Chapter 7 Chemical Bonding Review Quiz 7 Thermodynamics

Chapter 7 Chemical Bonding

Review Quiz 7 • Thermodynamics • Effective Nuclear Charge

How do we determine the type of bond that occurs between atoms?
Electronegativity • Electronegativity is the ability of an atom in a molecule to attract shared electrons to it. • We can use the Periodic Table to predict the relative electronegativity of the elements.
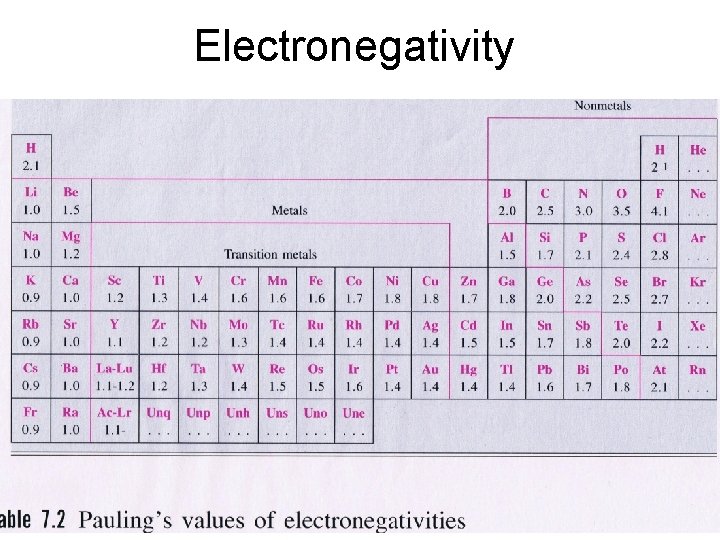
Electronegativity • Inert Table 7. 2
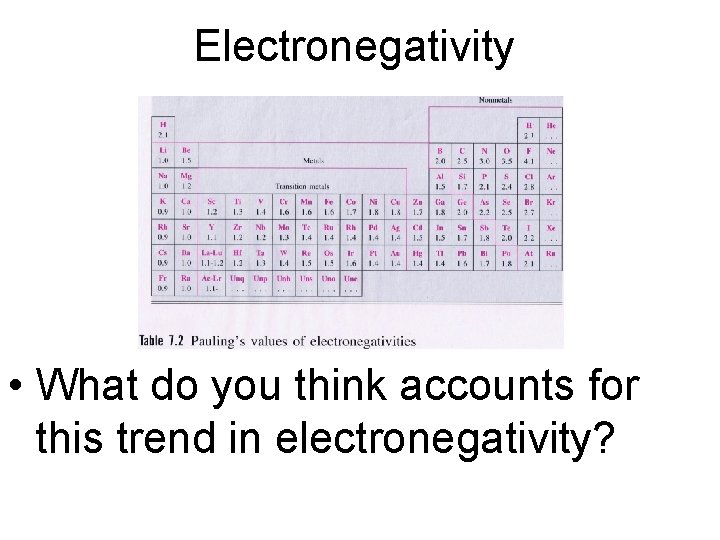
Electronegativity • What do you think accounts for this trend in electronegativity?
Electronegativity Difference • When valence electrons are shared between atoms of identical electronegativity a nonpolar covalent bond results. • When valence electrons are shared between atoms of different electronegativity a polar covalent bond results.
Note on covalent bonding • When the electronegativity of the elements involved in a bond is very close we often consider the bonds to be nonpolar even though they may have a very slight polarity. • For this course however we will consider nonpolar covalent bonds to have electronegativity differences of zero.
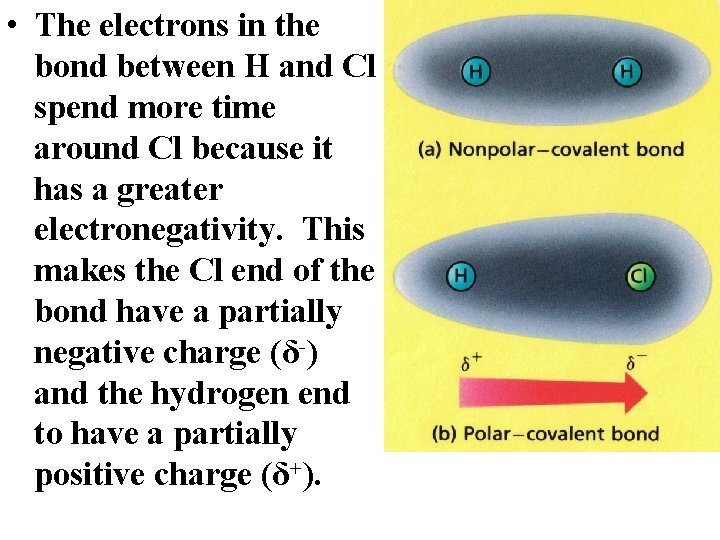
• The electrons in the bond between H and Cl spend more time around Cl because it has a greater electronegativity. This makes the Cl end of the bond have a partially negative charge (δ-) and the hydrogen end to have a partially positive charge (δ+).
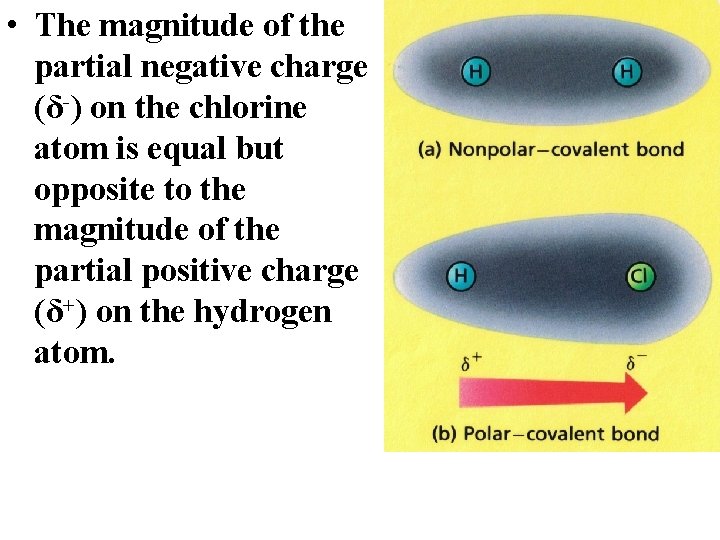
• The magnitude of the partial negative charge (δ-) on the chlorine atom is equal but opposite to the magnitude of the partial positive charge (δ+) on the hydrogen atom.
Polar and Nonpolar Bonds • A polar covalent bond has a positive end a negative end. It typically is a bond between two different nonmetal atoms. • A nonpolar covalent bond has an equal charge distribution with neither end of the bond being charged. It typically is a bond between two of the same nonmetal atoms.
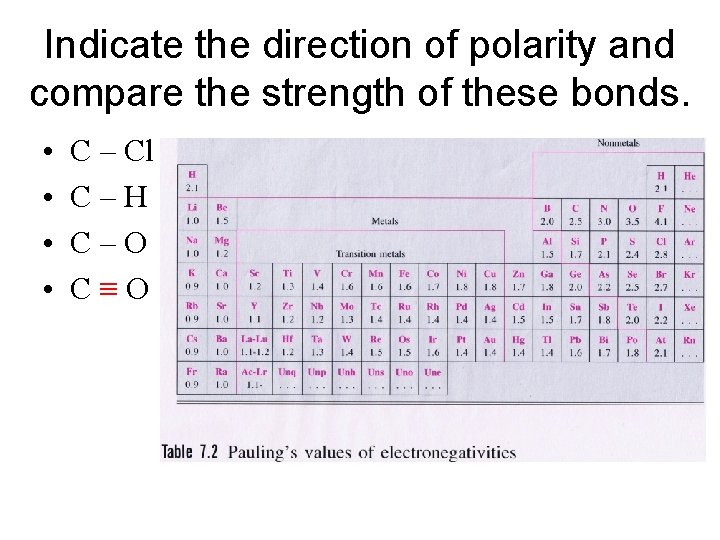
Indicate the direction of polarity and compare the strength of these bonds. • • C – Cl C–H C–O C≡O
Covalent Bonds • A molecular compound is made up of discrete units called molecules, which typically consist of two or more nonmetal atoms held together by covalent bonds. A covalent bond results when two atoms share electrons.
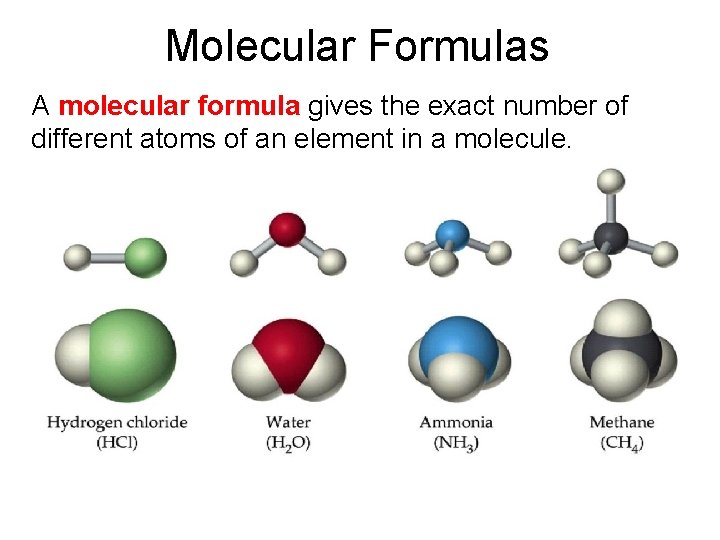
Molecular Formulas A molecular formula gives the exact number of different atoms of an element in a molecule.
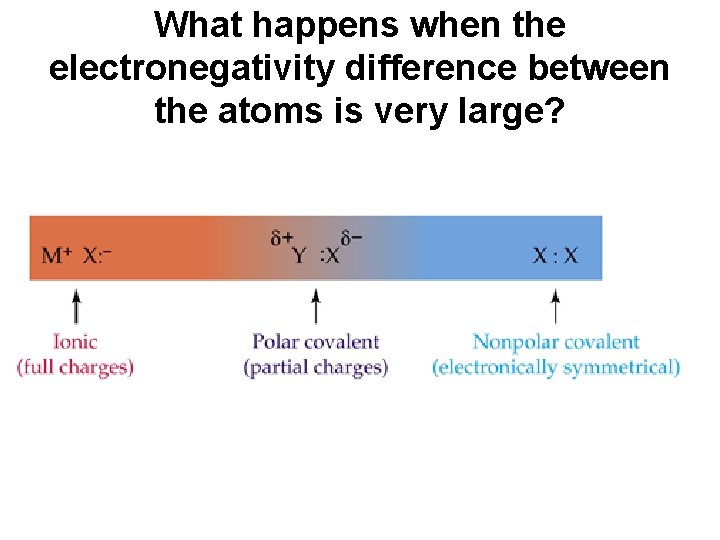
What happens when the electronegativity difference between the atoms is very large?
Definitions • Ionic bonds result from the transfer of one or more electrons from a metal atom to a nonmetal atom. • A cation is a positive ion. • An anion is a negative ion. • Ionic bonds are electrostatic forces of attraction between ions.
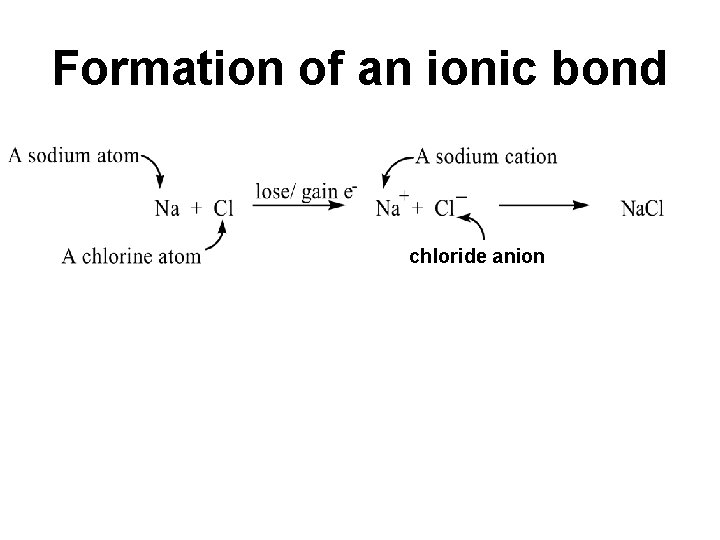
Formation of an ionic bond chloride anion
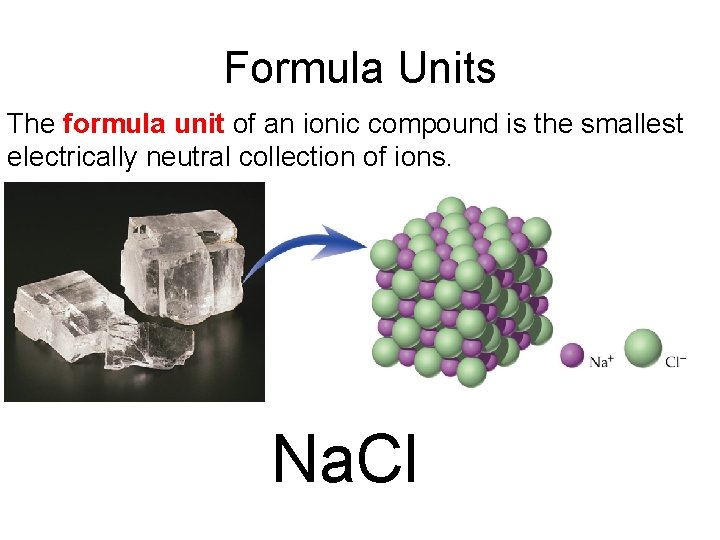
Formula Units The formula unit of an ionic compound is the smallest electrically neutral collection of ions. Na. Cl

Na vs. Na+

So when does a bond change from covalent to ionic? • All bonds have some ionic character, and the difference between ionic and covalent is not distinct but rather a continuum. • The difference in electronegativity is not the only factor in determining whether a bond is designated ionic or covalent.
So when does a bond change from covalent to ionic? • Generally, bonds between nonmetal atoms are covalent and bonds between a metal and a nonmetal are ionic. • Examination of the properties of a compound is really the best way to determine the type of bonds that exist within the compound.

Valence electrons using the periodic table.
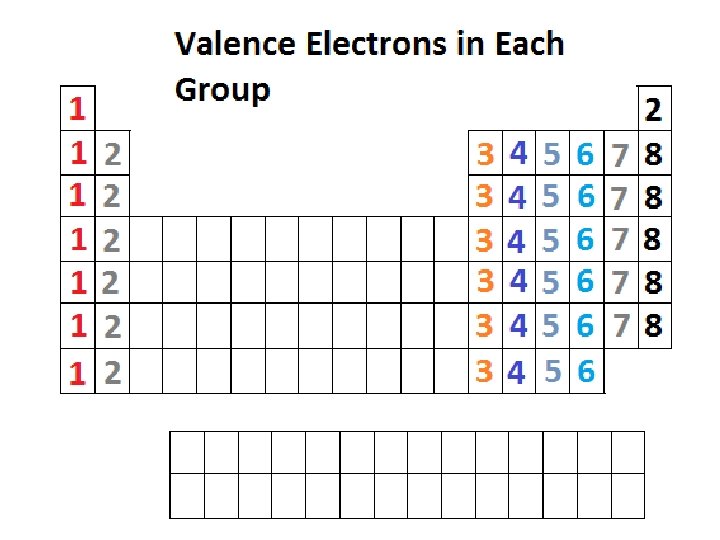

We can use valence electrons to write electron dot (lewis structures) for atoms.
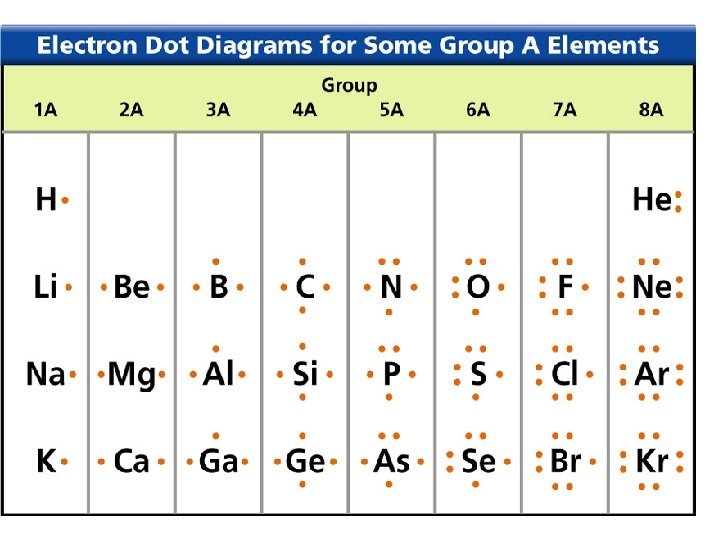

Lewis structures for molecules • See page in your notebook titled “Lewis Structures (AP Chapter 7)” for the steps involved in writing Lewis Structures.
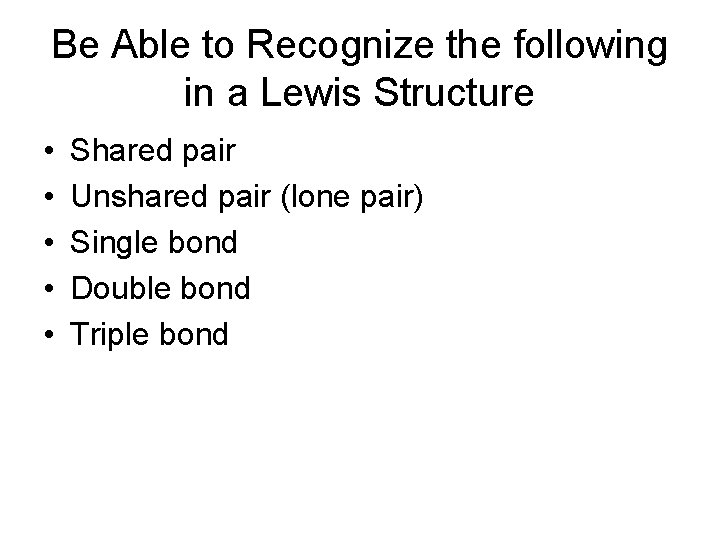
Be Able to Recognize the following in a Lewis Structure • • • Shared pair Unshared pair (lone pair) Single bond Double bond Triple bond
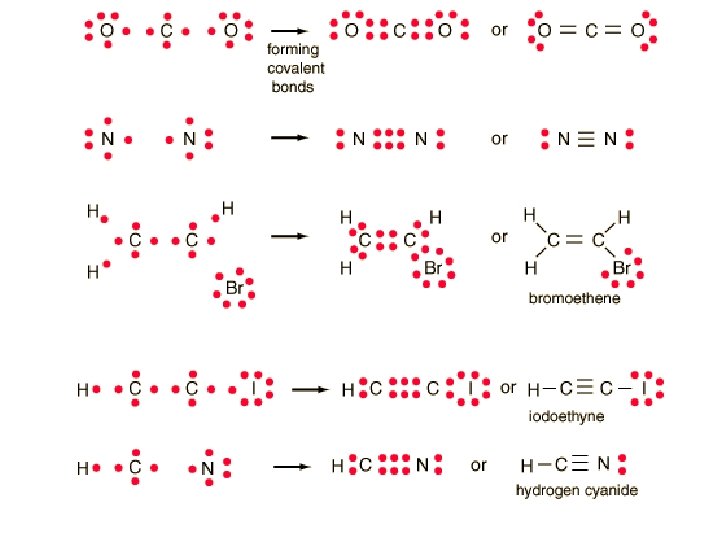
Double and Triple Bonds • Normally occur exclusively between C, N and O atoms. • Can occur rarely with other atoms in the same groups.
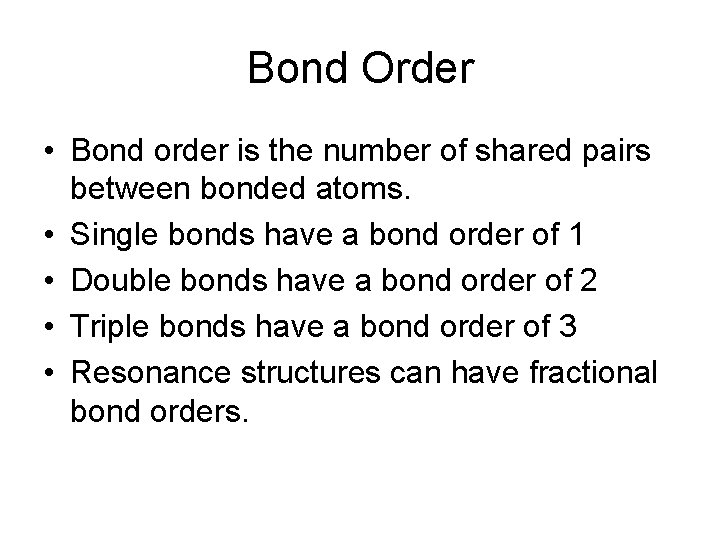
Bond Order • Bond order is the number of shared pairs between bonded atoms. • Single bonds have a bond order of 1 • Double bonds have a bond order of 2 • Triple bonds have a bond order of 3 • Resonance structures can have fractional bond orders.
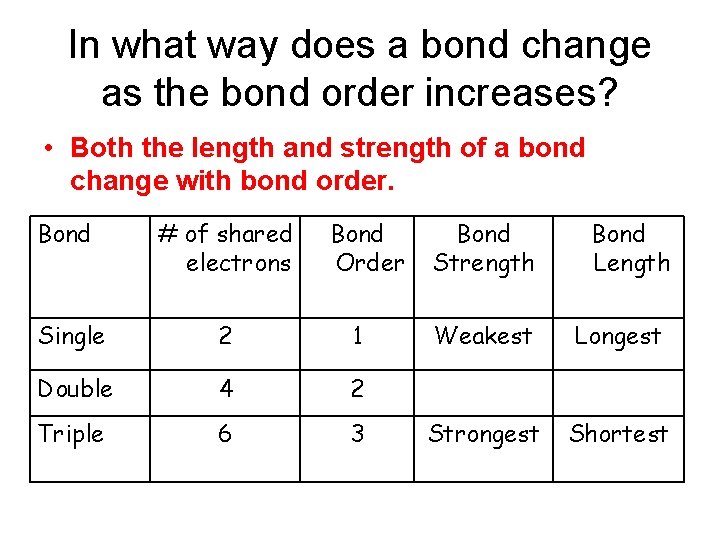
In what way does a bond change as the bond order increases? • Both the length and strength of a bond change with bond order. Bond # of shared electrons Bond Order Single 2 1 Double 4 2 Triple 6 3 Bond Strength Bond Length Weakest Longest Strongest Shortest
Non-Octet Structures • Deficient Octet structures are stable with less than 8 electrons in their valence shell. • Expanded Octet structures are stable with more than 8 electrons in their valence shell.
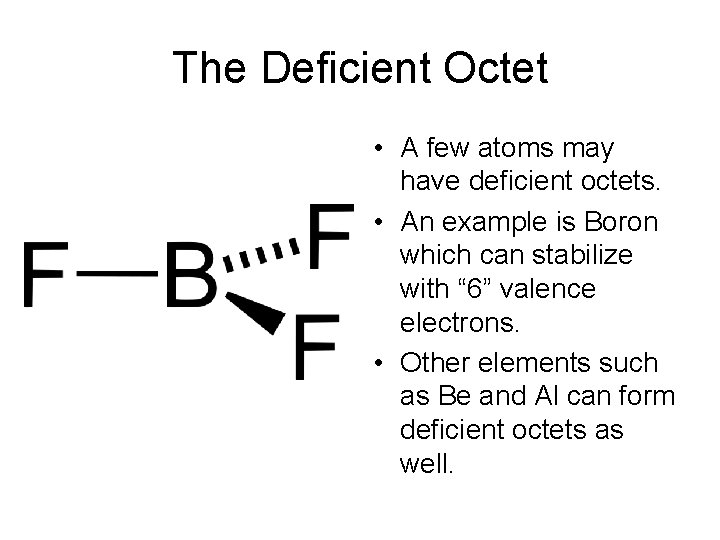
The Deficient Octet • A few atoms may have deficient octets. • An example is Boron which can stabilize with “ 6” valence electrons. • Other elements such as Be and Al can form deficient octets as well.
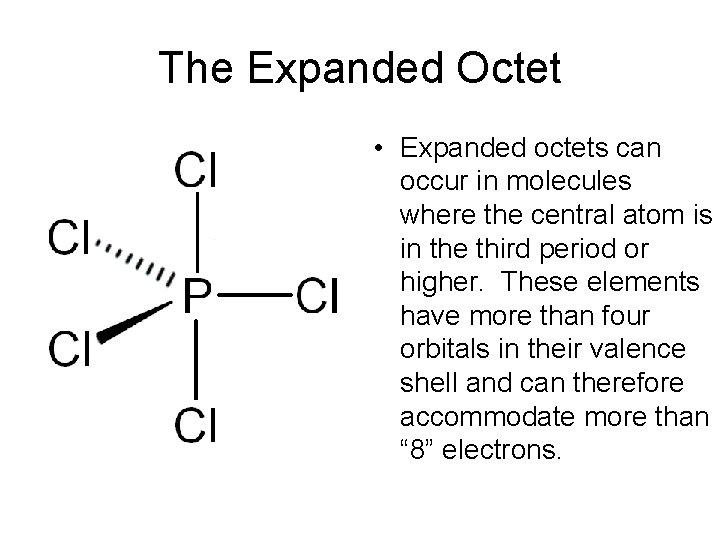
The Expanded Octet • Expanded octets can occur in molecules where the central atom is in the third period or higher. These elements have more than four orbitals in their valence shell and can therefore accommodate more than “ 8” electrons.
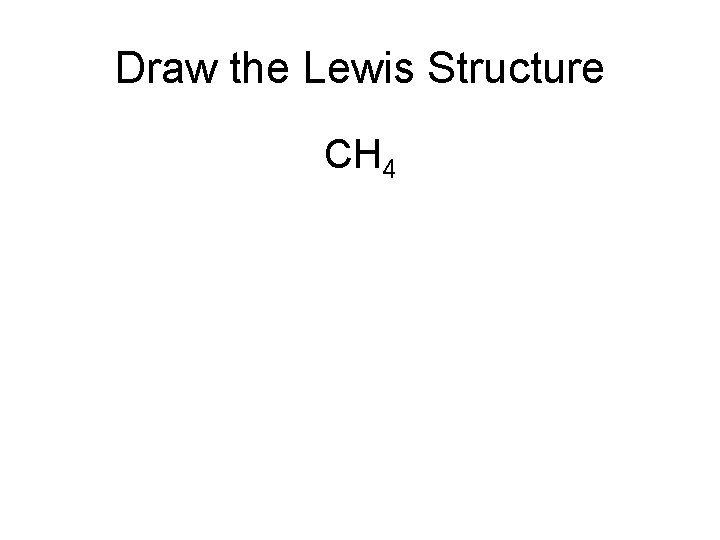
Draw the Lewis Structure CH 4
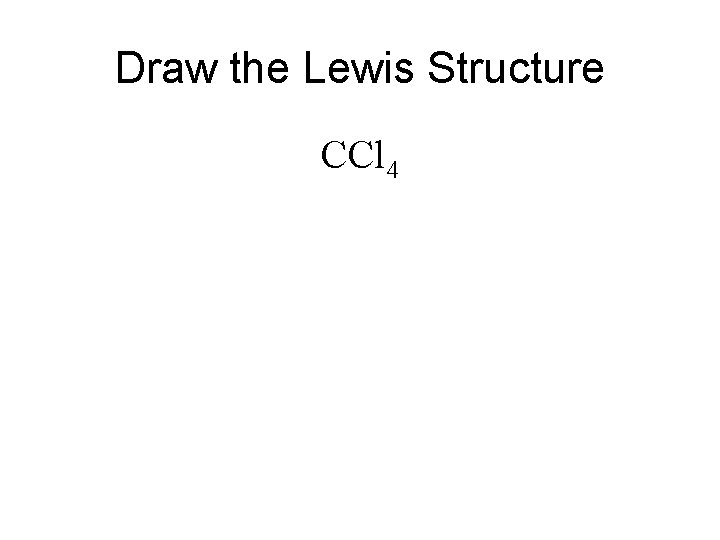
Draw the Lewis Structure CCl 4

Draw the Lewis Structure Cl. NO
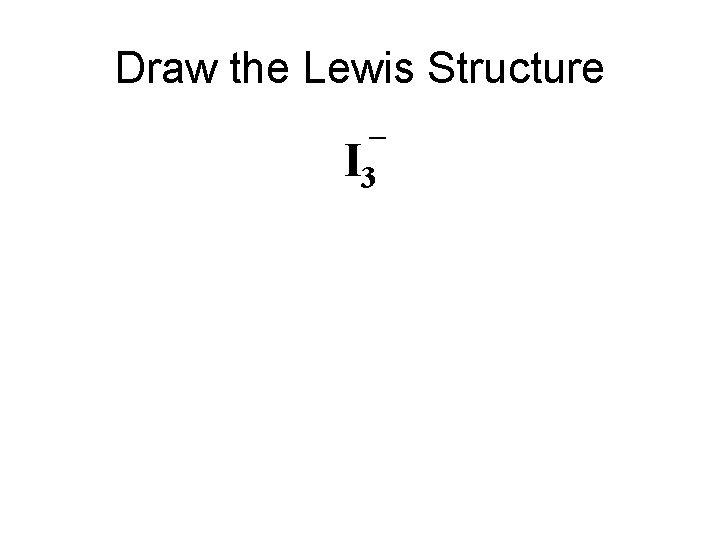
Draw the Lewis Structure I 3

Draw the Lewis Structure SF 4
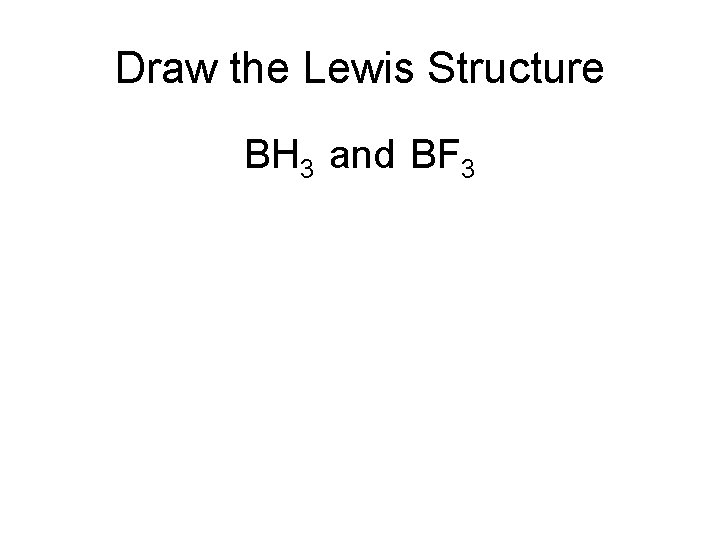
Draw the Lewis Structure BH 3 and BF 3

Draw the Lewis Structure Be. H 2
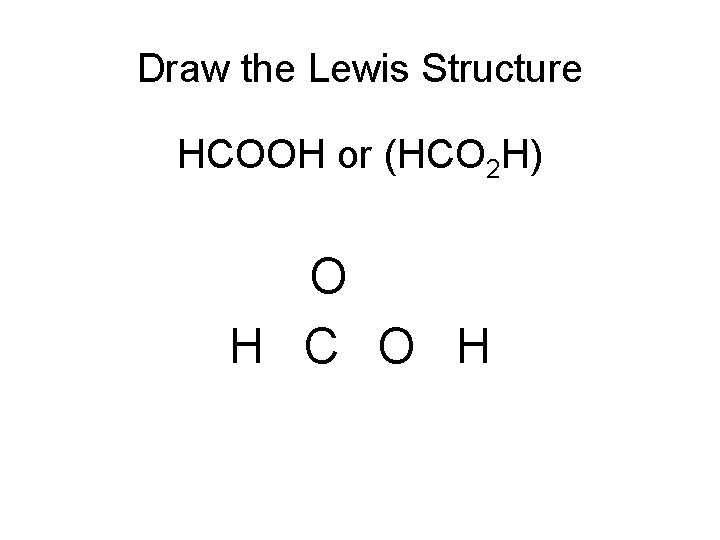
Draw the Lewis Structure HCOOH or (HCO 2 H) O H C O H
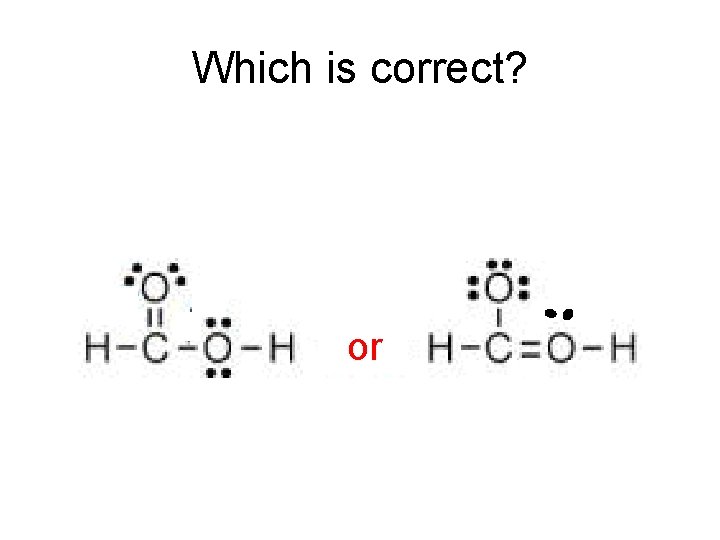
Which is correct? A A or
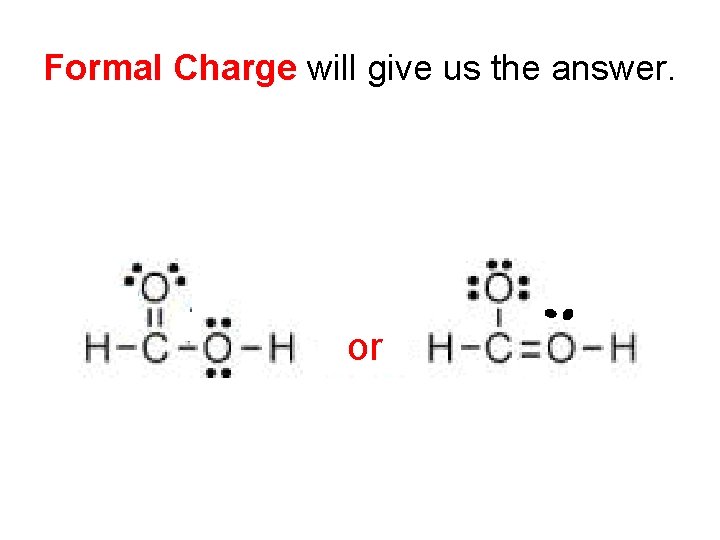
Formal Charge will give us the answer. A A or

Formal Charge • Each atom in a molecule or polyatomic ion has a formal charge. • Formal charge is not the oxidation number (charge on the atom in the molecule). It is simply a calculated value. • Formal charge is helpful for determining which Lewis structure is most likely when two or more different Lewis structures can be drawn for a molecule or ion. • The sum of the formal charges in a molecule = 0 • The sum of the formal charges in an ion = the charge overall charge of the ion.
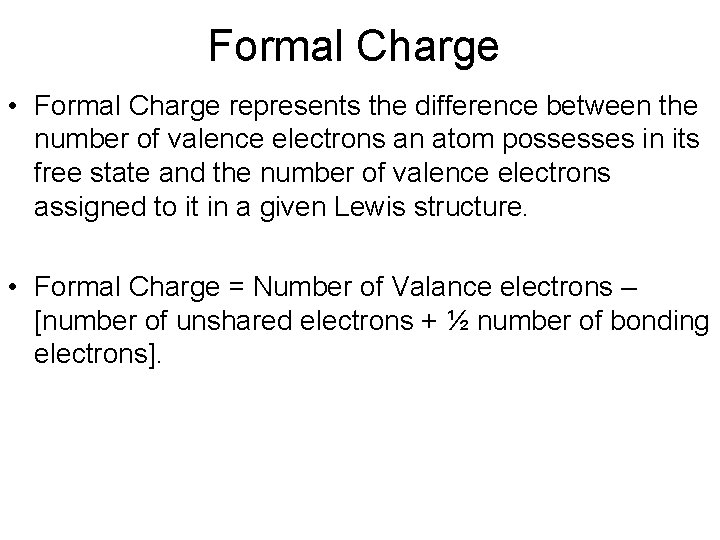
Formal Charge • Formal Charge represents the difference between the number of valence electrons an atom possesses in its free state and the number of valence electrons assigned to it in a given Lewis structure. • Formal Charge = Number of Valance electrons – [number of unshared electrons + ½ number of bonding electrons].
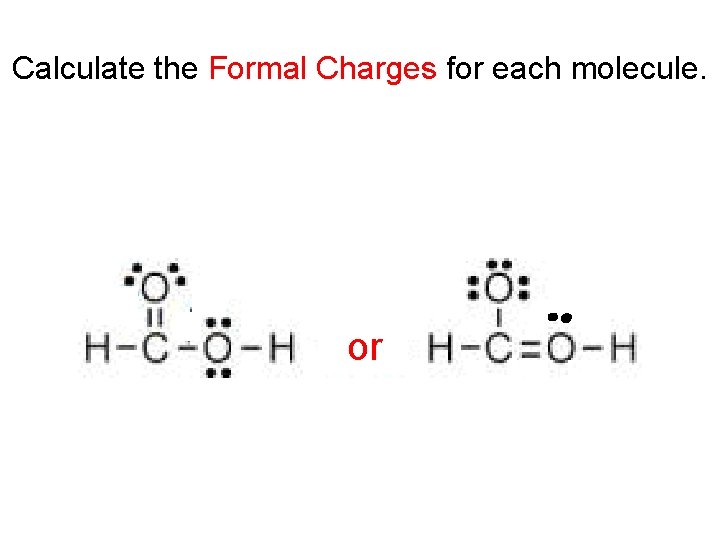
Calculate the Formal Charges for each molecule. A A or

Rules for Formal Charge 1. Structures with the lowest magnitude of formal charges are more stable. 2. More electronegative atoms should have negative formal charges. 3. Adjacent atoms should have opposite formal charges (or zero formal charge).
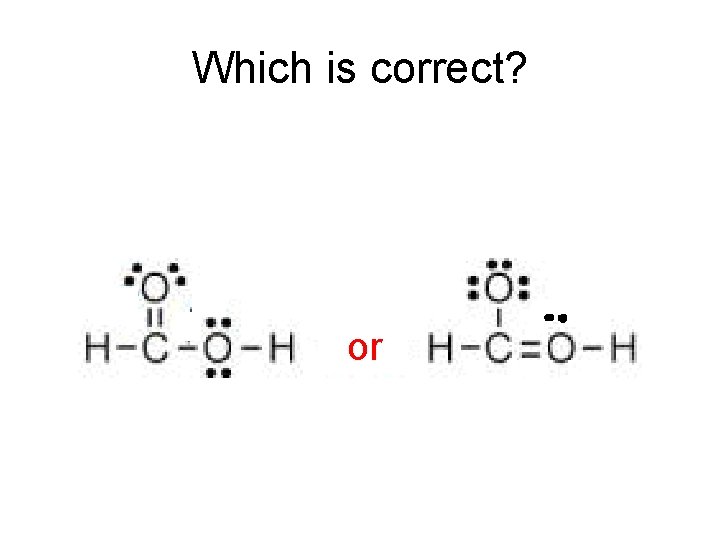
Which is correct? A A or
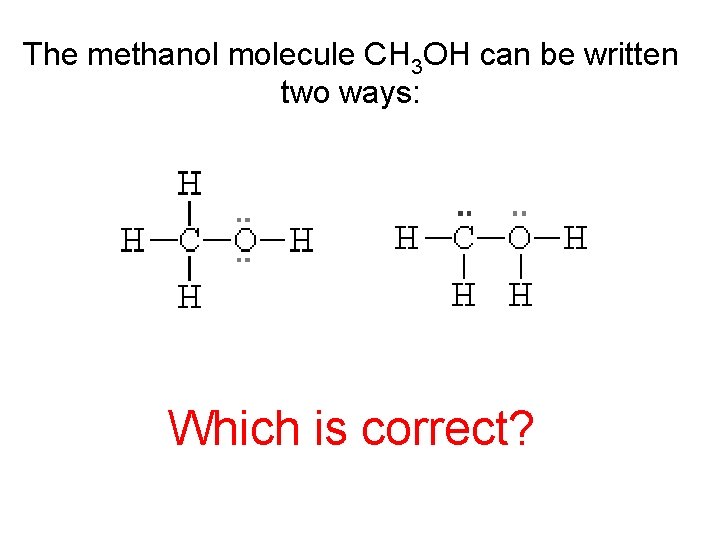
The methanol molecule CH 3 OH can be written two ways: Which is correct?

Use formal charge to explain why BCl 3 is more stable with a deficient octet than with a double bond?
Limitations to Lewis Structures • One limitation when drawing Lewis structures is that on rare occasions an odd number of valence electrons exist (example: NO 2). • We tend to handle this by using the flexibility of the central atom within a molecule.
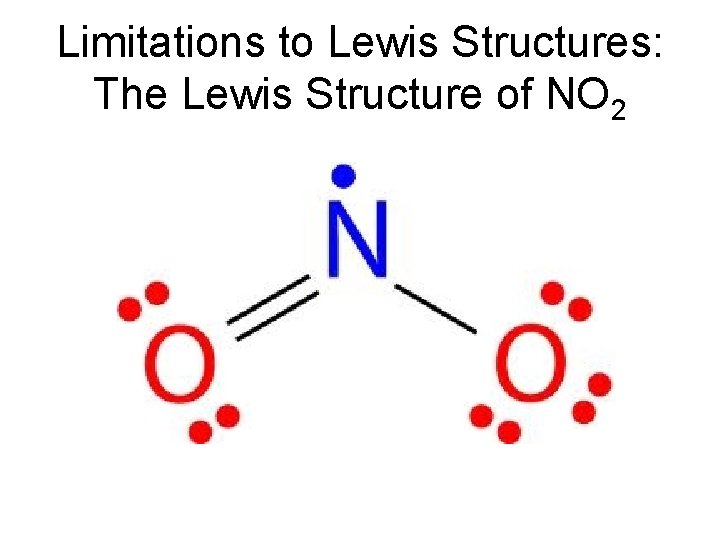
Limitations to Lewis Structures: The Lewis Structure of NO 2
Limitations to Lewis Structures • One of the most common problems that arises when drawing Lewis structures occurs when no single Lewis structure can properly represent the bonding within a molecule. • We use the concept of resonance when this situation arises.
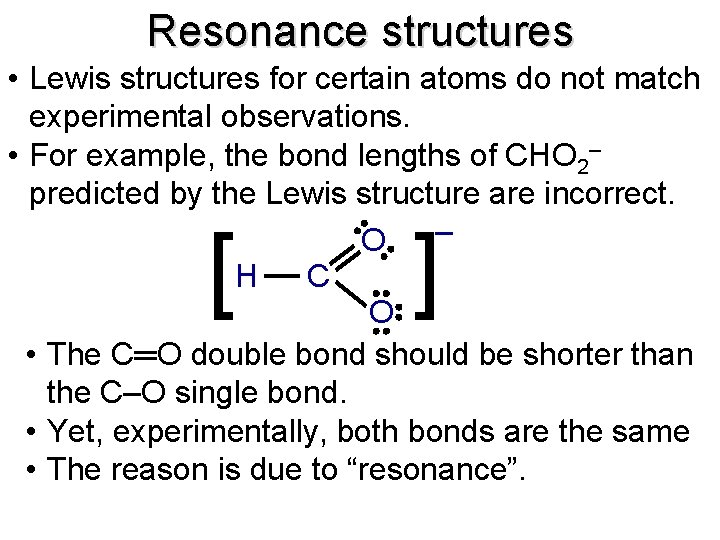
Resonance structures • Lewis structures for certain atoms do not match experimental observations. • For example, the bond lengths of CHO 2– predicted by the Lewis structure are incorrect. – O H C O • The C═O double bond should be shorter than the C‒O single bond. • Yet, experimentally, both bonds are the same • The reason is due to “resonance”. [ ]

Resonance • If two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion, the actual distribution of electrons is an average of that shown by the various Lewis structures.
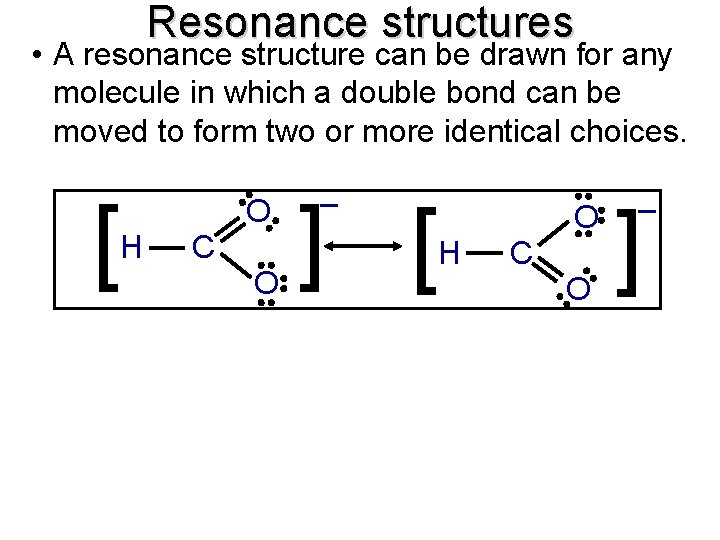
Resonance structures • A resonance structure can be drawn for any molecule in which a double bond can be moved to form two or more identical choices. C O ] [ H C O H – O [ O – ]
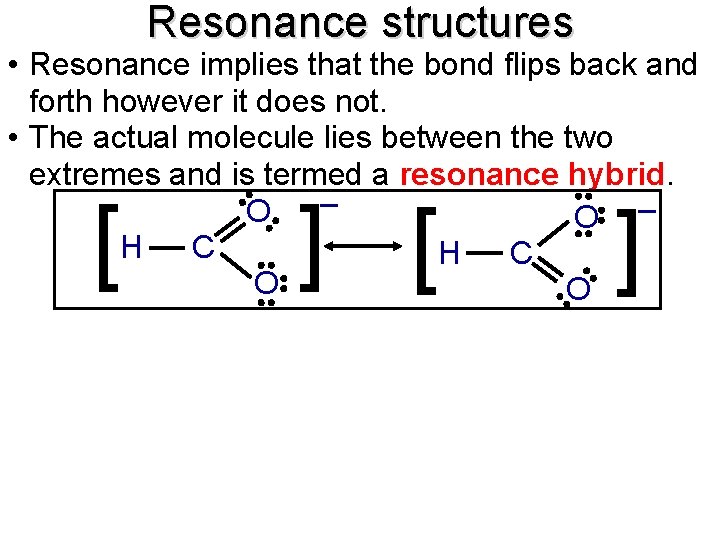
Resonance structures • Resonance implies that the bond flips back and forth however it does not. • The actual molecule lies between the two extremes and is termed a resonance hybrid. – – O H C O O ] [ O [ ]

The Mule Analogy • “A Mule is part donkey and part horse but is never a donkey or a horse. ”
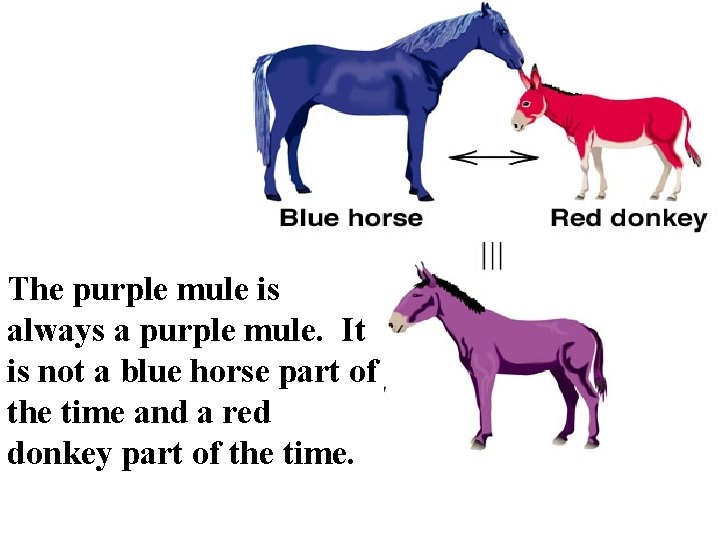
The purple mule is always a purple mule. It is not a blue horse part of the time and a red donkey part of the time.
![Resonance structures C O ] [ H C O H – O [ O Resonance structures C O ] [ H C O H – O [ O](http://slidetodoc.com/presentation_image_h2/c91b256c9d54edc99eeef2eb843dfc30/image-61.jpg)
Resonance structures C O ] [ H C O H – O [ O – ]

Resonance Misconceptions • It should be emphasized that resonance occurs by the movement of bonds and not the movement of atoms.

The forces of attraction in a covalent bond. • The covalent bond creates a region of high electron density between the atoms. • The positively charged nucleus of each bonded atom attracts toward this region of negative charge created by the bond between them. • The bonded atoms therefore attract toward each other and stick together.
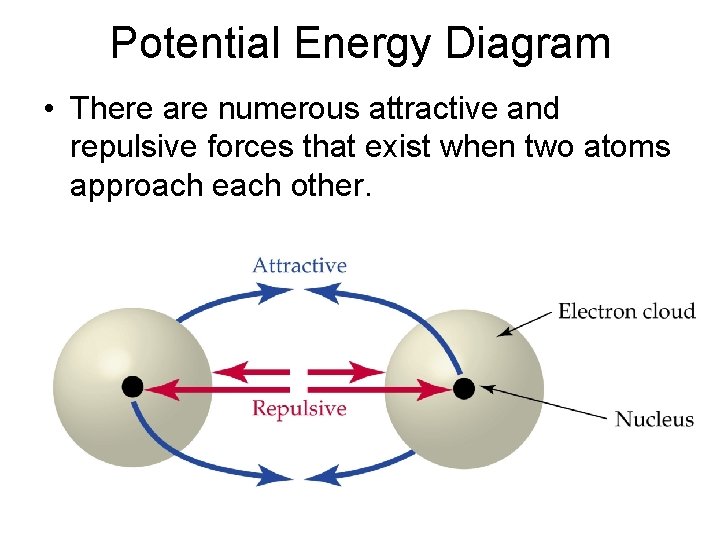
Potential Energy Diagram • There are numerous attractive and repulsive forces that exist when two atoms approach each other.
Potential Energy Diagram • Consider the formation of a H 2 molecule. • As the atoms approach one another, the electrons concentrate between the nuclei, and attraction occurs. • At very short distances, the electrons would be ‘squeezed out’ from between the nuclei and the two positively charged nuclei would repel each other.

Potential Energy Diagram • There is a distance at which the energy between the atoms is the lowest. • This is point of greatest stability for the molecule and it determines the bond length between the atoms where the attractive and repulsive forces are balanced. • It can also determine whether a bond forms or not.
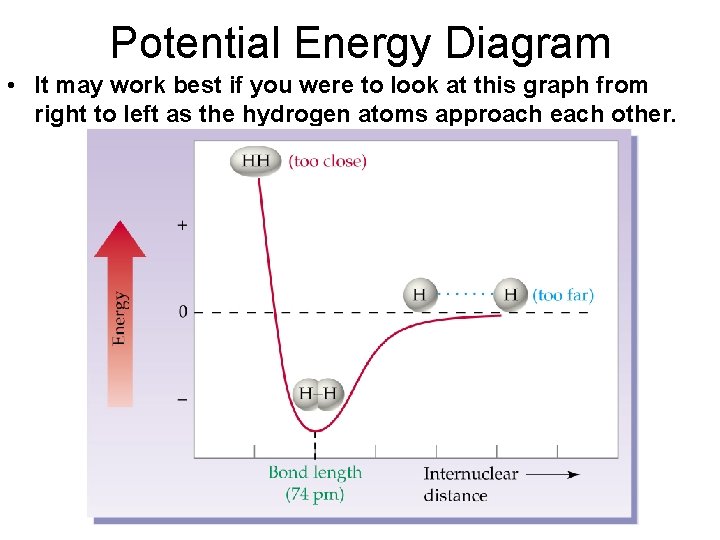
Potential Energy Diagram • It may work best if you were to look at this graph from right to left as the hydrogen atoms approach each other.
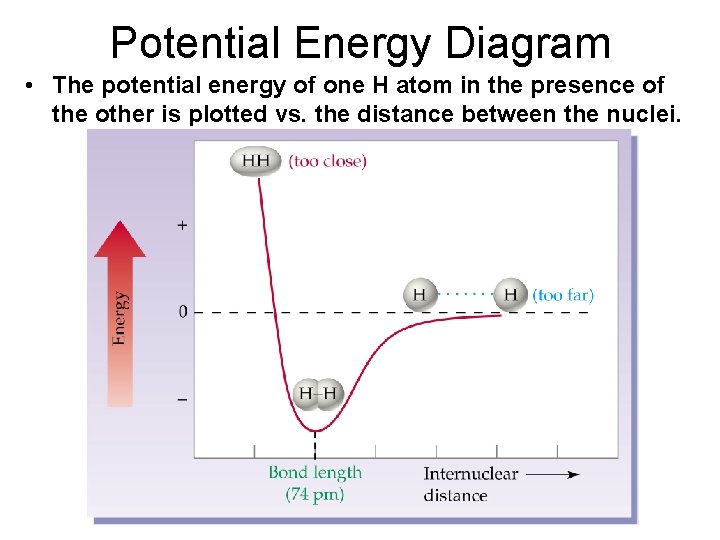
Potential Energy Diagram • The potential energy of one H atom in the presence of the other is plotted vs. the distance between the nuclei.
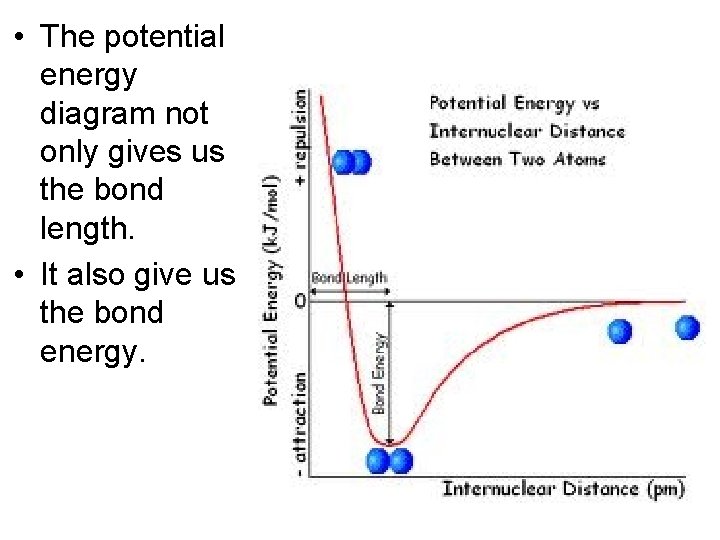
• The potential energy diagram not only gives us the bond length. • It also give us the bond energy.
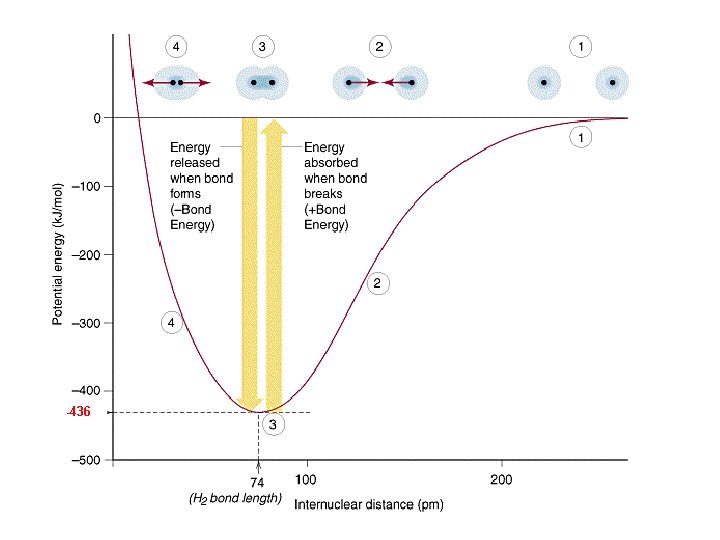
-436
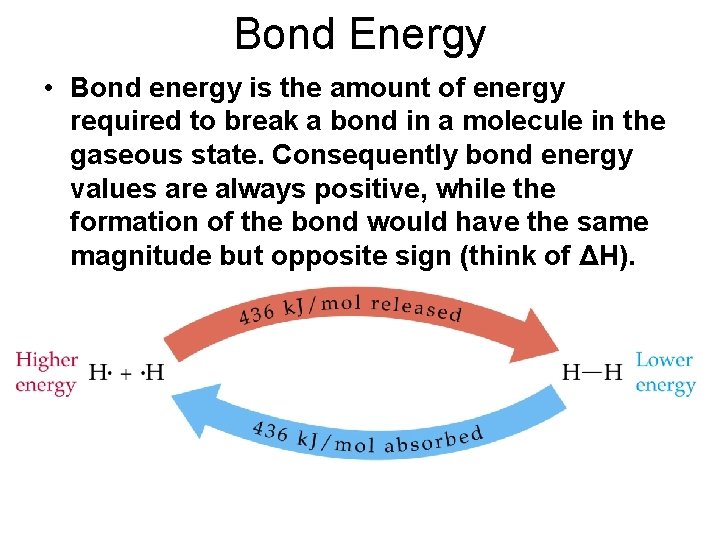
Bond Energy • Bond energy is the amount of energy required to break a bond in a molecule in the gaseous state. Consequently bond energy values are always positive, while the formation of the bond would have the same magnitude but opposite sign (think of ΔH).

Bond Energy • Bond energies can be used to determine the ΔH of a reaction.
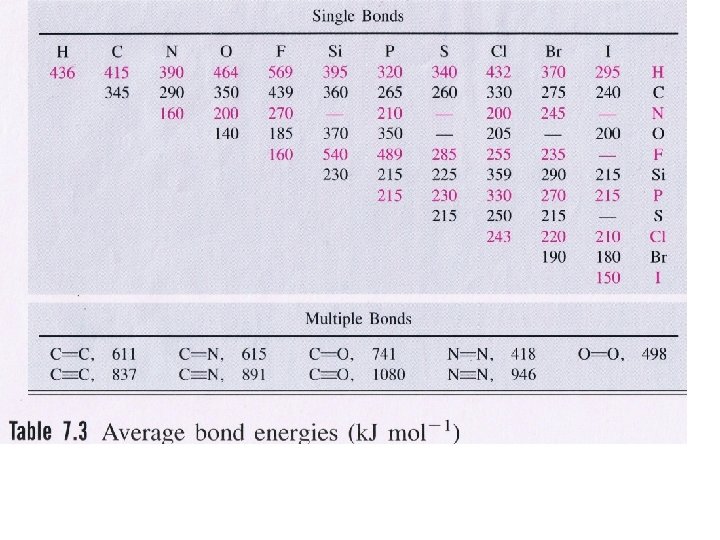
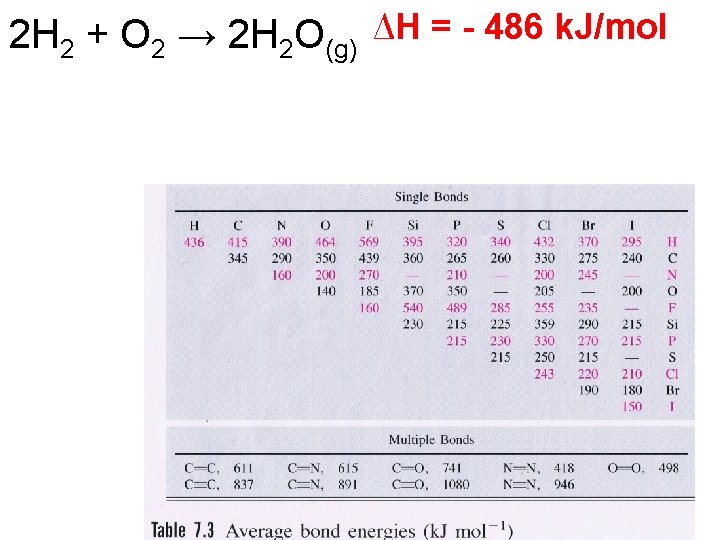
2 H 2 + O 2 → 2 H 2 O(g) ∆H = - 486 k. J/mol
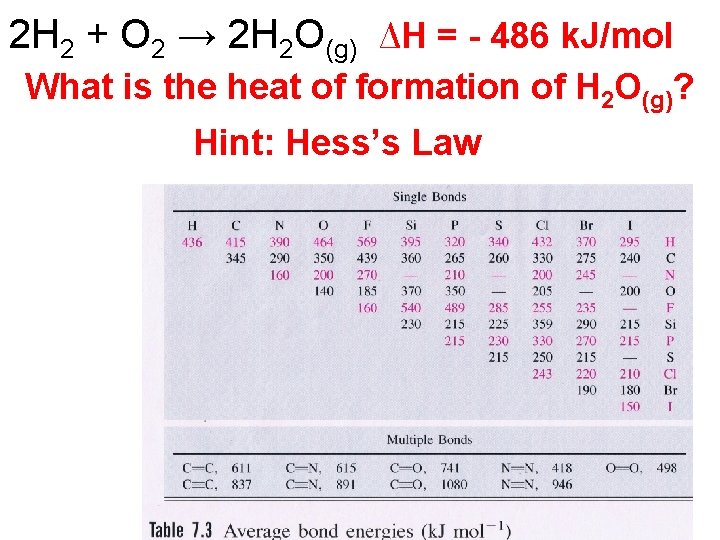
2 H 2 + O 2 → 2 H 2 O(g) ∆H = - 486 k. J/mol What is the heat of formation of H 2 O(g)? Hint: Hess’s Law
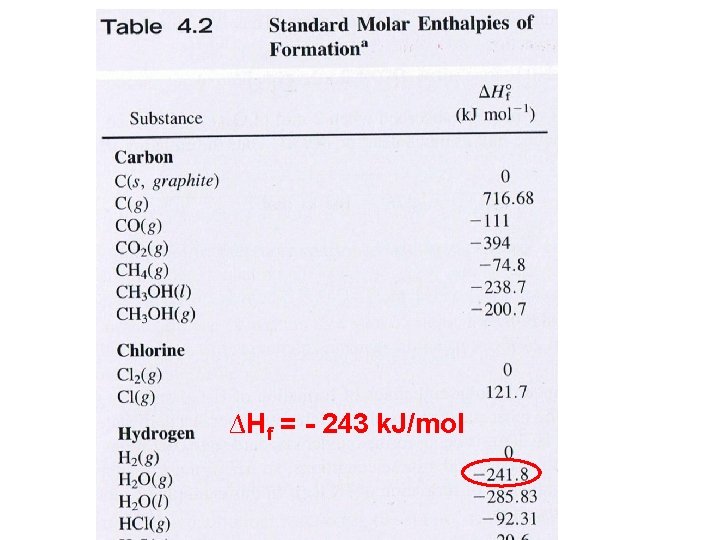
∆Hf = - 243 k. J/mol
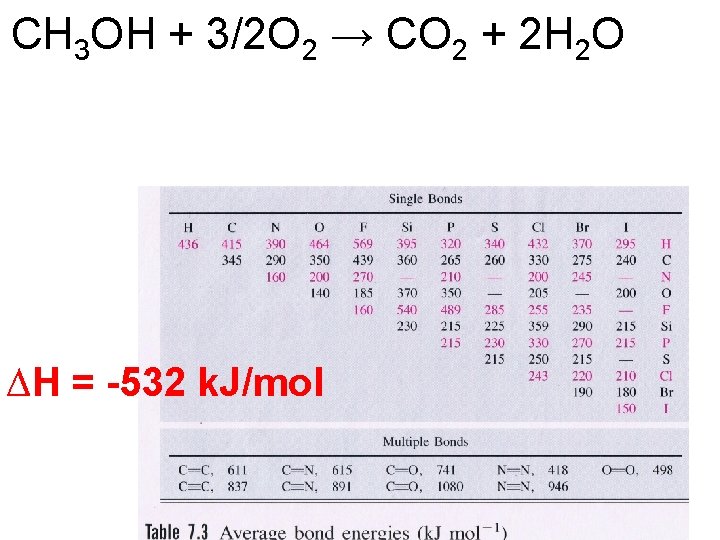
CH 3 OH + 3/2 O 2 → CO 2 + 2 H 2 O ∆H = -532 k. J/mol
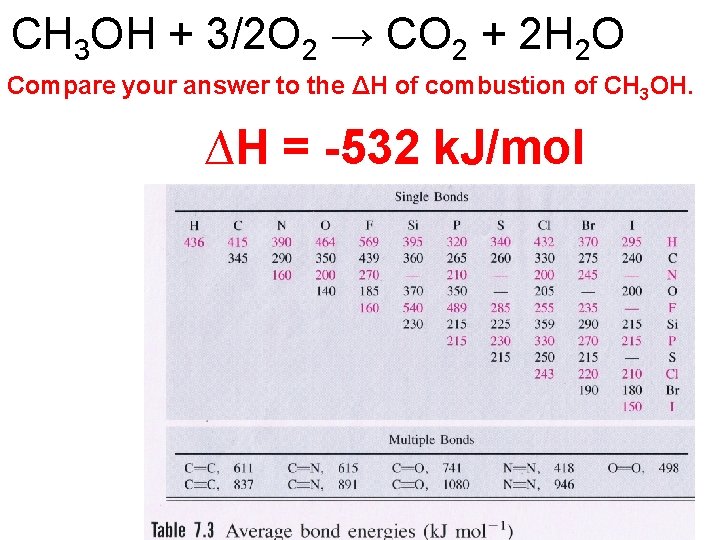
CH 3 OH + 3/2 O 2 → CO 2 + 2 H 2 O Compare your answer to the ΔH of combustion of CH 3 OH. ∆H = -532 k. J/mol

What is Bond Energy?
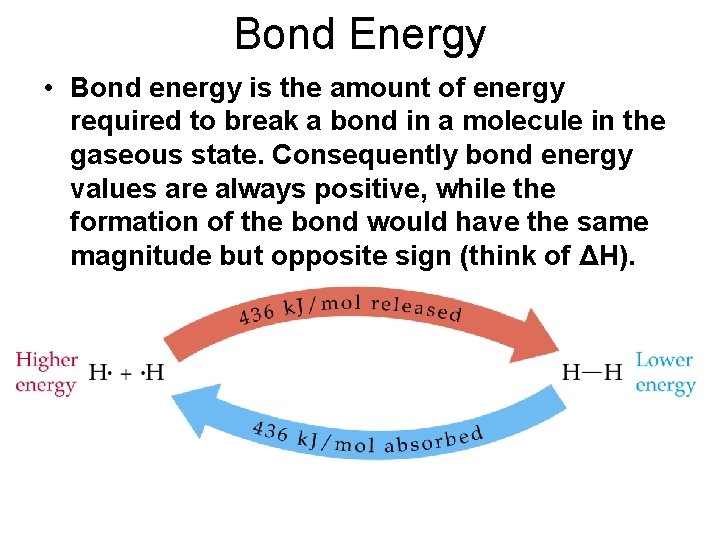
Bond Energy • Bond energy is the amount of energy required to break a bond in a molecule in the gaseous state. Consequently bond energy values are always positive, while the formation of the bond would have the same magnitude but opposite sign (think of ΔH).
Metallic Bonding • When metal atoms combine together the valence electrons become delocalized and are not associated with any particular atom. • Metallic bonding can be visualized as an array of positive metal ions with delocalized electrons moving in the spaces between the ions.
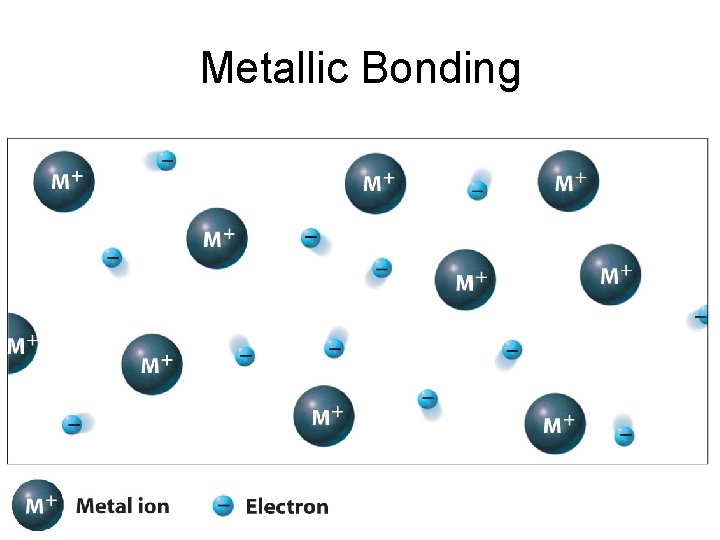
Metallic Bonding

Metallic Bonding • The positively charged metal ions are arranged in a regular fashion in a metallic lattice. • Loosely held valence electrons, surround each of these ions. • Being loosely held to the metal ions, the valence electrons enjoy complete freedom in the metallic lattice and are regarded as mobile electrons (delocalized).

Metallic Bonding • A metal can be regarded as a sea of electrons (common pool of valence electrons) in which there is a three dimensional ordered arrangement of positively charged metal ions, surrounded throughout by the mobile valence electrons.
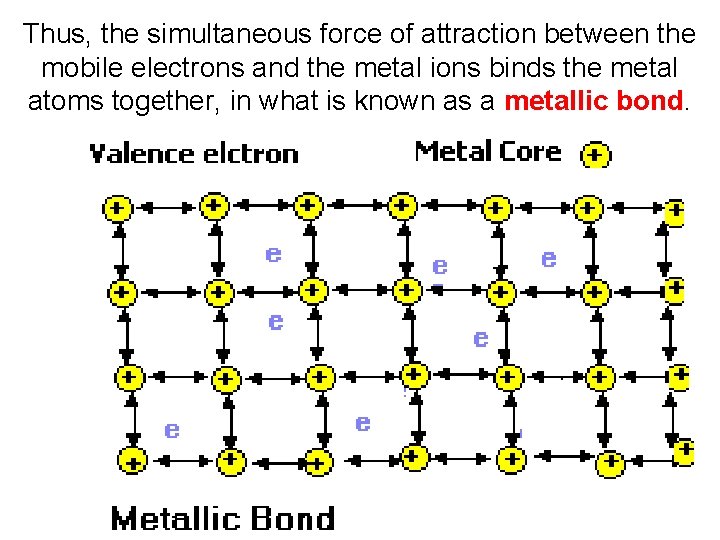
Thus, the simultaneous force of attraction between the mobile electrons and the metal ions binds the metal atoms together, in what is known as a metallic bond.

Properties of Metals/Metallic Bonding • • Metallic luster When light falls on the surface of the metal, the delocalized electrons absorb photons of light and become excited. In many metals most colors of the visible spectrum are absorbed and released (exceptions would include gold and copper) by the delocalized electrons. Therefore these oscillating electrons readily return all the visible light that hits them. Light appears to be reflected from the metal surface and the surface acquires a shining appearance, which is known as metallic luster.
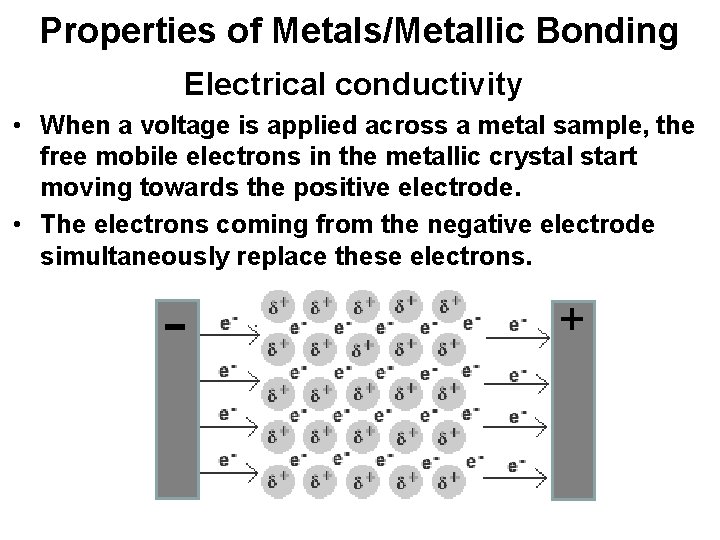
Properties of Metals/Metallic Bonding Electrical conductivity • When a voltage is applied across a metal sample, the free mobile electrons in the metallic crystal start moving towards the positive electrode. • The electrons coming from the negative electrode simultaneously replace these electrons. - +
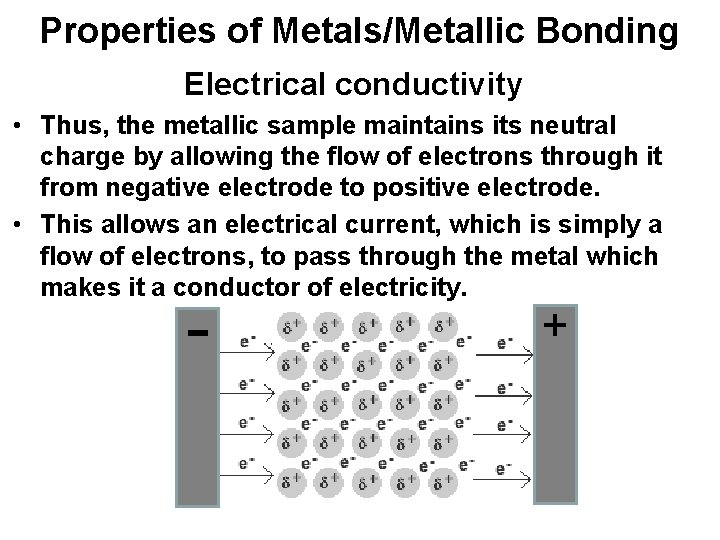
Properties of Metals/Metallic Bonding Electrical conductivity • Thus, the metallic sample maintains its neutral charge by allowing the flow of electrons through it from negative electrode to positive electrode. • This allows an electrical current, which is simply a flow of electrons, to pass through the metal which makes it a conductor of electricity. - +
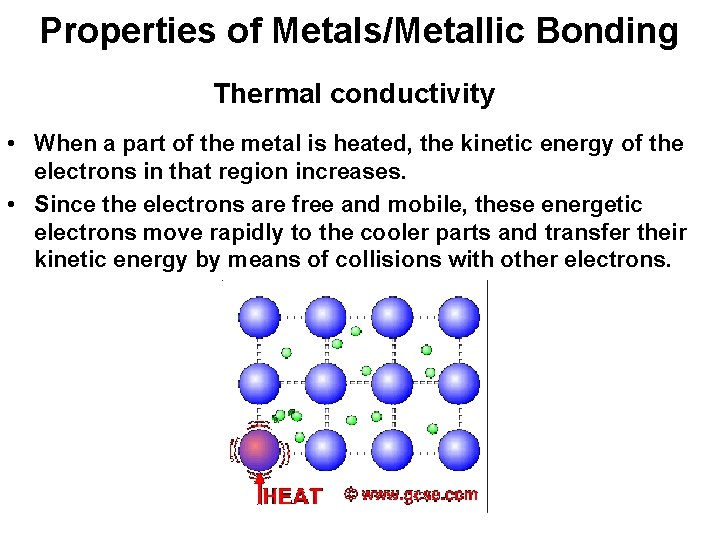
Properties of Metals/Metallic Bonding Thermal conductivity • When a part of the metal is heated, the kinetic energy of the electrons in that region increases. • Since the electrons are free and mobile, these energetic electrons move rapidly to the cooler parts and transfer their kinetic energy by means of collisions with other electrons.
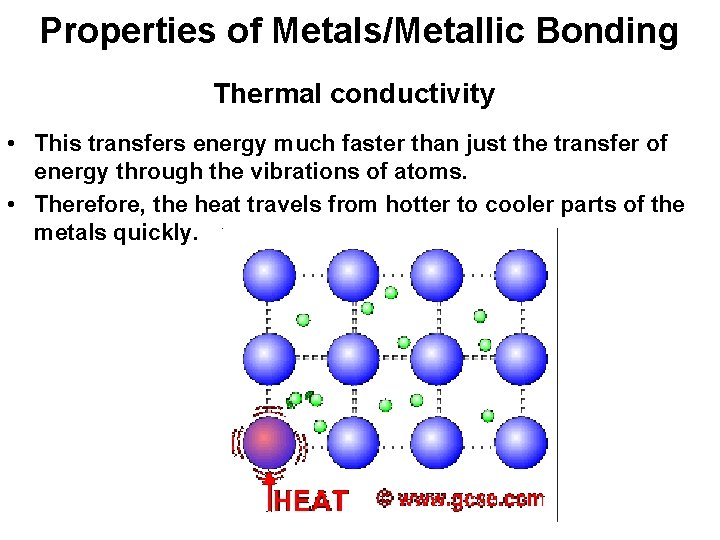
Properties of Metals/Metallic Bonding Thermal conductivity • This transfers energy much faster than just the transfer of energy through the vibrations of atoms. • Therefore, the heat travels from hotter to cooler parts of the metals quickly.
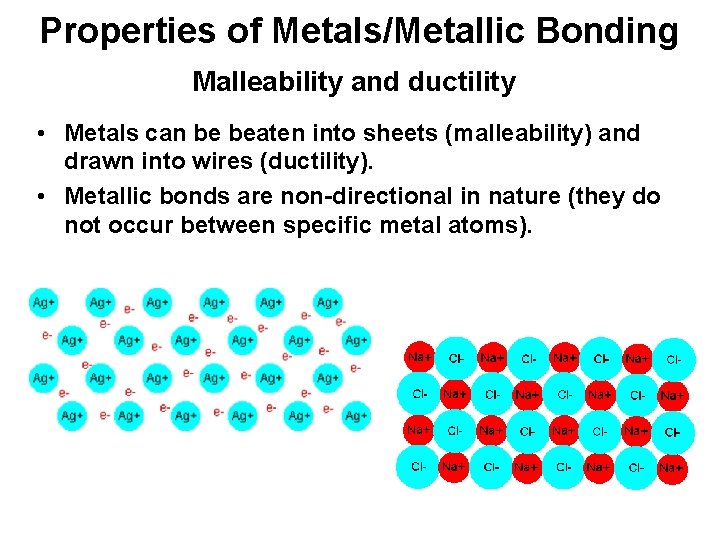
Properties of Metals/Metallic Bonding Malleability and ductility • Metals can be beaten into sheets (malleability) and drawn into wires (ductility). • Metallic bonds are non-directional in nature (they do not occur between specific metal atoms).
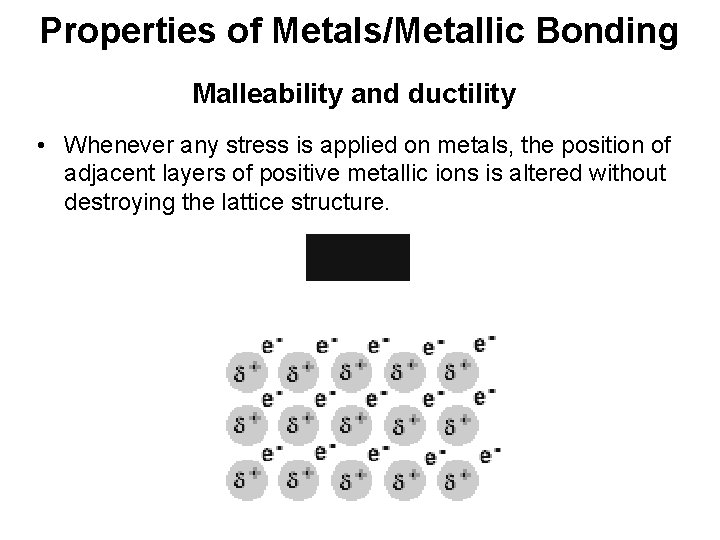
Properties of Metals/Metallic Bonding Malleability and ductility • Whenever any stress is applied on metals, the position of adjacent layers of positive metallic ions is altered without destroying the lattice structure.
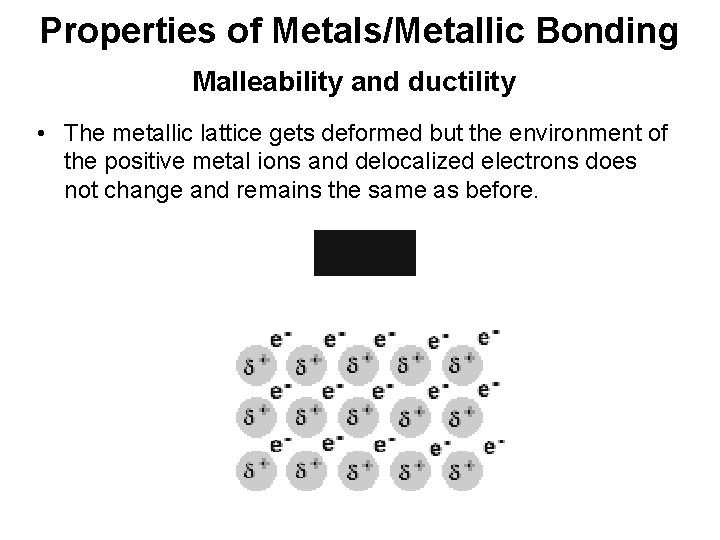
Properties of Metals/Metallic Bonding Malleability and ductility • The metallic lattice gets deformed but the environment of the positive metal ions and delocalized electrons does not change and remains the same as before.
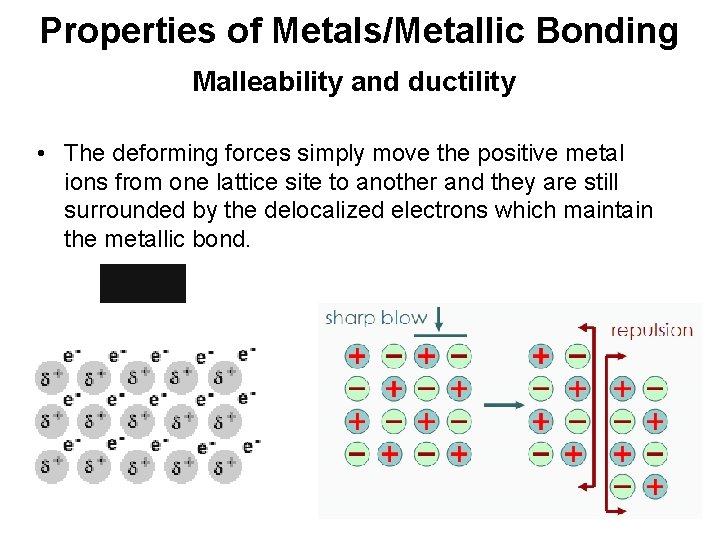
Properties of Metals/Metallic Bonding Malleability and ductility • The deforming forces simply move the positive metal ions from one lattice site to another and they are still surrounded by the delocalized electrons which maintain the metallic bond.

Properties of Metals/Metallic Bonding Hardness of metals • The hardness of metals is due to the strength of the metallic bond. • In general, the strength of a metallic bond depends upon: – The greater the number of valence electrons for delocalization the stronger is the metallic bond. – The greater the charge of the metal ion the stronger the metallic bond. – For metal ions of like charge the smaller the size of the metal ion, the greater is the attraction for the delocalized electrons. Consequently, the stronger the metallic bond.

Properties of Metals/Metallic Bonding Hardness of metals • For example, alkali metals have only one valence electron and are amongst the largest metal ions, which makes the metallic bonds weak. • Consequently these metals are soft metals.

Alloys • An alloy is a solid solution of two or more metals. • Alloys are normally made to enhance the properties of a metal. • Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy.

Alloys • Interstitial alloys form between different size atoms, where the smaller atoms feel the interstitial spaces between the larger atoms. • This causes a large increase in the mass of a sample without much change in volume. • What does this do the density of the resulting alloy?
Alloys • An example of an interstitial alloy is steel. • Steel is an alloy of iron and other elements, including carbon.

Alloys • The smaller carbon atoms fill the spaces between the larger iron atoms.

Alloys • This makes the lattice structure of steel more rigid increasing its strength and lowering its malleability. This makes steel an excellent building material.

Alloys • Substitutional alloys form between atoms of similar size, where one atom substitutes for another within the lattice.

Alloys • Substitutional alloys include bronze and brass, in which some of the copper atoms are substituted with either tin or zinc atoms.
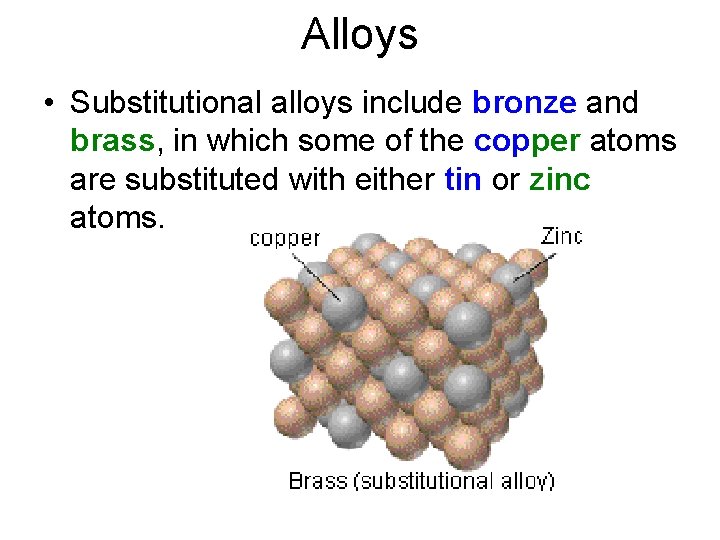
Alloys • Substitutional alloys include bronze and brass, in which some of the copper atoms are substituted with either tin or zinc atoms.

Alloys • The density of substitutional alloys lie between the substances within the alloy and the alloy remains malleable and ductile. • Advantages of substitutional alloys are normally that they retain some of the beneficial properties of both substances. • All alloys tend to maintain their “sea of mobile electrons” and therefore their conductivity.

Alloys • Stainless steel is an example of a combination of interstitial and substitutional alloys, because the carbon atoms fit into the interstices, but some of the iron atoms are replaced with nickel and chromium atoms. • This actually alters the chemistry of the surface of the metal allowing for the formation of a chemically inert oxide layer which prevents rusting.

Lab: Reaction Potential. • In this lab you will learn how to use a standard reduction potentials table to predict the products of single replacement reactions. • A piece of copper is dropped into a solution of silver nitrate. • Write the net ionic equation for the reaction that occurs.
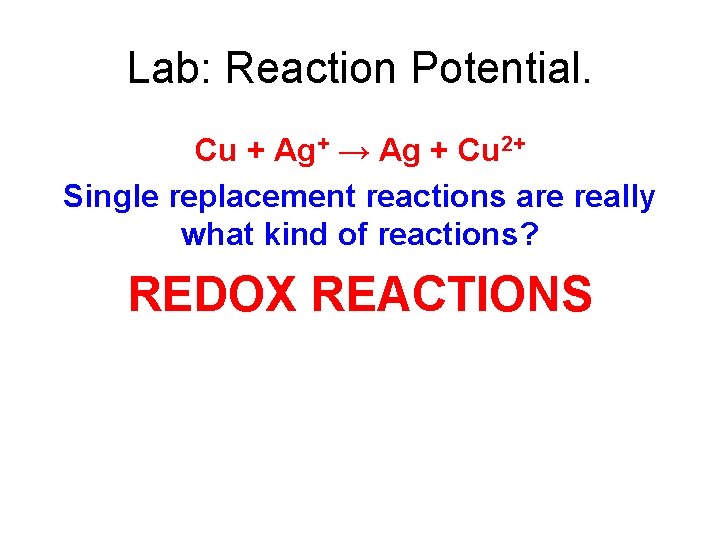
Lab: Reaction Potential. Cu + Ag+ → Ag + Cu 2+ Single replacement reactions are really what kind of reactions? REDOX REACTIONS

LEO the lion says GER Lose Electrons Oxidation Gain Electrons Reduction

Oxidation – Reduction (Redox) • Reduction is the decrease in oxidation number caused by the gain of electrons. • Oxidation is the increase in oxidation number caused by the loss of electrons.
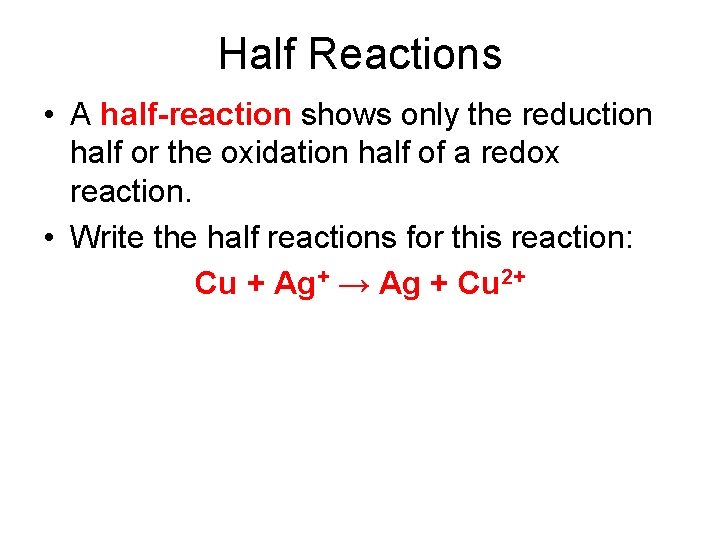
Half Reactions • A half-reaction shows only the reduction half or the oxidation half of a redox reaction. • Write the half reactions for this reaction: Cu + Ag+ → Ag + Cu 2+
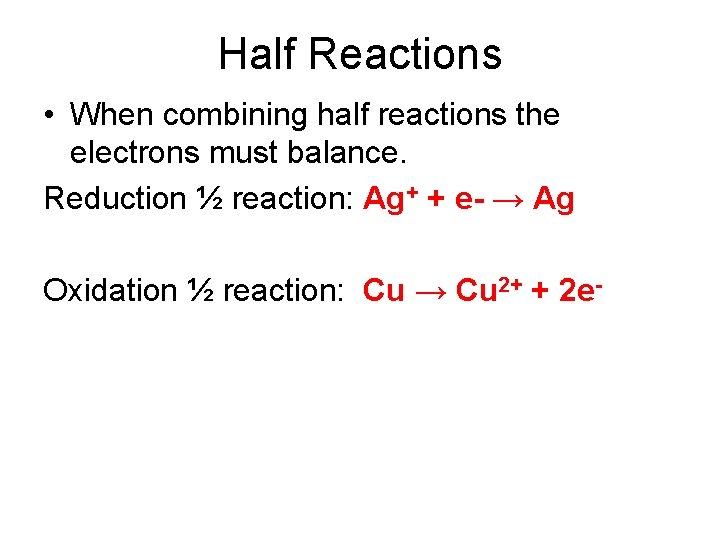
Half Reactions • When combining half reactions the electrons must balance. Reduction ½ reaction: Ag+ + e- → Ag Oxidation ½ reaction: Cu → Cu 2+ + 2 e-
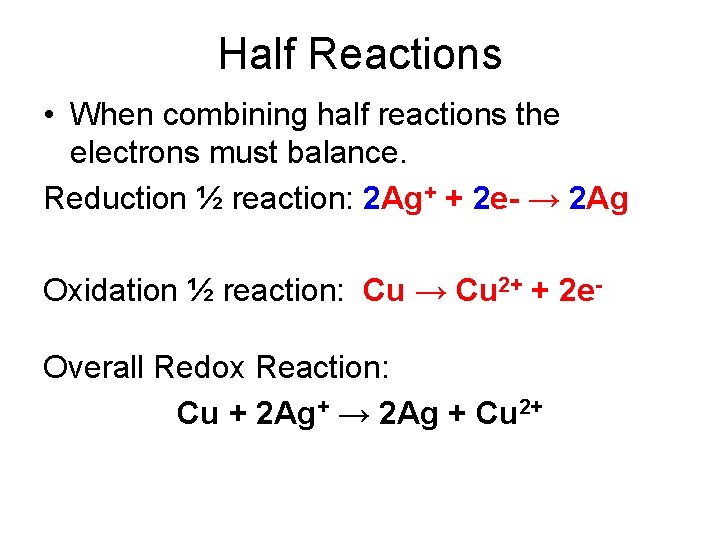
Half Reactions • When combining half reactions the electrons must balance. Reduction ½ reaction: 2 Ag+ + 2 e- → 2 Ag Oxidation ½ reaction: Cu → Cu 2+ + 2 e. Overall Redox Reaction: Cu + 2 Ag+ → 2 Ag + Cu 2+
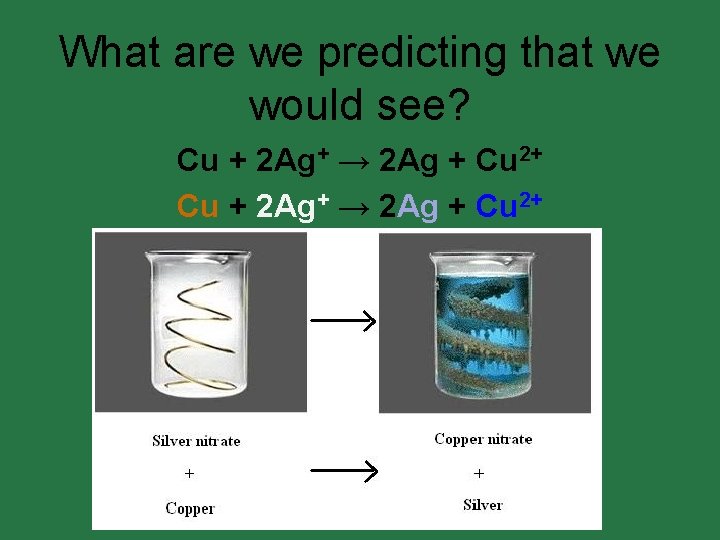
What are we predicting that we would see? Cu + 2 Ag+ → 2 Ag + Cu 2+
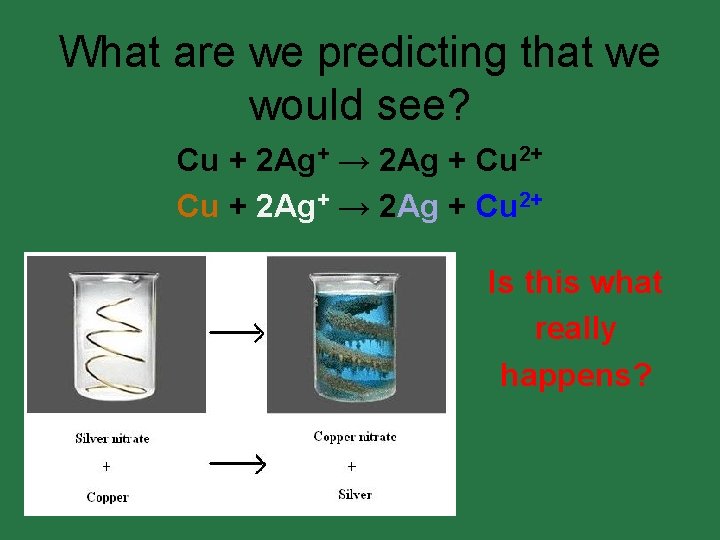
What are we predicting that we would see? Cu + 2 Ag+ → 2 Ag + Cu 2+ • • • Is this what really happens?
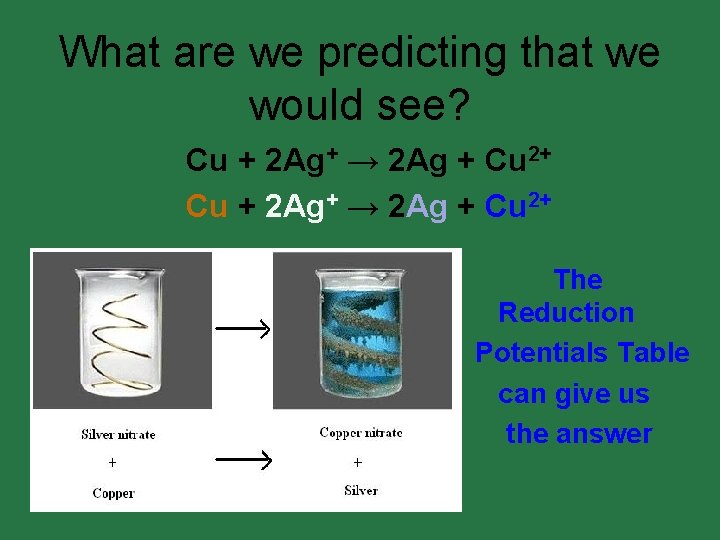
What are we predicting that we would see? Cu + 2 Ag+ → 2 Ag + Cu 2+ The Reduction Potentials Table can give us the answer
The Standard Reduction Potentials Table • In order for a redox reaction to occur it must have a positive reaction (cell) potential. • A negative reaction (cell) potential indicates that the reaction will not occur.


A piece of gold (Au) is dropped in a solution of Pb(NO 3)2. Does the reaction occur? If so write the balanced redox reaction (net ionic form).
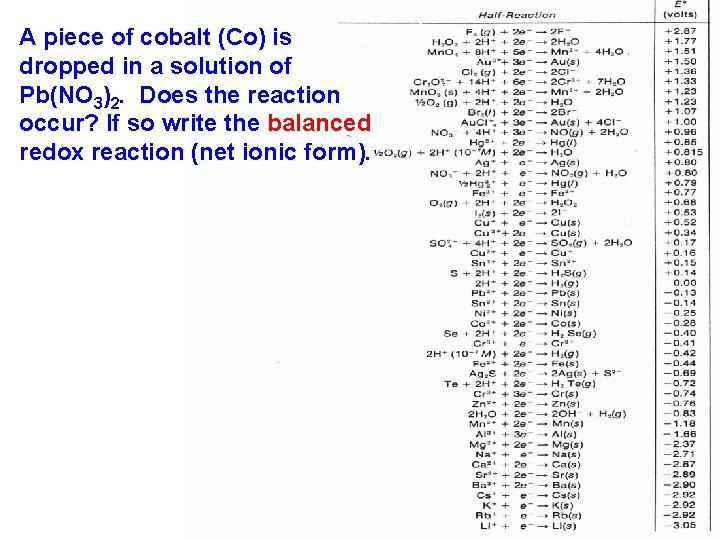
A piece of cobalt (Co) is dropped in a solution of Pb(NO 3)2. Does the reaction occur? If so write the balanced redox reaction (net ionic form).

Which metal is more reactive • Metals tend to oxidize (lose electrons) when they react (LEO). • The previous problem shows us that cobalt is a more reactive (active) metal than lead. • This is evident since the cobalt is able to replace the lead. • An activity series is a list of substances ranked in order of relative reactivity.

• Cobalt is a more reactive metal than lead. • In other words it is easier for cobalt to oxidize than it is for lead to oxidize.
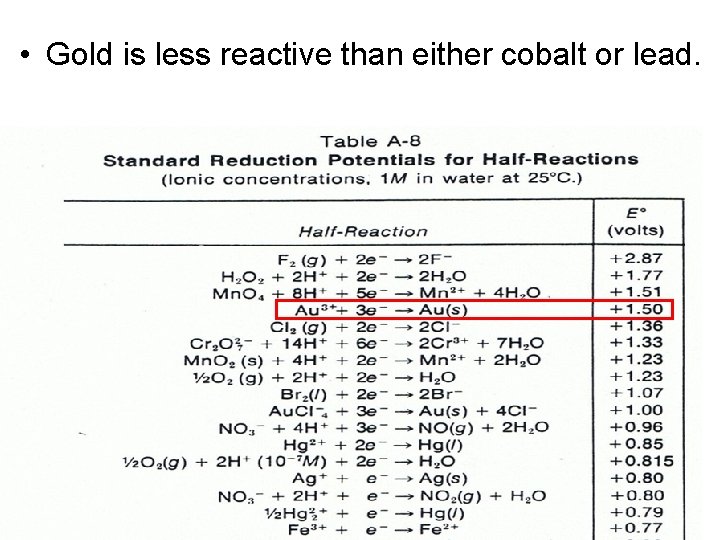
• Gold is less reactive than either cobalt or lead.

An Activity Series • An activity series is a list of substances ranked in order of relative reactivity. • In an activity series each element is listed above the element(s) that it is more reactive than. • An element on the list replaces from a compound any of the element(s) below it in a single replacement reaction.

An Activity Series • The last problem indicates that cobalt is a more reactive metal than lead. • Therefore your activity series for metals would look like this: Cobalt Lead Gold

An Activity Series • It might be expected that metals with lower ionization energies and lower electronegativities would be more active, since they would be expected to more easily lose electrons in a displacement reaction.

• However while ionization energy and electronegativity do affect a metal's ranking in the series, other factors (free energies, heats of sublimation, hydration, etc. ) have a strong and complex influence on relative activity since the activity series is based on aqueous solutions at room temperature.

Lab: “Reaction Cell Potential” • The following slides discuss the lab procedure.
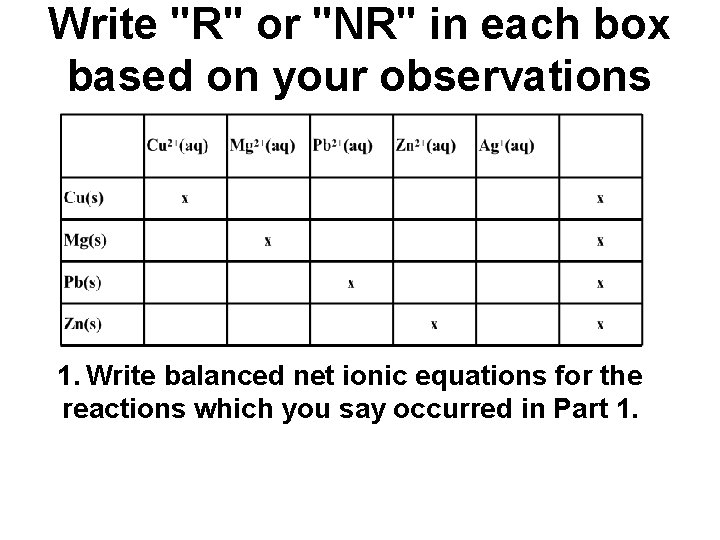
Write "R" or "NR" in each box based on your observations 1. Write balanced net ionic equations for the reactions which you say occurred in Part 1.
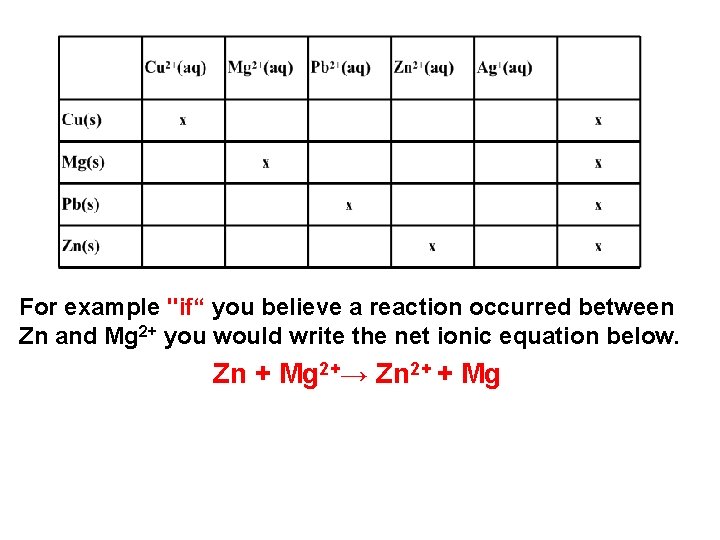
For example "if“ you believe a reaction occurred between Zn and Mg 2+ you would write the net ionic equation below. Zn + Mg 2+→ Zn 2+ + Mg
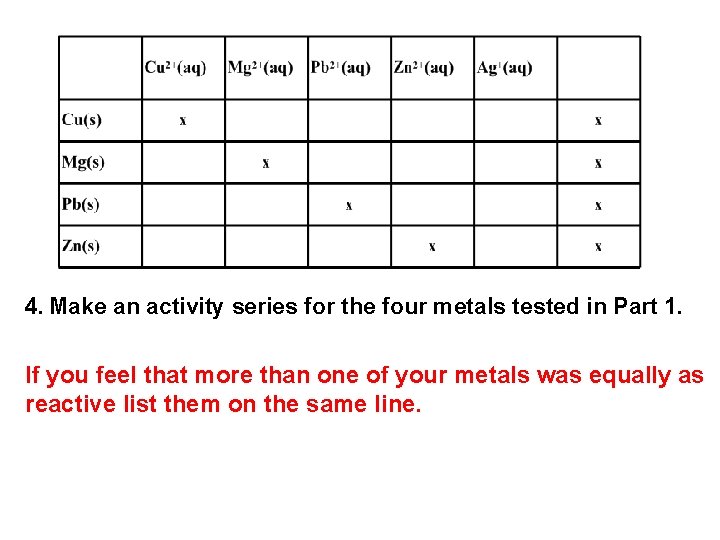
4. Make an activity series for the four metals tested in Part 1. If you feel that more than one of your metals was equally as reactive list them on the same line.
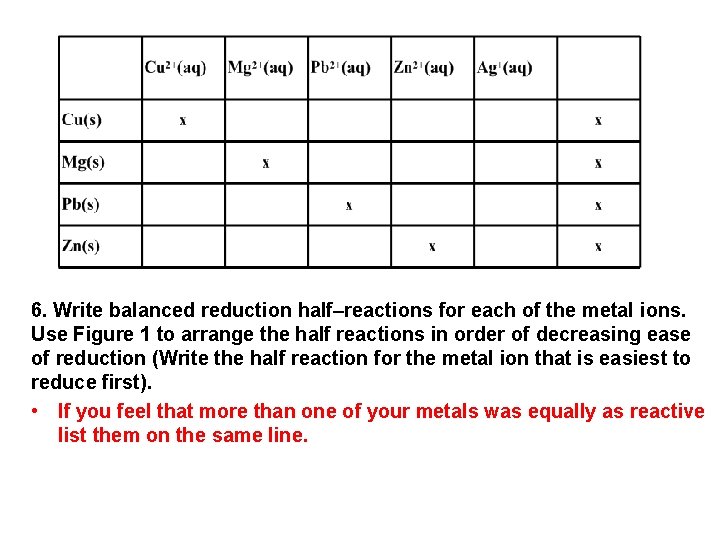
6. Write balanced reduction half–reactions for each of the metal ions. Use Figure 1 to arrange the half reactions in order of decreasing ease of reduction (Write the half reaction for the metal ion that is easiest to reduce first). • If you feel that more than one of your metals was equally as reactive list them on the same line.

Part 2: Determine an Activity Series for Nonmetals: • ow do we determine whether a reaction occurs or not in each of the 6 test tubes in part 2?
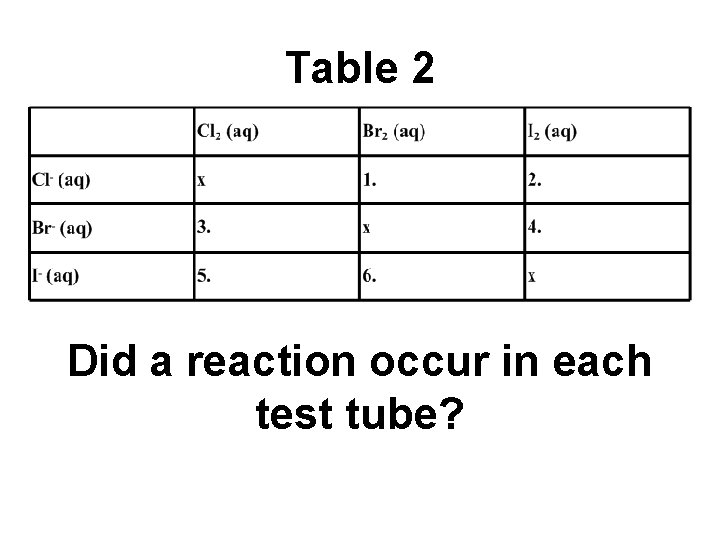
Table 2. . Did a reaction occur in each test tube?
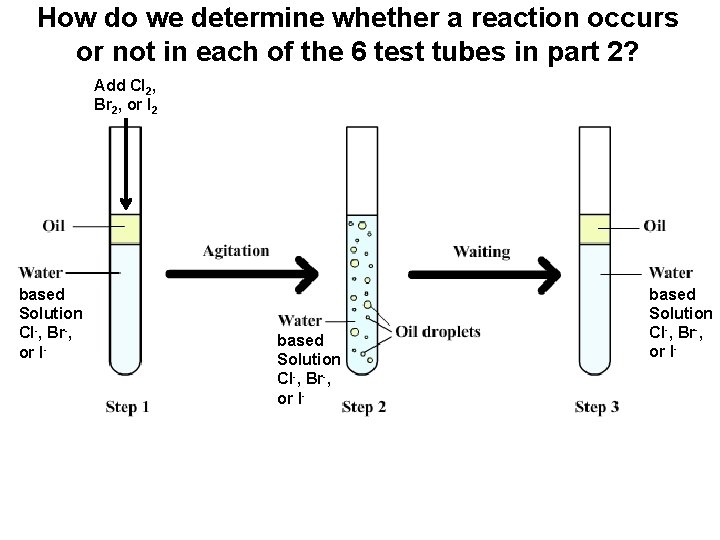
How do we determine whether a reaction occurs or not in each of the 6 test tubes in part 2? Add Cl 2, Br 2, or I 2 • based solution based Solution Cl-, Br-, or I-

"Like dissolves Like" • Based on the Polarity of the Substance. • Water vs. Oil • Halides vs. Halogens.
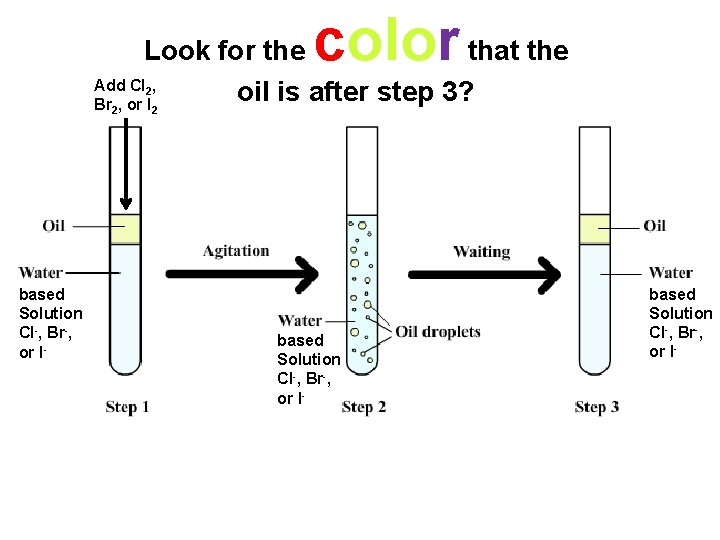
color Look for the that the Add Cl , oil is after step 3? Br , or I 2 2 2 • based solution based Solution Cl-, Br-, or I-

Color of the Oil Layer • chlorine - yellowish/green • bromine - reddish/orange • iodine - purple

Color of the Oil Layer chlorine - yellowish/green bromine - reddish/orange iodine - purple Oil Layer

Sample Data for Part 2 • Test tube 1: Bromine is added to a chloride solution. A red color is observed in the oil layer. • Test tube 6: Bromine is added to an iodide solution. A purple color is observed in the oil layer.
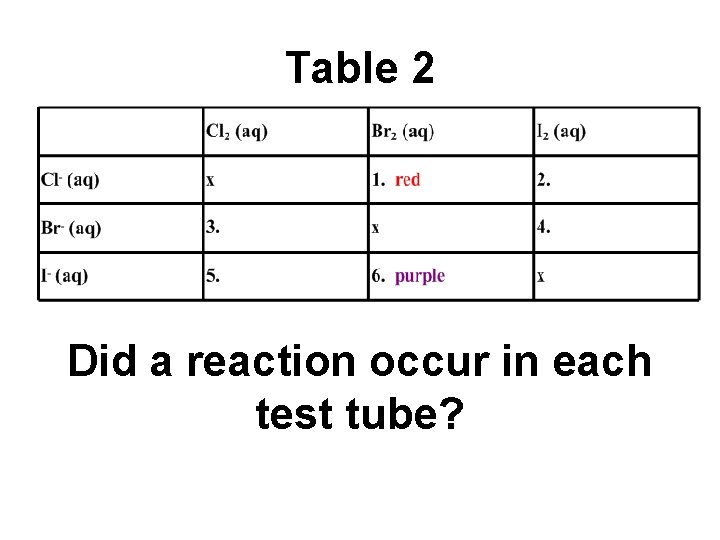
Table 2 Did a reaction occur in each test tube?
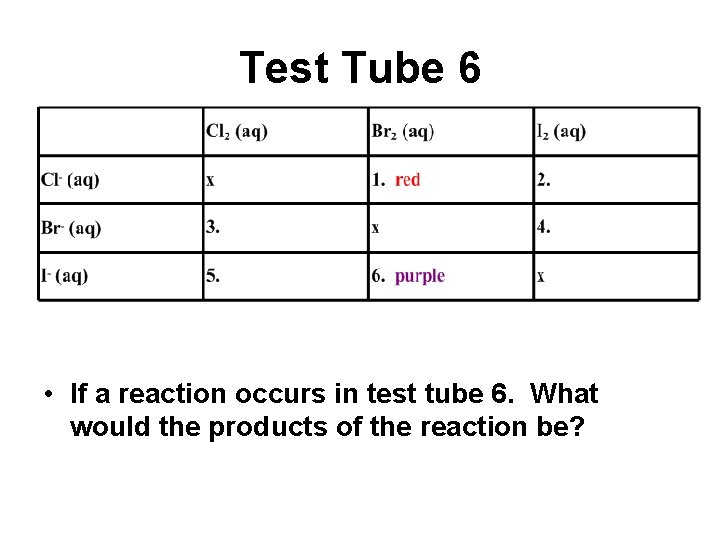
Test Tube 6 • If a reaction occurs in test tube 6. What would the products of the reaction be?
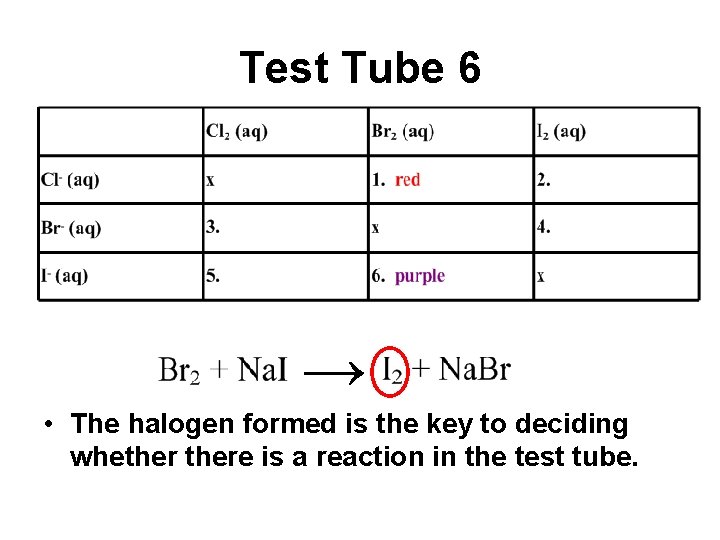
Test Tube 6 → • The halogen formed is the key to deciding whethere is a reaction in the test tube.
- Slides: 144