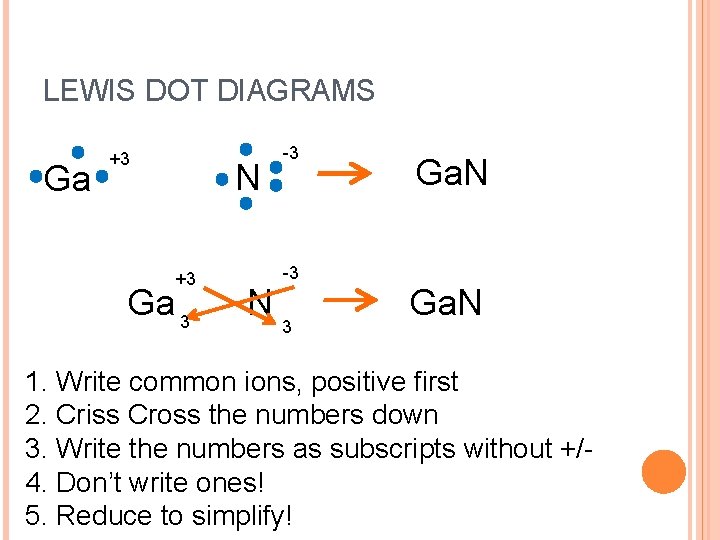
LEWIS DOT DIAGRAMS Ga +3 N +3 Ga 3 N -3 -3 3 Ga. N 1. Write common ions, positive first 2. Criss Cross the numbers down 3. Write the numbers as subscripts without +/4. Don’t write ones! 5. Reduce to simplify!
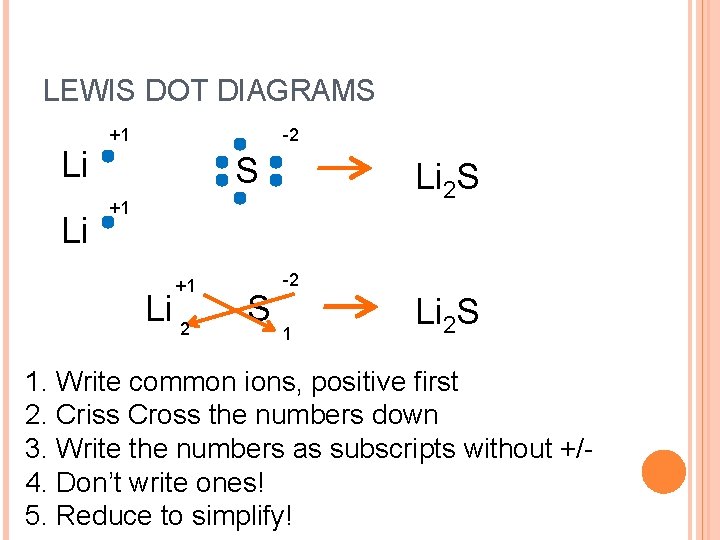
LEWIS DOT DIAGRAMS Li Li +1 -2 S Li 2 S +1 +1 Li 2 S -2 1 Li 2 S 1. Write common ions, positive first 2. Criss Cross the numbers down 3. Write the numbers as subscripts without +/4. Don’t write ones! 5. Reduce to simplify!
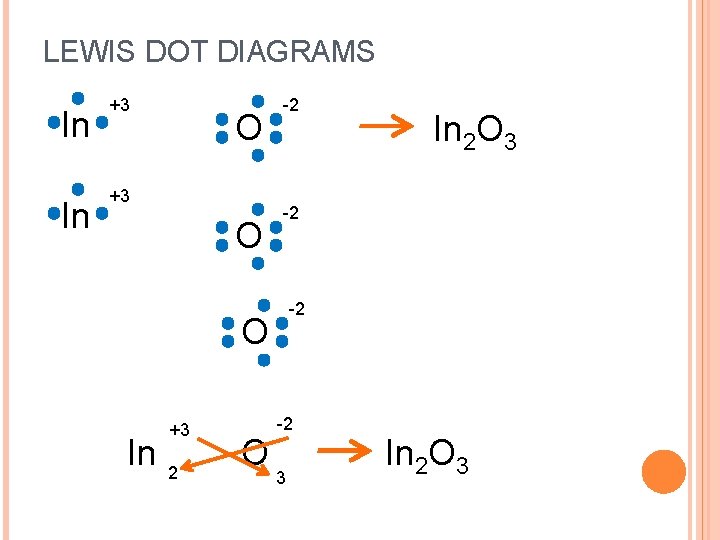
LEWIS DOT DIAGRAMS In In +3 O -2 -2 -2 O In +3 2 O In 2 O 3 -2 3 In 2 O 3

LET’S TRY SOME Criss Cross #1

1/24/16 Today I will use the criss cross method with polyatomic ions Warm Up - Use criss cross to determine the formula of the compound that forms between: a. Sr and P b. Al and As c. Rb and S
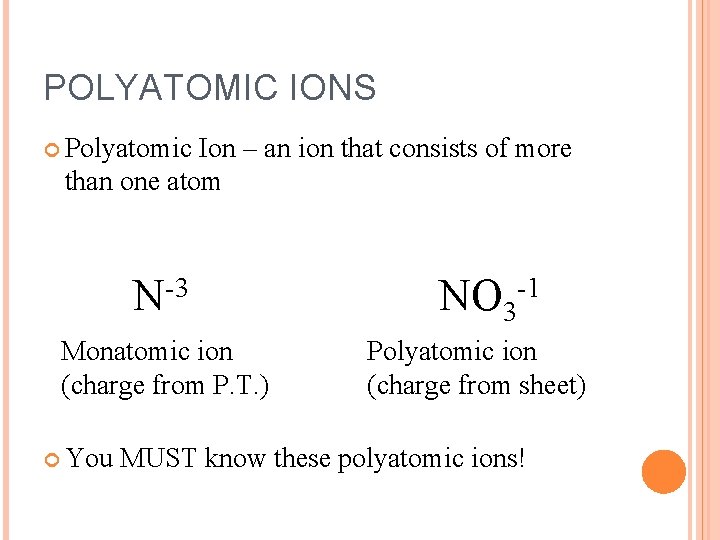
POLYATOMIC IONS Polyatomic Ion – an ion that consists of more than one atom N-3 Monatomic ion (charge from P. T. ) You NO 3 -1 Polyatomic ion (charge from sheet) MUST know these polyatomic ions!
CRISS CROSS METHOD Criss Cross will also work with compounds containing polyatomic ions Polyatomic bonding! ions act like a single atom in ionic
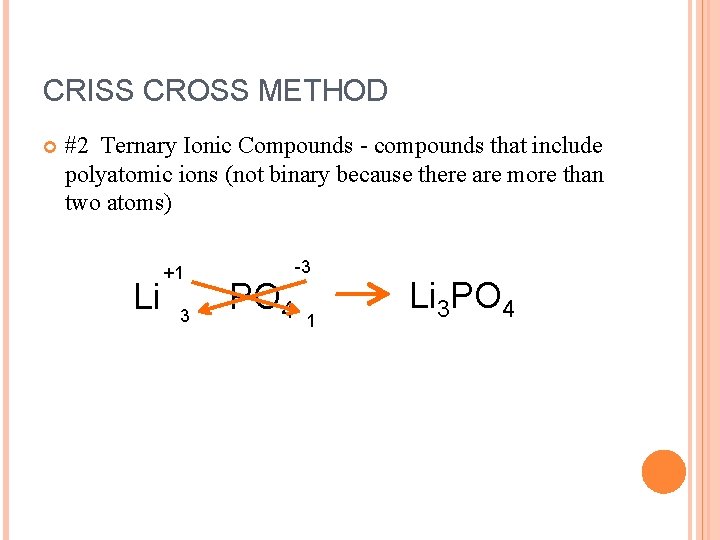
CRISS CROSS METHOD #2 Ternary Ionic Compounds - compounds that include polyatomic ions (not binary because there are more than two atoms) Li +1 3 PO 4 -3 1 Li 3 PO 4
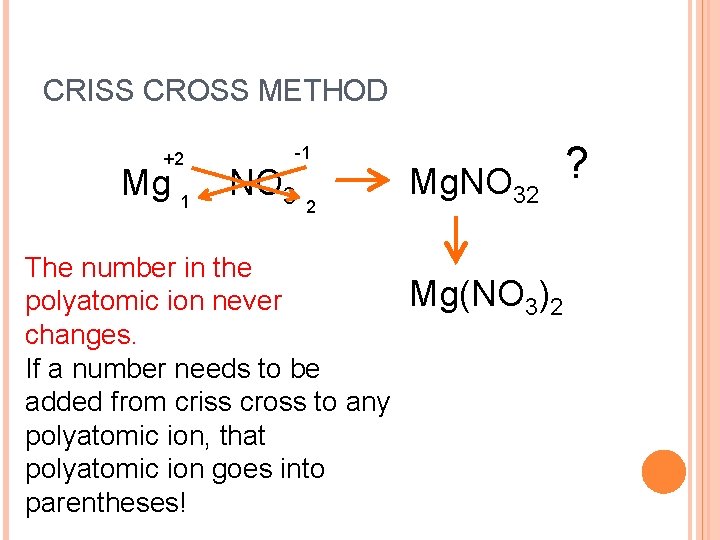
CRISS CROSS METHOD +2 Mg 1 -1 NO 3 2 The number in the polyatomic ion never changes. If a number needs to be added from criss cross to any polyatomic ion, that polyatomic ion goes into parentheses! Mg. NO 32 Mg(NO 3)2 ?
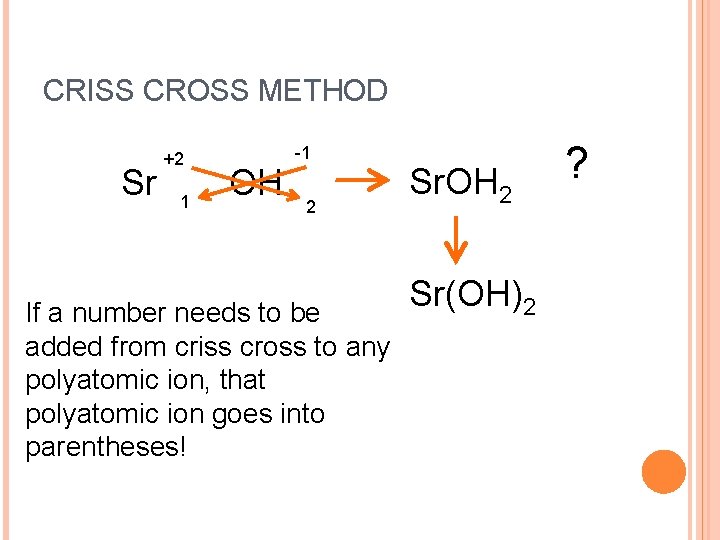
CRISS CROSS METHOD Sr +2 1 OH -1 2 If a number needs to be added from criss cross to any polyatomic ion, that polyatomic ion goes into parentheses! Sr. OH 2 Sr(OH)2 ?
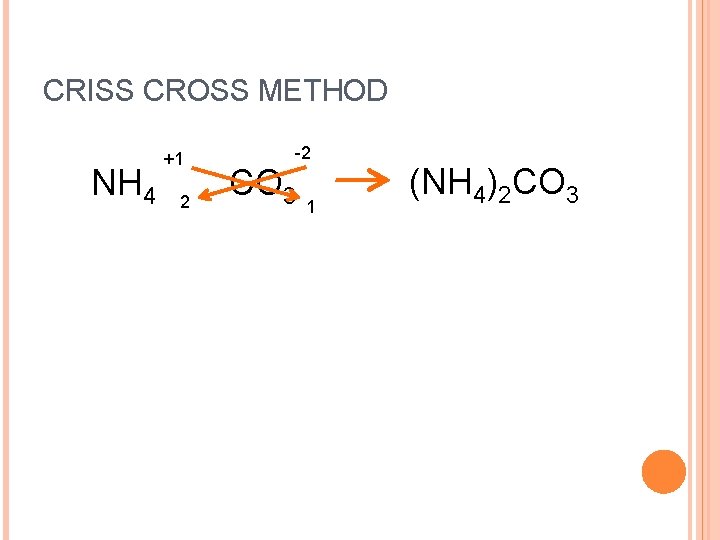
CRISS CROSS METHOD NH 4 +1 2 -2 CO 3 1 (NH 4)2 CO 3

HOMEWORK Complete Criss Cross #2

1/25 Today I will define covalent bonds and draw simple Lewis Structures. Warm Up - Use criss cross to determine the compound that is formed between the following Cs and N Al and NO 3 Be and PO 3 Mg and P Ba and Cr. O 4
COVALENT BONDING Ionic Bond – occurs when oppositely charged ions are held together by electrical attraction caused by transferring electrons. **We will consider an ionic bond to be any bond between a metal and a nonmetal OR any bond that contains a polyatomic ion! Covalent Bond – occurs when electrons are shared between atoms. **We will consider a covalent bond to be any bond between two non-metals!
COVALENT BONDING Each unit of a covalent compound is called a MOLECULE. (As opposed to ionic formula units) Covalent compounds do NOT have a set ratio between elements. (Ionic AWLAYS does) You CANNOT use criss cross or any other method to determine the formula! (You must with ionic compounds) Covalent compounds form a wide range of substances with a wide range of properties!

COVALENT BONDING CO 2 C 12 H 22 O 11 C 6 H 12 O 6
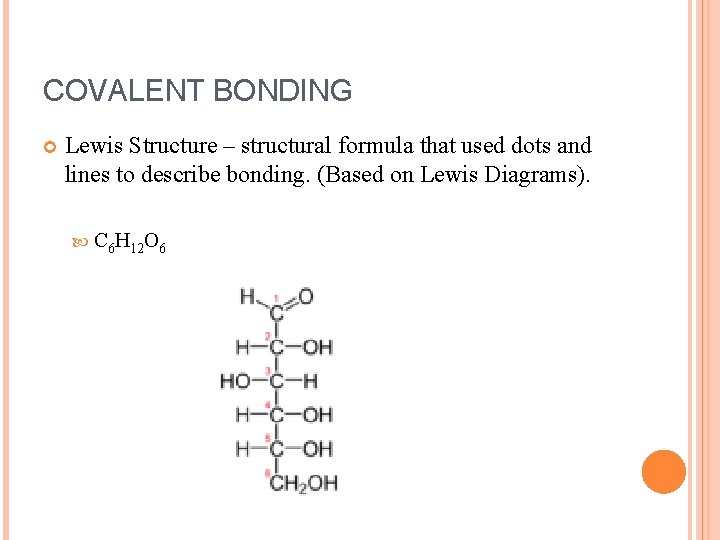
COVALENT BONDING Lewis Structure – structural formula that used dots and lines to describe bonding. (Based on Lewis Diagrams). C 6 H 12 O 6
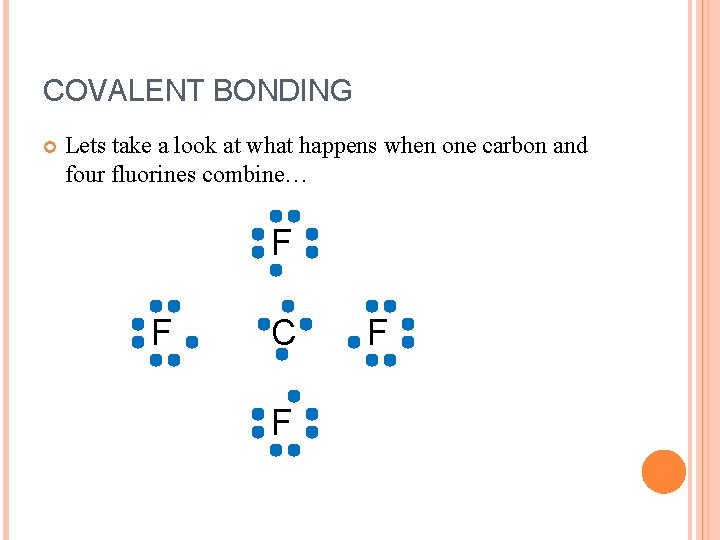
COVALENT BONDING Lets take a look at what happens when one carbon and four fluorines combine… F F C F F
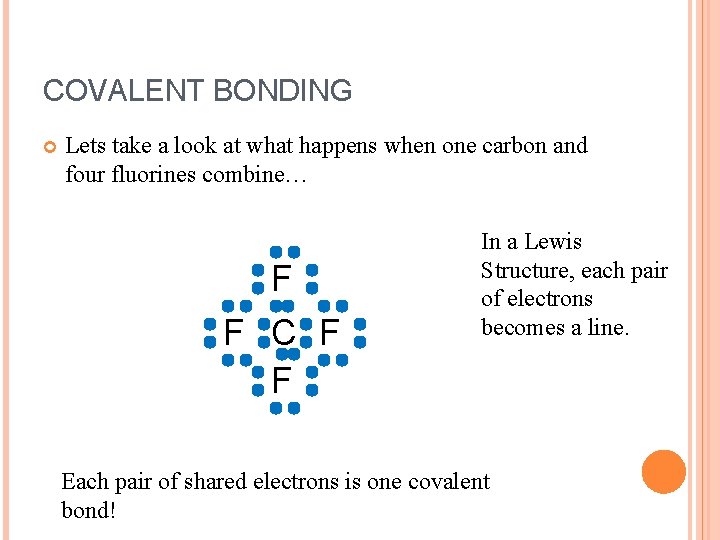
COVALENT BONDING Lets take a look at what happens when one carbon and four fluorines combine… F F C F F In a Lewis Structure, each pair of electrons becomes a line. Each pair of shared electrons is one covalent bond!
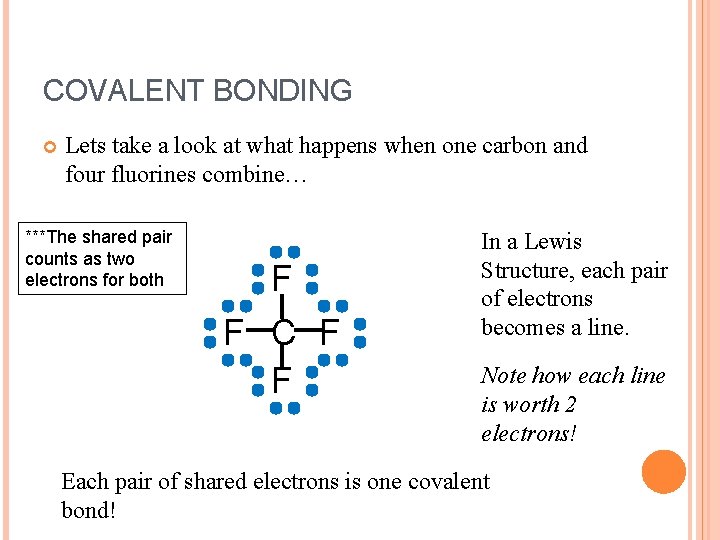
COVALENT BONDING Lets take a look at what happens when one carbon and four fluorines combine… ***The shared pair counts as two electrons for both F F C F F In a Lewis Structure, each pair of electrons becomes a line. Note how each line is worth 2 electrons! Each pair of shared electrons is one covalent bond!
LEWIS STRUCTURES Steps to drawing simple Lewis Structures CF 4 1. Count up the total number of valence electrons present.
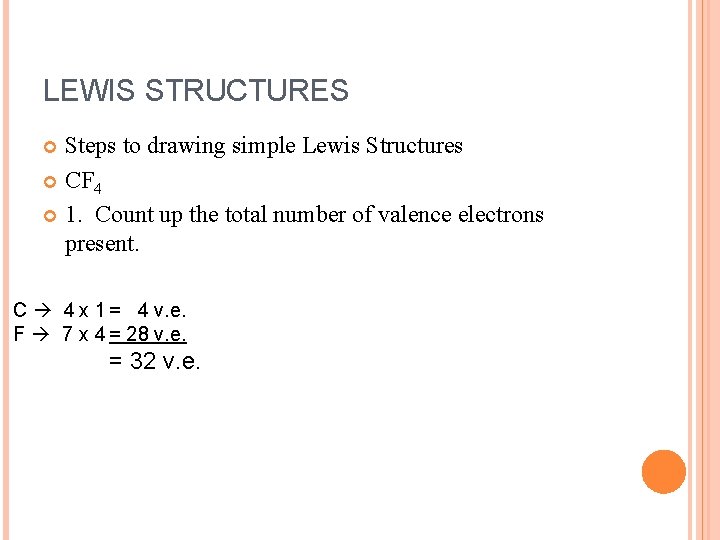
LEWIS STRUCTURES Steps to drawing simple Lewis Structures CF 4 1. Count up the total number of valence electrons present. C 4 x 1 = 4 v. e. F 7 x 4 = 28 v. e. = 32 v. e.
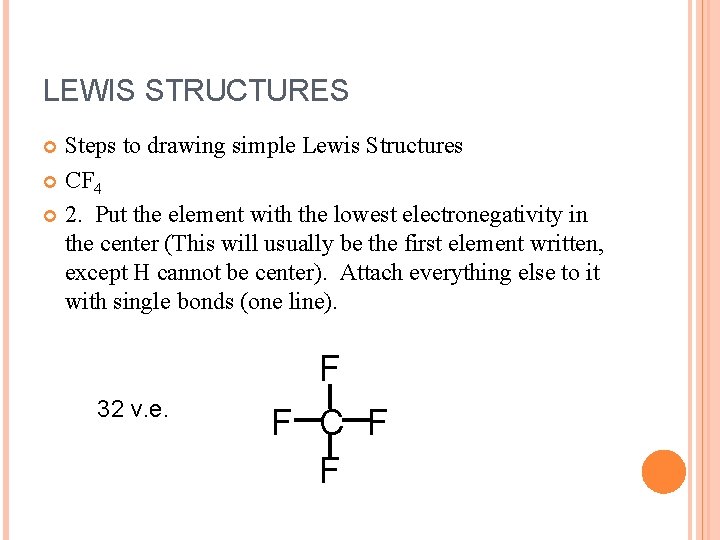
LEWIS STRUCTURES Steps to drawing simple Lewis Structures CF 4 2. Put the element with the lowest electronegativity in the center (This will usually be the first element written, except H cannot be center). Attach everything else to it with single bonds (one line). 32 v. e. F F C F F
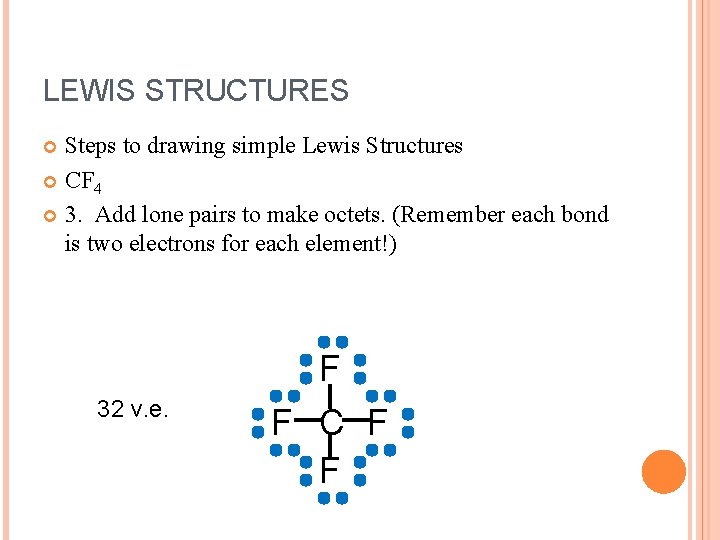
LEWIS STRUCTURES Steps to drawing simple Lewis Structures CF 4 3. Add lone pairs to make octets. (Remember each bond is two electrons for each element!) 32 v. e. F F C F F
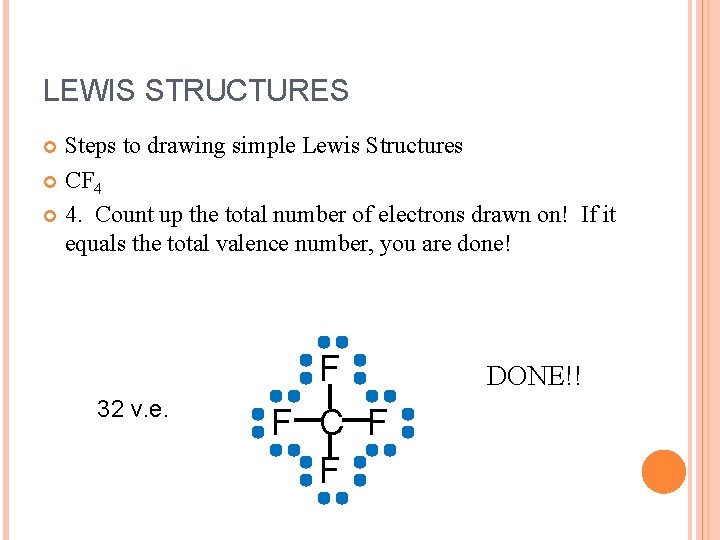
LEWIS STRUCTURES Steps to drawing simple Lewis Structures CF 4 4. Count up the total number of electrons drawn on! If it equals the total valence number, you are done! 32 v. e. F F C F F DONE!!
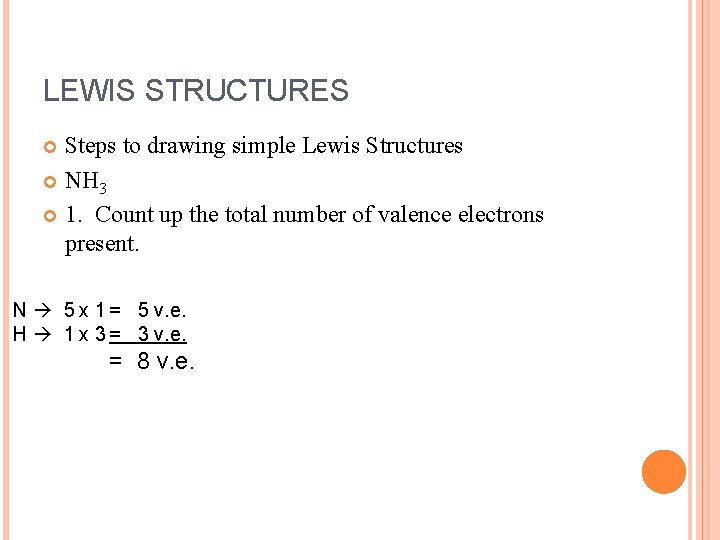
LEWIS STRUCTURES Steps to drawing simple Lewis Structures NH 3 1. Count up the total number of valence electrons present. N 5 x 1 = 5 v. e. H 1 x 3 = 3 v. e. = 8 v. e.
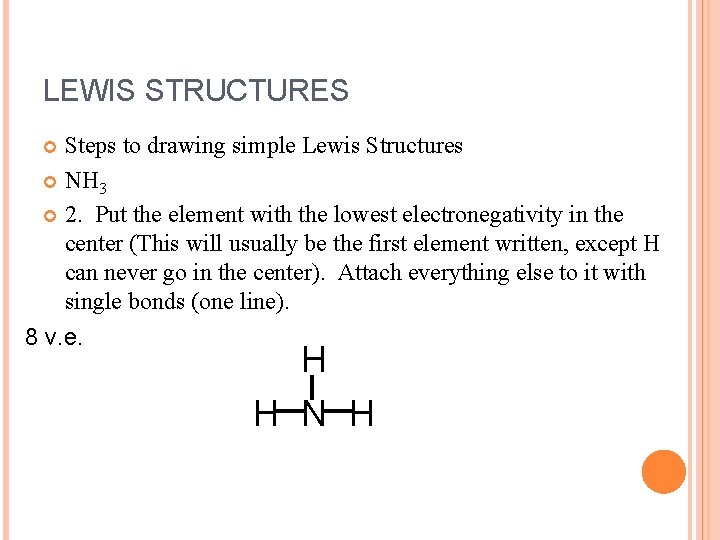
LEWIS STRUCTURES Steps to drawing simple Lewis Structures NH 3 2. Put the element with the lowest electronegativity in the center (This will usually be the first element written, except H can never go in the center). Attach everything else to it with single bonds (one line). 8 v. e. H H N H
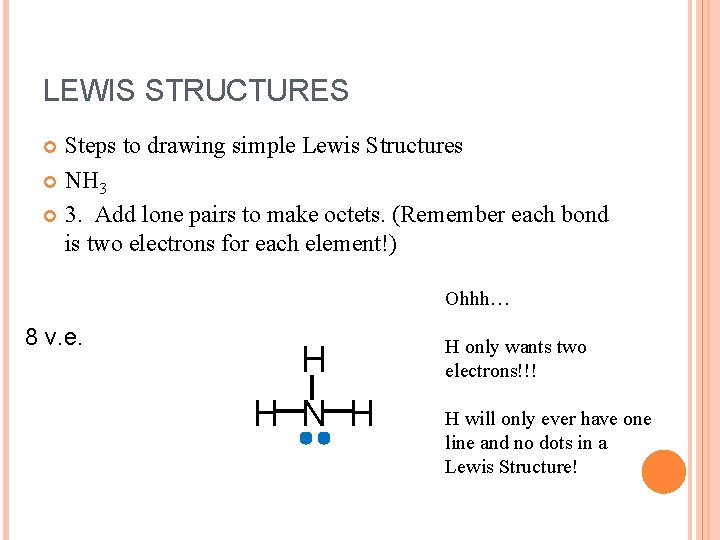
LEWIS STRUCTURES Steps to drawing simple Lewis Structures NH 3 3. Add lone pairs to make octets. (Remember each bond is two electrons for each element!) Ohhh… 8 v. e. H H N H H only wants two electrons!!! H will only ever have one line and no dots in a Lewis Structure!
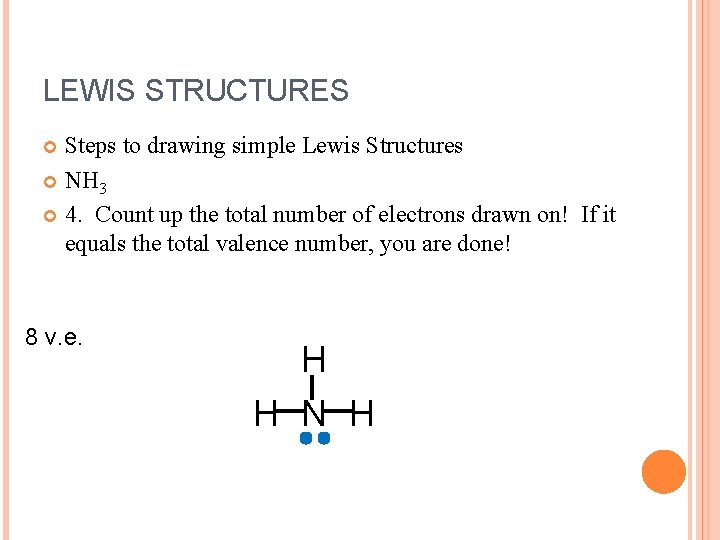
LEWIS STRUCTURES Steps to drawing simple Lewis Structures NH 3 4. Count up the total number of electrons drawn on! If it equals the total valence number, you are done! 8 v. e. H H N H

HOMEWORK Writing Simple Lewis Structures #1

1/26/16 Today I will draw Lewis Structures with multiple bonds. Warm (H 2 O) Up – Draw the Lewis Structure for water
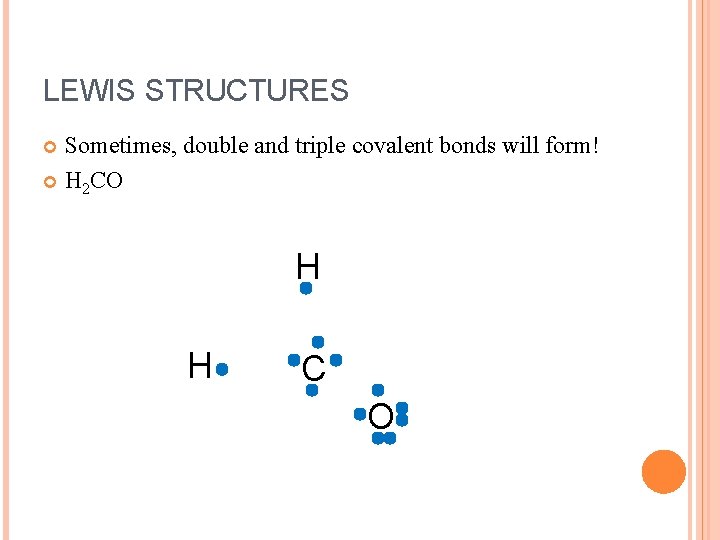
LEWIS STRUCTURES Sometimes, double and triple covalent bonds will form! H 2 CO H H C O
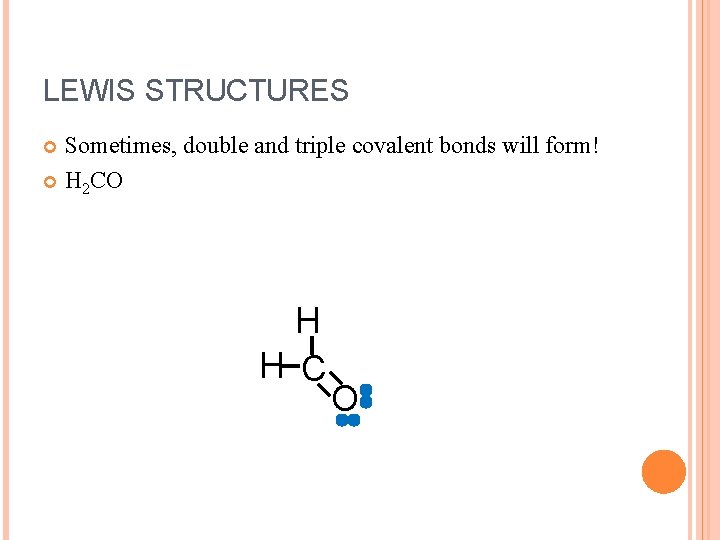
LEWIS STRUCTURES Sometimes, double and triple covalent bonds will form! H 2 CO H H C O
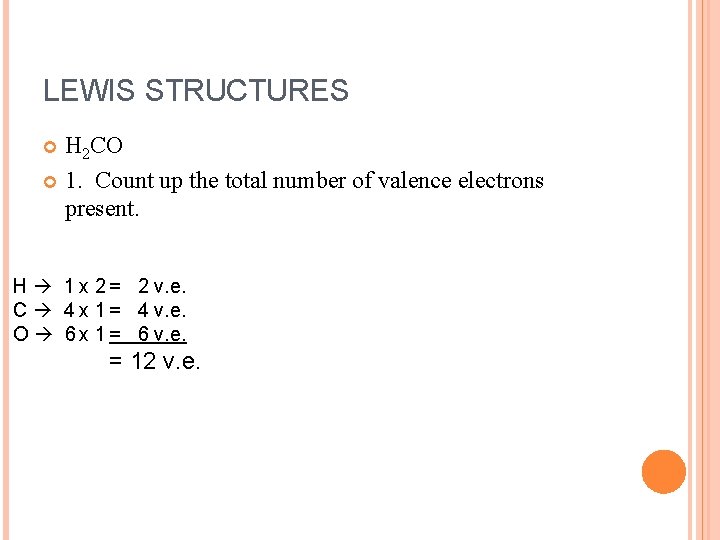
LEWIS STRUCTURES H 2 CO 1. Count up the total number of valence electrons present. H 1 x 2 = 2 v. e. C 4 x 1 = 4 v. e. O 6 x 1 = 6 v. e. = 12 v. e.
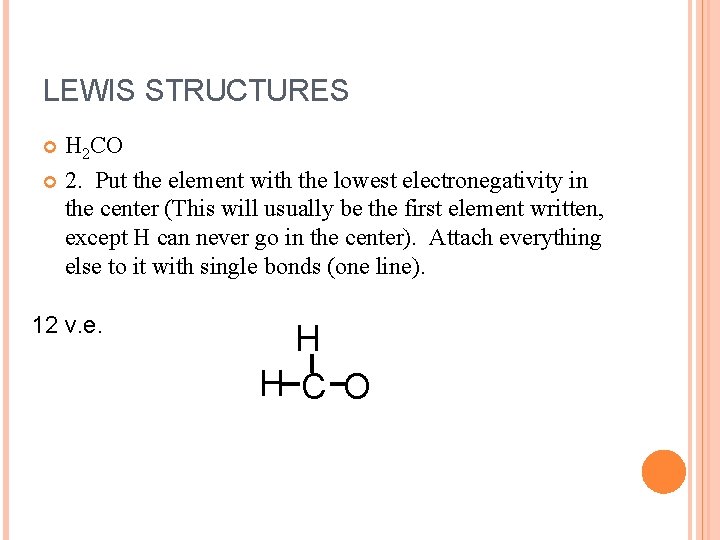
LEWIS STRUCTURES H 2 CO 2. Put the element with the lowest electronegativity in the center (This will usually be the first element written, except H can never go in the center). Attach everything else to it with single bonds (one line). 12 v. e. H H C O
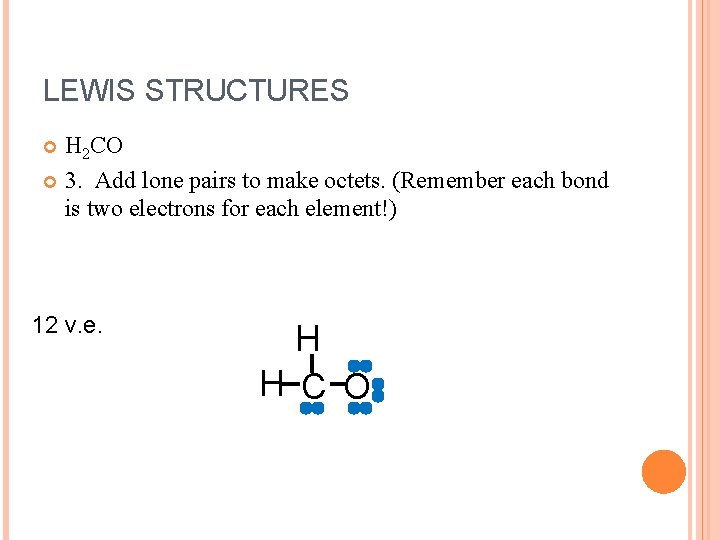
LEWIS STRUCTURES H 2 CO 3. Add lone pairs to make octets. (Remember each bond is two electrons for each element!) 12 v. e. H H C O
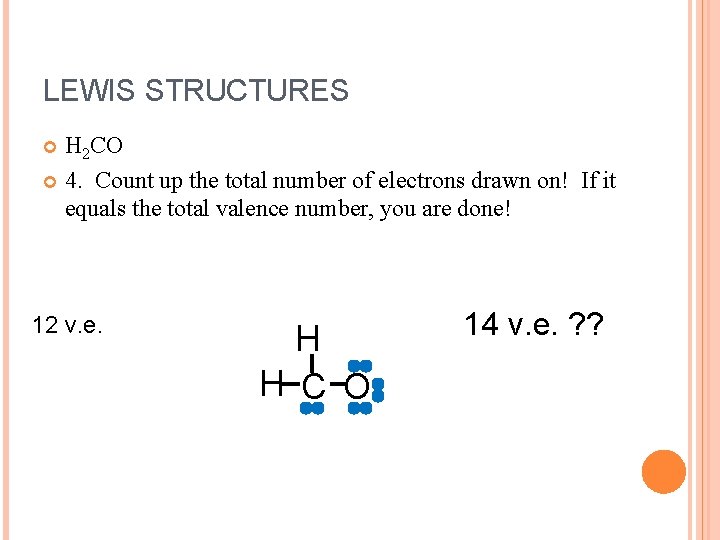
LEWIS STRUCTURES H 2 CO 4. Count up the total number of electrons drawn on! If it equals the total valence number, you are done! 12 v. e. H H C O 14 v. e. ? ?
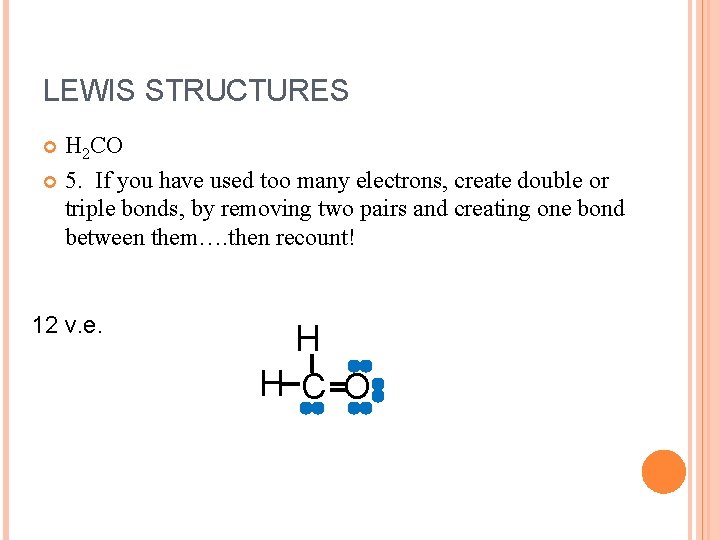
LEWIS STRUCTURES H 2 CO 5. If you have used too many electrons, create double or triple bonds, by removing two pairs and creating one bond between them…. then recount! 12 v. e. H H C O
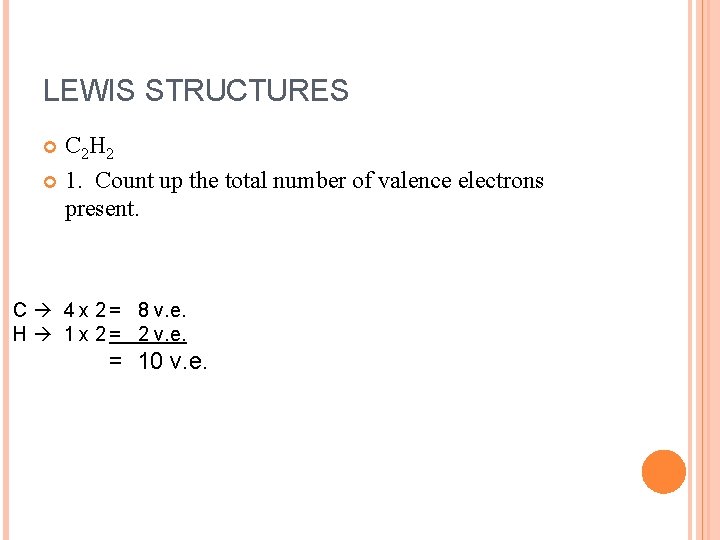
LEWIS STRUCTURES C 2 H 2 1. Count up the total number of valence electrons present. C 4 x 2 = 8 v. e. H 1 x 2 = 2 v. e. = 10 v. e.
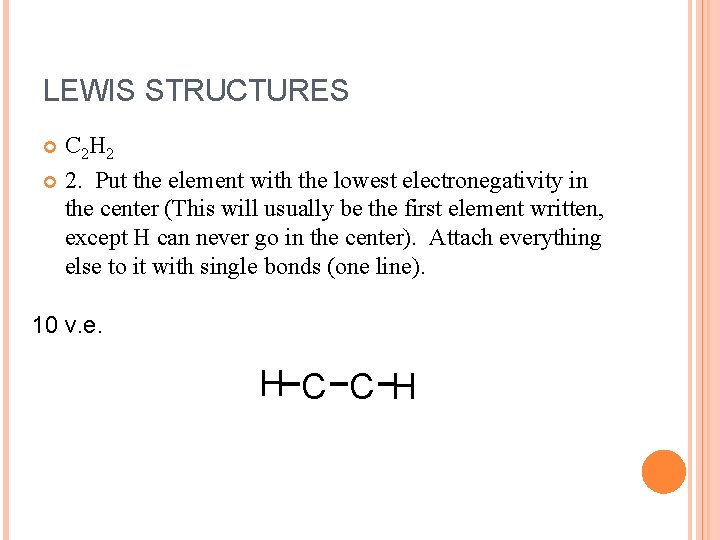
LEWIS STRUCTURES C 2 H 2 2. Put the element with the lowest electronegativity in the center (This will usually be the first element written, except H can never go in the center). Attach everything else to it with single bonds (one line). 10 v. e. H C C H
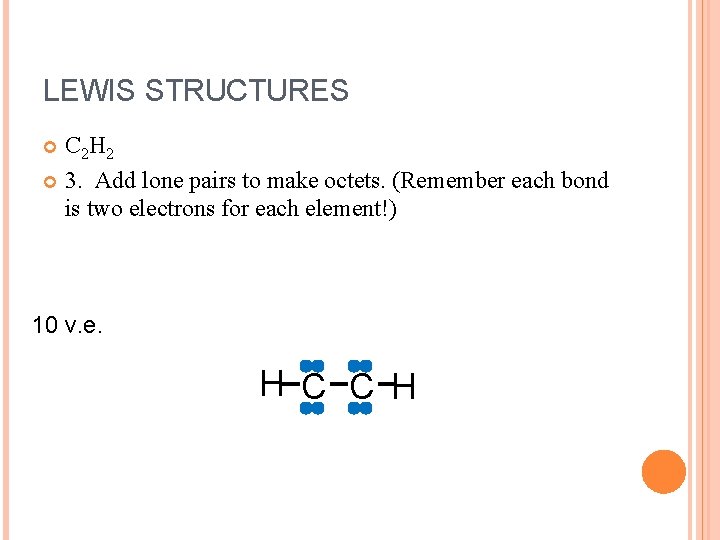
LEWIS STRUCTURES C 2 H 2 3. Add lone pairs to make octets. (Remember each bond is two electrons for each element!) 10 v. e. H C C H
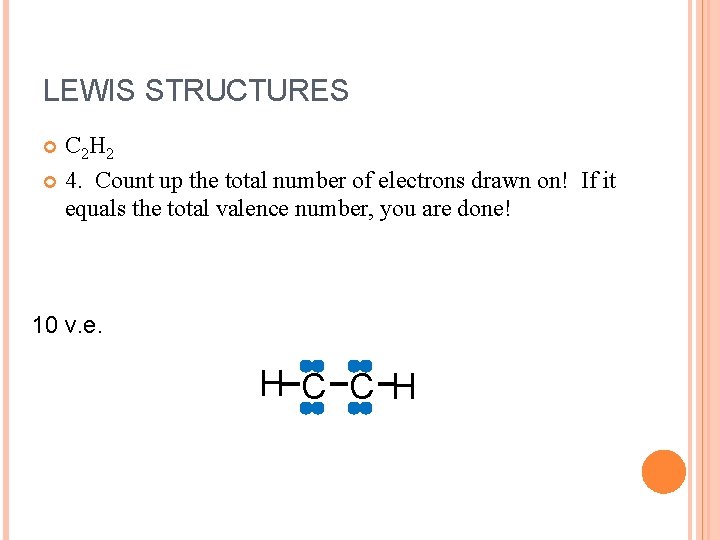
LEWIS STRUCTURES C 2 H 2 4. Count up the total number of electrons drawn on! If it equals the total valence number, you are done! 10 v. e. H C C H
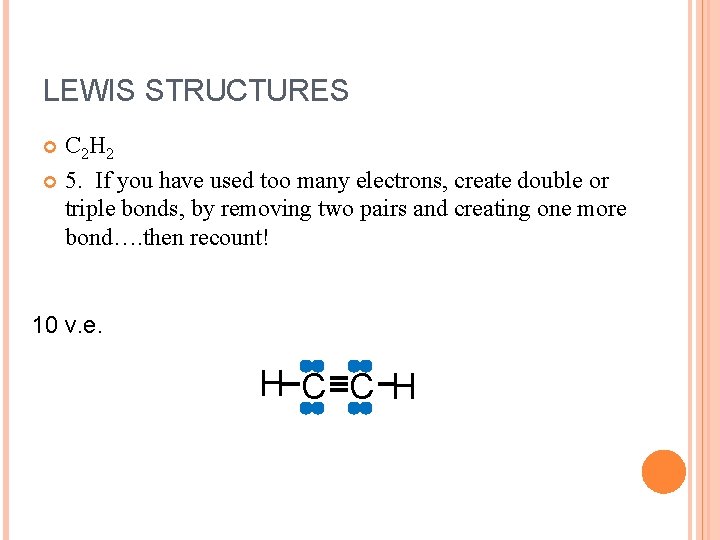
LEWIS STRUCTURES C 2 H 2 5. If you have used too many electrons, create double or triple bonds, by removing two pairs and creating one more bond…. then recount! 10 v. e. H C C H
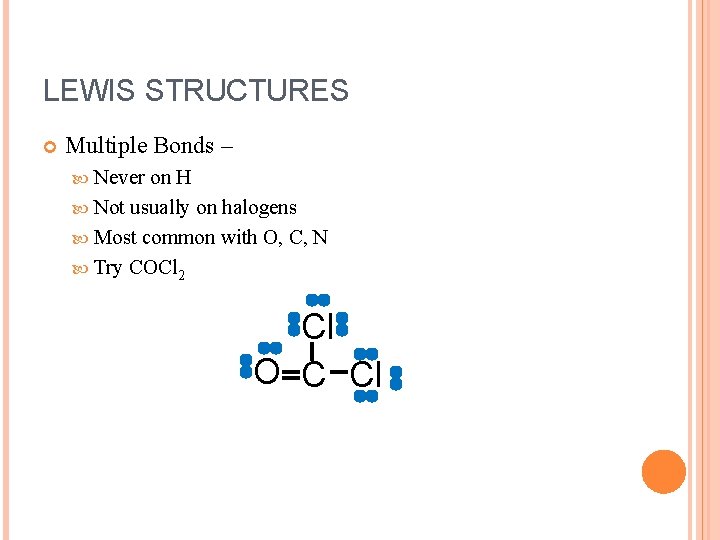
LEWIS STRUCTURES Multiple Bonds – Never on H Not usually on halogens Most common with O, C, N Try COCl 2 Cl O C Cl

HOMEWORK Writing Lewis Structures with Multiple Bonds #2

1/27 Today I will draw lewis structures of ions & other exceptions Warm Up – Use criss cross to write formulas: a. b. c. d. e. Rb and PO 4 Al and OH B and N NH 4 and O Sr and As
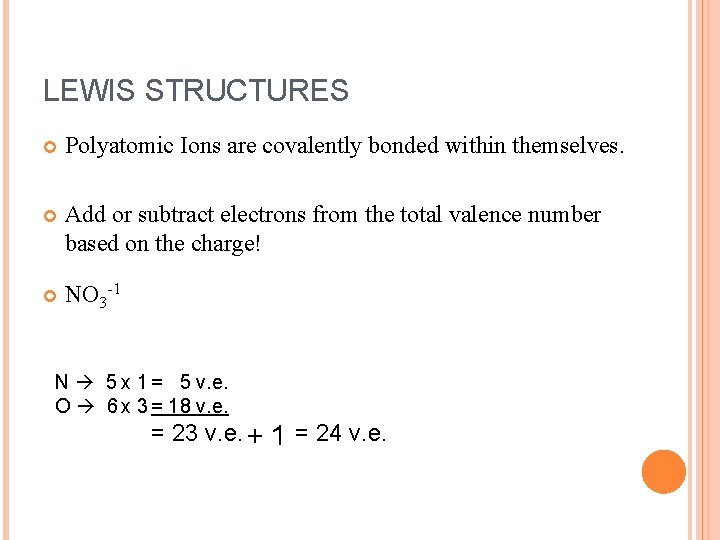
LEWIS STRUCTURES Polyatomic Ions are covalently bonded within themselves. Add or subtract electrons from the total valence number based on the charge! NO 3 -1 N 5 x 1 = 5 v. e. O 6 x 3 = 18 v. e. = 23 v. e. + 1 = 24 v. e.
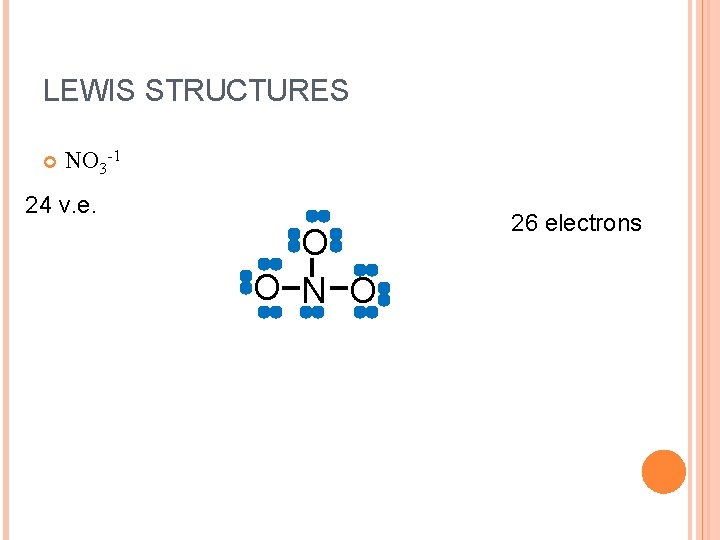
LEWIS STRUCTURES NO 3 -1 24 v. e. O O N O 26 electrons
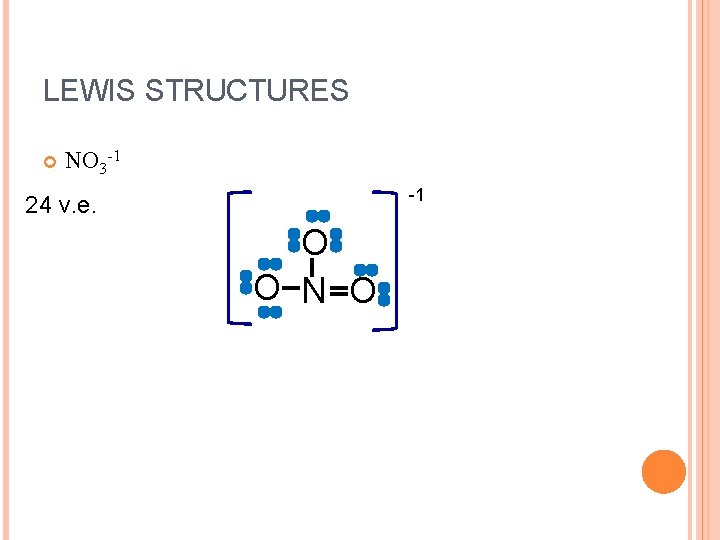
LEWIS STRUCTURES NO 3 -1 -1 24 v. e. O O N O
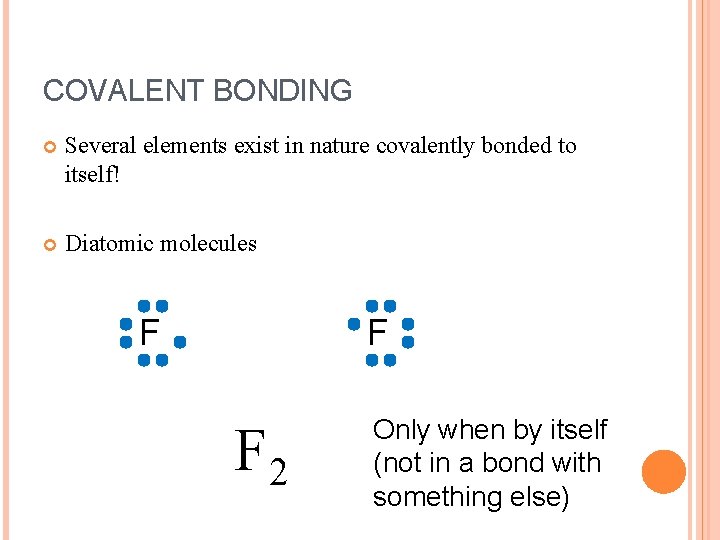
COVALENT BONDING Several elements exist in nature covalently bonded to itself! Diatomic molecules F F F 2 Only when by itself (not in a bond with something else)
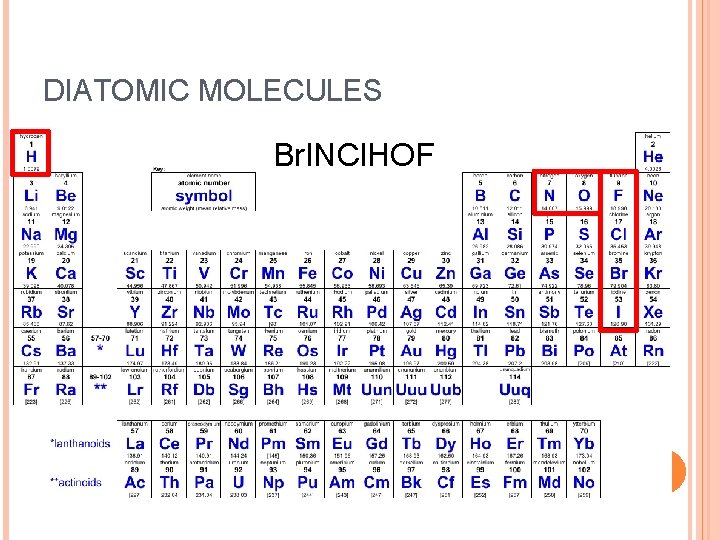
DIATOMIC MOLECULES Br. INCl. HOF

HOMEWORK Lewis Structures #3 (Skip #10)

1/30 Today I will name ionic compounds Warm Up – Draw the Lewis Structure for NI 3

NAMING IONIC COMPOUNDS Ionic Compounds can be identified because they typically form between a metal and a non-metal OR contain a polyatomic ion!! Rules for naming ionic compounds 1. Name the cation (metal or polyatomic ion) 2. Name the anion but… If the anion is an element, change the ending to –ide If the anion is a polyatomic ion, simply name it (no change) 3. Rule three to come!
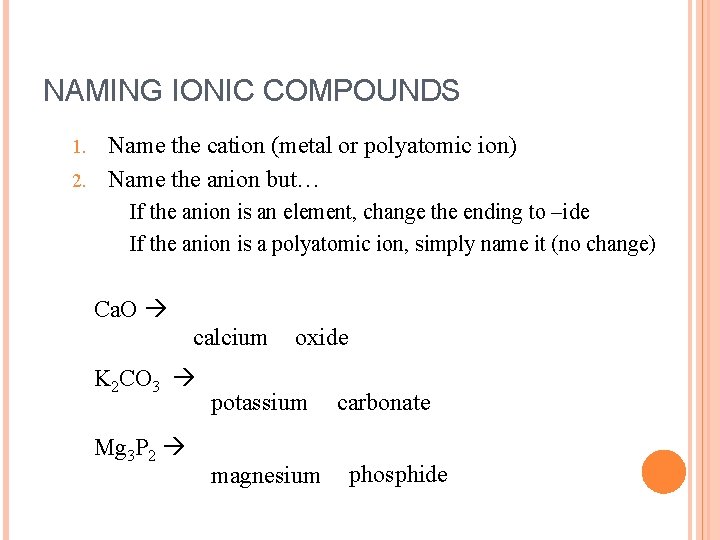
NAMING IONIC COMPOUNDS Name the cation (metal or polyatomic ion) 2. Name the anion but… 1. If the anion is an element, change the ending to –ide If the anion is a polyatomic ion, simply name it (no change) Ca. O calcium K 2 CO 3 Mg 3 P 2 oxide potassium magnesium carbonate phosphide
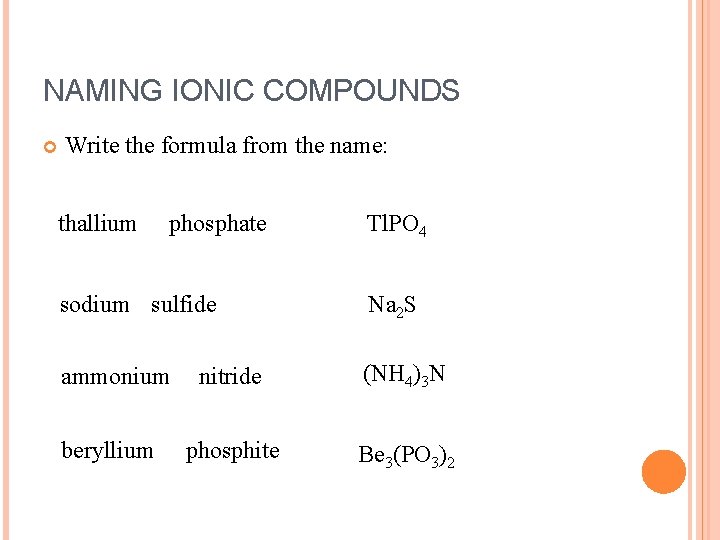
NAMING IONIC COMPOUNDS Write the formula from the name: thallium phosphate Tl. PO 4 sodium sulfide Na 2 S ammonium nitride (NH 4)3 N phosphite Be 3(PO 3)2 beryllium

HOMEWORK Ionic Compounds – Formulas and Names #1

1/31 Today I will name ionic compounds with Type II Cations Warm Up – Name the following Compounds KCl Be. Br 2 Al 2 S 3

NAMING IONIC COMPOUNDS Transition (and other Type II) Metals! Some metals can form more than one charge! These are called Type II cations! This is typically seen in the transition metals (also, lead and tin) Except zinc (always +2) and silver (always +1) Rule #3! If a type II cation is present, place the charge of the ion in roman numerals in parenthesis after the cation name.
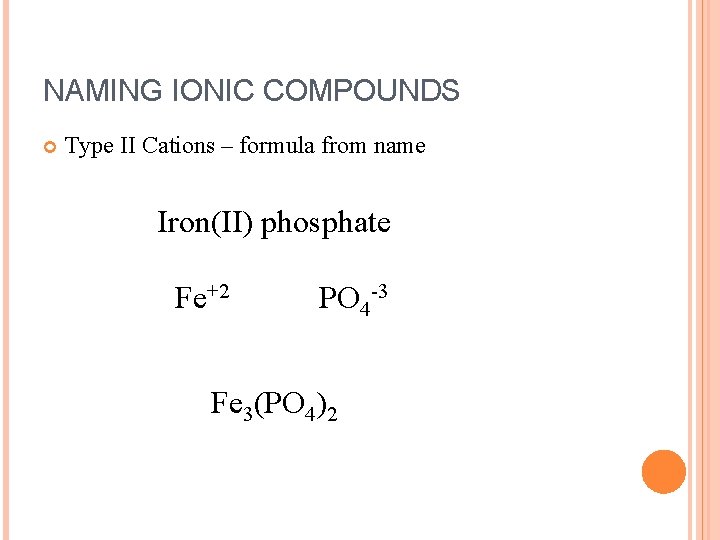
NAMING IONIC COMPOUNDS Type II Cations – formula from name Iron(II) phosphate Fe+2 PO 4 -3 Fe 3(PO 4)2

NAMING IONIC COMPOUNDS Type II Cations – name from formula When naming an ionic compound from the formula, you must recognize the type II cations and give them a number by un-crisscrossing! Co. F 2 Cobalt (II)fluoride Co 2(SO 3)3 Cobalt (III)sulfite Fe. O Iron (II) oxide Sn. O 2 Tin (IV) oxide Cu. OH Copper (I)hydroxide

HOMEWORK Ionic Compounds – Formulas and Names #2 (#3 is extra practice!)

2/1 Today Warm I will name covalent compounds Up – Name the following: Ca(OH)2 Al. Cl 3 Ni. S Write the formulas for the following: Calcium chloride Lead(II) phosphide Magnesium phosphate

NAMING COVALENT COMPOUNDS Covalent Compounds can be identified because they typically form between two non-metals!! Rules for naming covalent compounds 1. Name the first element 2. Name second element and change the ending to ide 3. Add prefixes EXCEPT where the first element is a one! (When two vowels are together, you may eliminate one. )
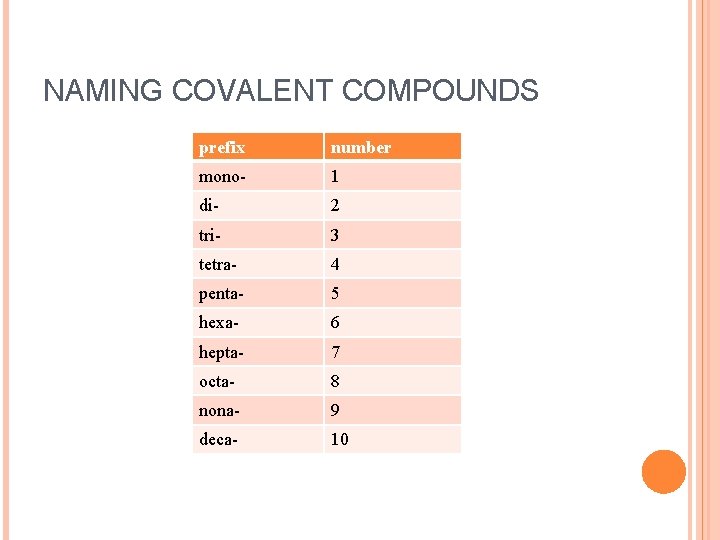
NAMING COVALENT COMPOUNDS prefix number mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa- 6 hepta- 7 octa- 8 nona- 9 deca- 10
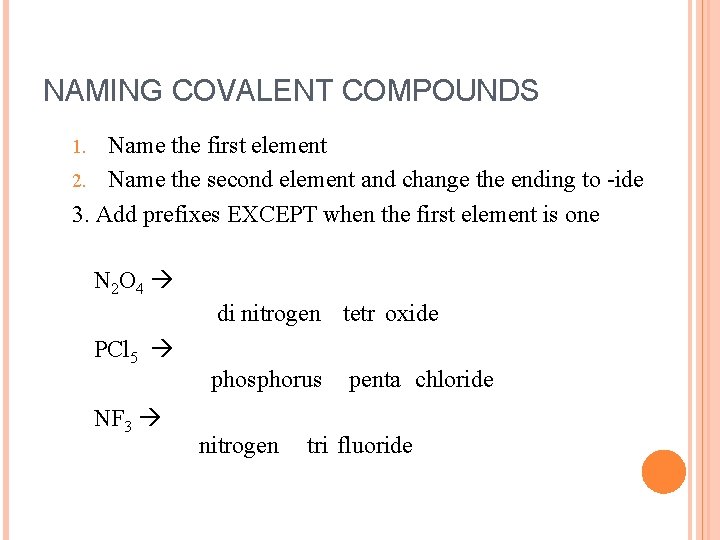
NAMING COVALENT COMPOUNDS Name the first element 2. Name the second element and change the ending to -ide 3. Add prefixes EXCEPT when the first element is one 1. N 2 O 4 di nitrogen tetr oxide PCl 5 NF 3 phosphorus nitrogen penta chloride tri fluoride
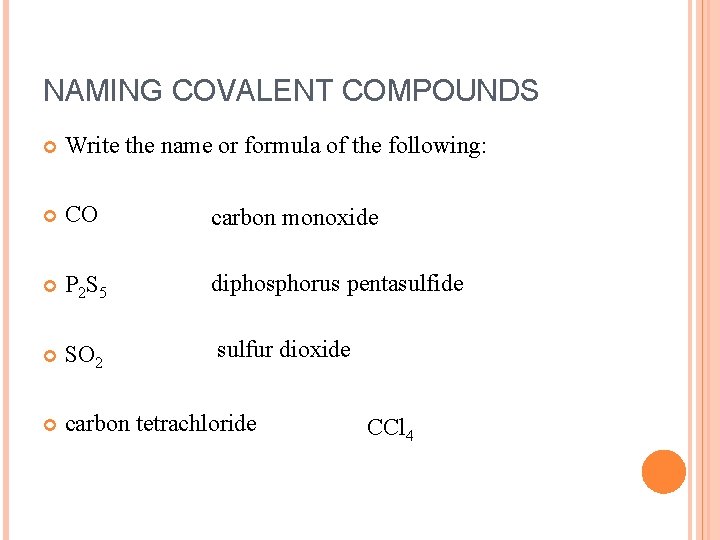
NAMING COVALENT COMPOUNDS Write the name or formula of the following: CO carbon monoxide P 2 S 5 diphosphorus pentasulfide SO 2 sulfur dioxide carbon tetrachloride CCl 4

MIXED NAMING When naming, the first step is always to decide whether the compound is ionic or covalent.

HOMEWORK Covalent Compounds – Formulas and Names

2/2 Today I will practice naming compounds Warm Up – Tell whether each of the following compounds is ionic or covalent: a. Cl 2 b. SO 3 c. Na. OH d. (NH 4)3 P e. H 2 O 2
- Slides: 93