CHAPTER 7 Chemical Bonding Chemical bonds that hold

CHAPTER 7 Chemical Bonding
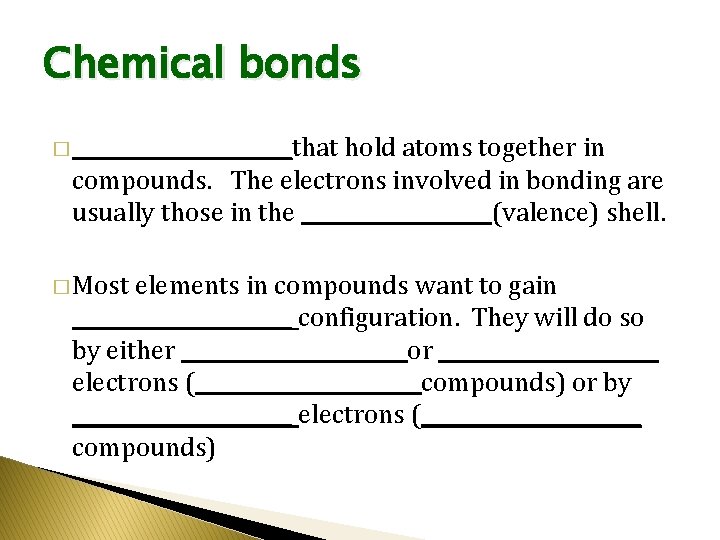
Chemical bonds � ___________that hold atoms together in compounds. The electrons involved in bonding are usually those in the __________(valence) shell. � Most elements in compounds want to gain ___________ configuration. They will do so by either ___________ or ___________ electrons (___________ compounds) or by ___________ electrons (___________ compounds)
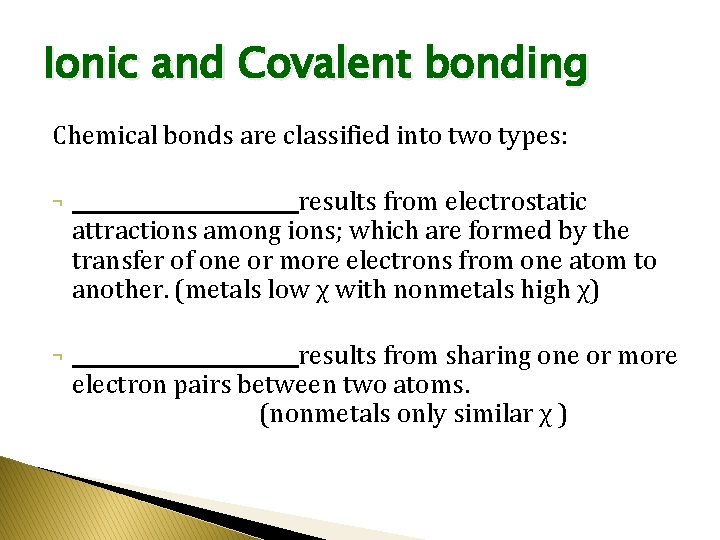
Ionic and Covalent bonding Chemical bonds are classified into two types: ¬ ___________ results from electrostatic attractions among ions; which are formed by the transfer of one or more electrons from one atom to another. (metals low χ with nonmetals high χ) ¬ ___________ results from sharing one or more electron pairs between two atoms. (nonmetals only similar χ )
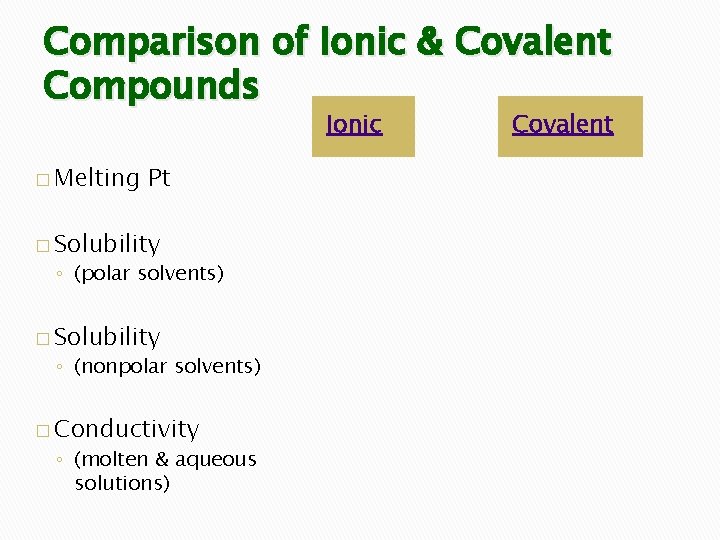
Comparison of Ionic & Covalent Compounds Ionic � Melting Pt � Solubility ◦ (polar solvents) � Solubility ◦ (nonpolar solvents) � Conductivity ◦ (molten & aqueous solutions) Covalent
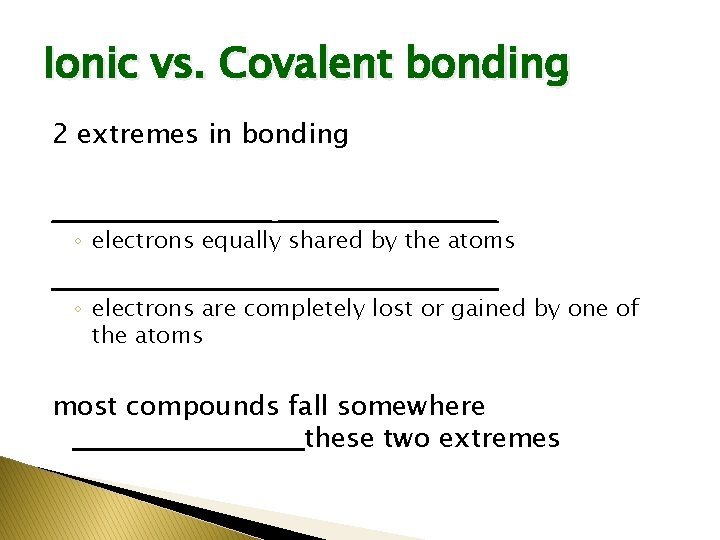
Ionic vs. Covalent bonding 2 extremes in bonding ______________________ ◦ electrons equally shared by the atoms ______________________ ◦ electrons are completely lost or gained by one of the atoms most compounds fall somewhere ___________ these two extremes
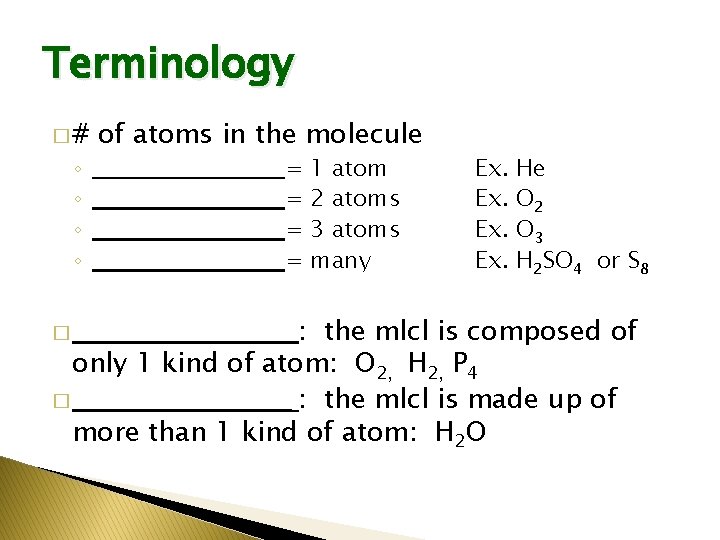
Terminology �# ◦ ◦ of atoms in the molecule ______________________ = 1 atom 2 atoms 3 atoms many Ex. Ex. He O 2 O 3 H 2 SO 4 or S 8 : the mlcl is composed of only 1 kind of atom: O 2, H 2, P 4 � ___________ : the mlcl is made up of more than 1 kind of atom: H 2 O � ___________
Lewis Dot Representations of Atoms or Lewis dot formulas, a convenient bookkeeping method for ______________________(electrons that are transferred or involved in chemical bonding) Only the electrons in the outermost s and p orbitals are shown as dots.
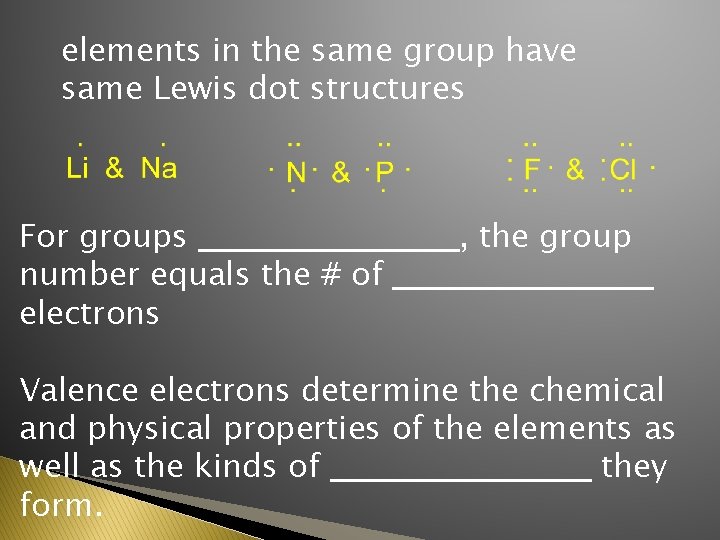
elements in the same group have same Lewis dot structures For groups ___________, the group number equals the # of ___________ electrons Valence electrons determine the chemical and physical properties of the elements as well as the kinds of ___________ they form.
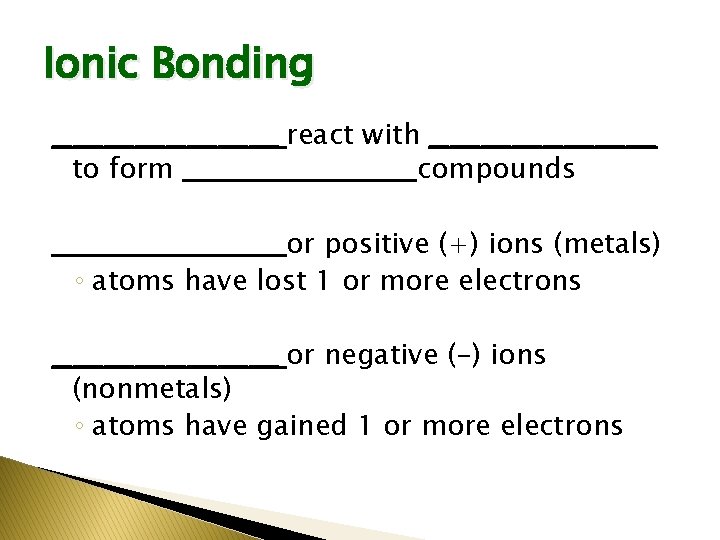
Ionic Bonding ___________ react with ___________ to form ___________ compounds ___________ or positive (+) ions (metals) ◦ atoms have lost 1 or more electrons ___________ or negative (-) ions (nonmetals) ◦ atoms have gained 1 or more electrons
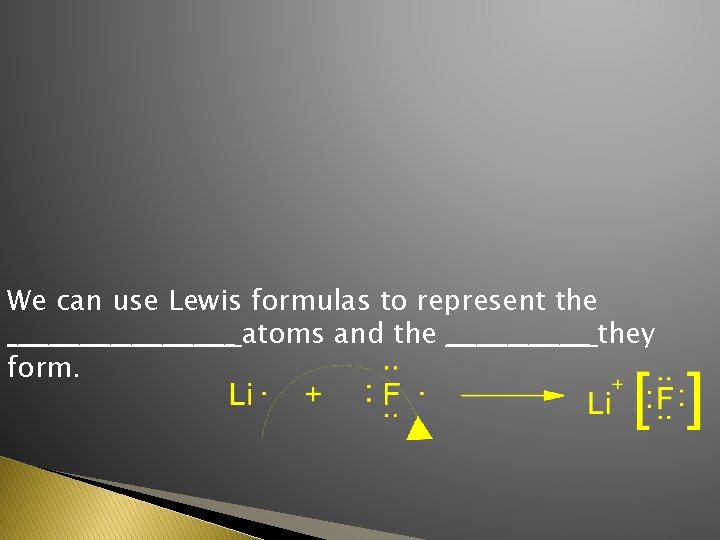
We can use Lewis formulas to represent the ___________ atoms and the _______ they form.

underlying reasons for Li. F formation 1 s Li ¯ F ¯ 2 s 2 p ¯ ¯ ¯ becomes Li+ ¯ [___] F- ¯ ¯ [___] ¯
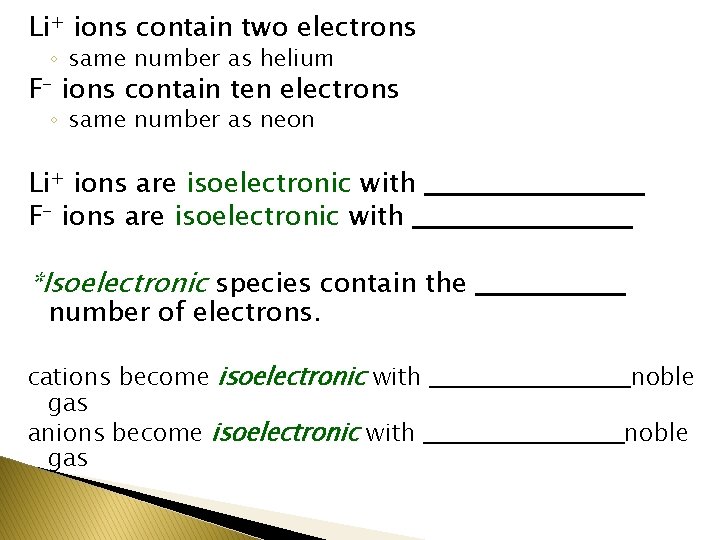
Li+ ions contain two electrons ◦ same number as helium F- ions contain ten electrons ◦ same number as neon Li+ ions are isoelectronic with ___________ F- ions are isoelectronic with ___________ *Isoelectronic species contain the ________ number of electrons. cations become isoelectronic with ___________ noble gas anions become isoelectronic with ___________ noble gas
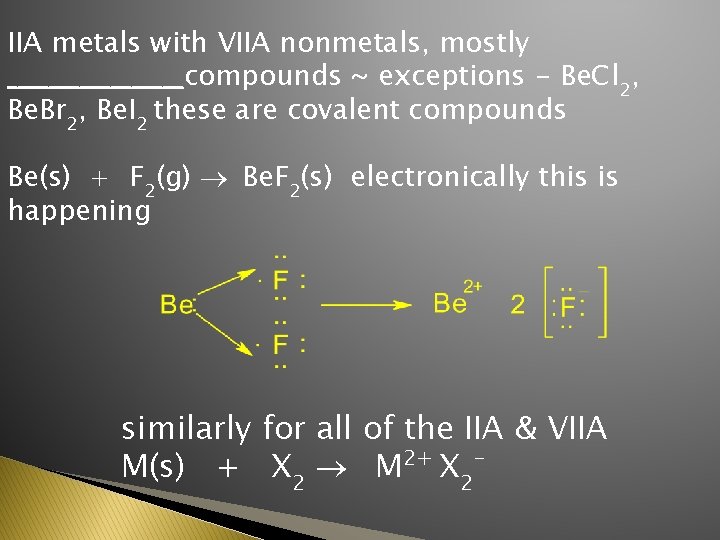
IIA metals with VIIA nonmetals, mostly _________compounds ~ exceptions - Be. Cl 2, Be. Br 2, Be. I 2 these are covalent compounds Be(s) + F 2(g) ® Be. F 2(s) electronically this is happening similarly for all of the IIA & VIIA M(s) + X 2 ® M 2+ X 2 -
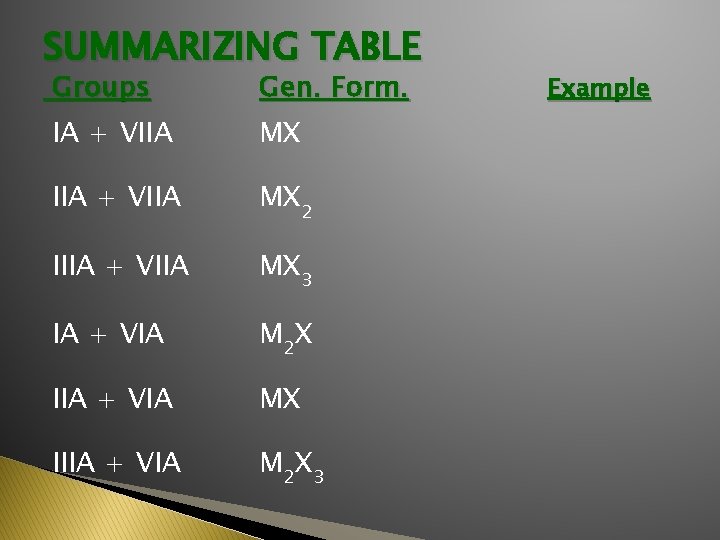
SUMMARIZING TABLE Groups Gen. Form. IA + VIIA MX IIA + VIIA MX 2 IIIA + VIIA MX 3 IA + VIA M 2 X IIA + VIA MX IIIA + VIA M 2 X 3 Example
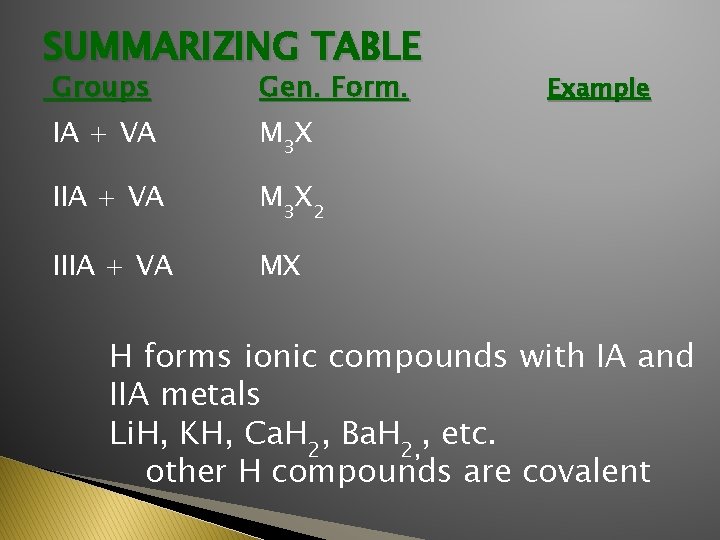
SUMMARIZING TABLE Groups Gen. Form. IA + VA M 3 X IIA + VA M 3 X 2 IIIA + VA MX Example H forms ionic compounds with IA and IIA metals Li. H, KH, Ca. H 2, Ba. H 2, , etc. other H compounds are covalent
lattice energy ___________ - the energy needed to separate oppositely charged ions. The ___________ the lattice energy, the ___________ the ionic bond. The stronger the ionic bond the ___________ in water at a given temperature, since the ions must __________________ from one another and attach to water in order to dissolve.

Coulomb’s Law ~ ions with high (big)charges = ___________ ~ ions with small (large) charges = ___________
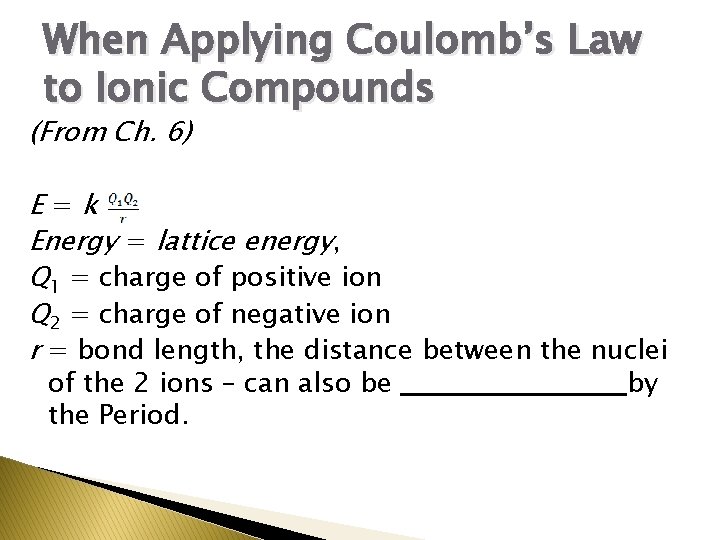
When Applying Coulomb’s Law to Ionic Compounds (From Ch. 6) E=k Energy = lattice energy, Q 1 = charge of positive ion Q 2 = charge of negative ion r = bond length, the distance between the nuclei of the 2 ions – can also be ___________ by the Period.

r (Use the Period of the element that is ___________ with the ion! � For � Na is in Period 3 and has 3 occupied energy levels. Na+ has lost an electron. It has 10 electrons and is isoelectronic with Ne in Period 2. Na+ has only 2 occupied energy levels. )

arrange these compounds in order of increasing attractions among ions KCl, Al 2 O 3, Ca. O

1. Which has a stronger ionic bond, Na. Cl or KCl? Explain why. The lattice energy of _________________, so this is the stronger ionic bond. Both have an electron charge of -1 and an effective nuclear charge of 1. But the valence electrons of ___ are in the ___ energy level, leading to a shorter bond length (measured as distance between ionic nuclei) and a stronger ionic bond than ___.

2. Which has a stronger ionic bond, Na. Cl or Al. Cl 3? Explain why. The _________has a stronger ionic bond. Both have the same -1 charge for the chloride ion, and both Na+ and Al+3 are isoelectronic with Ne and therefore have 2 occupied energy levels. But the higher positive charge of the ___________ leads to a stronger lattice energy and a stronger ionic bond.

3. Which is more soluble in 80° C water, Na. Cl or KCl? Explain why You can dissolve more grams of ____ in 100 grams of 80° C water since it has a ______________________ which requires less energy to separate/dissociate the ions from one another and allow them to attach to the polar water molecules.

4. Which is more soluble in 80° C water, Na. Cl or Al. Cl 3? Explain why _____is more soluble in 80° C water since it has a ______________________ which requires less energy to ___________ the ions from one another and allow them to attach to the polar water molecules.

5. Why is Na 2 O considered soluble in water while Al 2 O 3 is not Q 2 for oxygen is the same for both compounds, a -2. Radius of oxygen is the same for both, and Na+ and Al+3 are isoelectronic. So the larger Q 1 charge makes the ___________ of the ___________ greater, and since the ions stay bonded to one another it will not dissociate and dissolve.
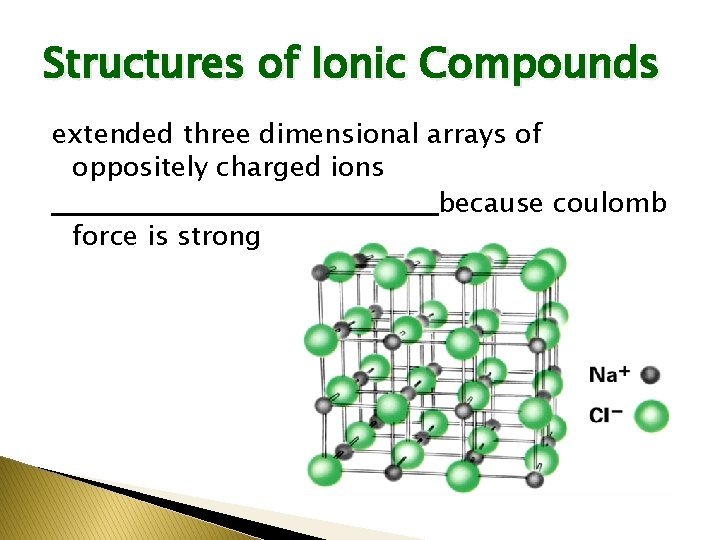
Structures of Ionic Compounds extended three dimensional arrays of oppositely charged ions ___________because coulomb force is strong
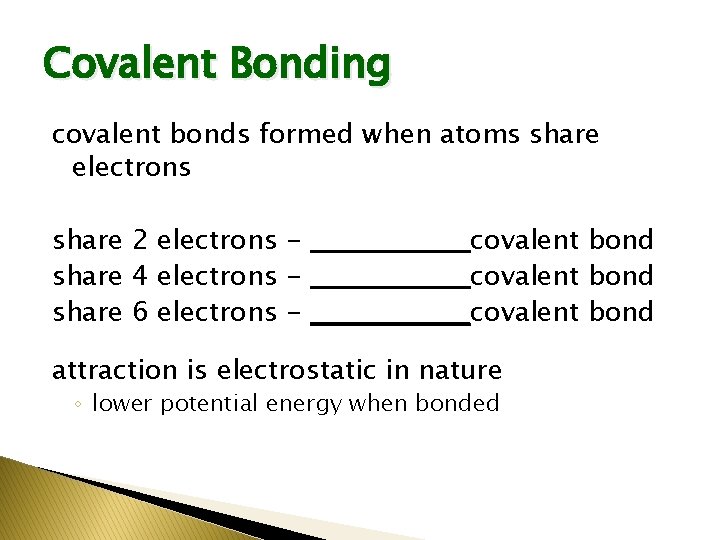
Covalent Bonding covalent bonds formed when atoms share electrons share 2 electrons - ________covalent bond share 4 electrons - ________covalent bond share 6 electrons - ________covalent bond attraction is electrostatic in nature ◦ lower potential energy when bonded
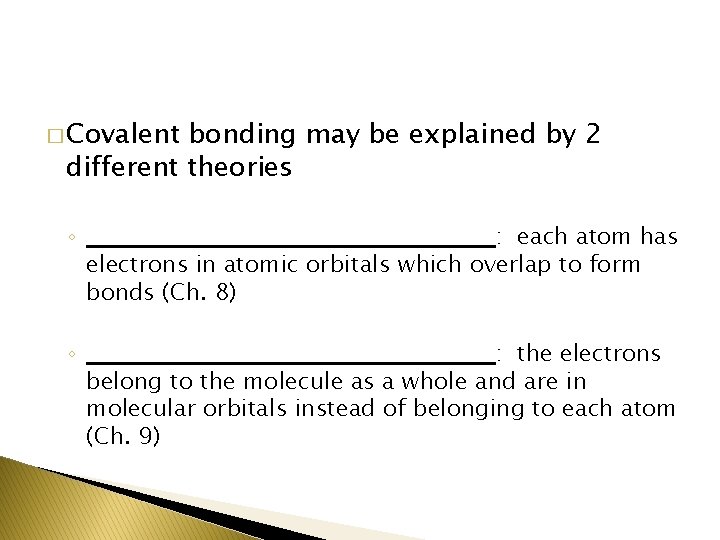
� Covalent bonding may be explained by 2 different theories ◦ ________________________: each atom has electrons in atomic orbitals which overlap to form bonds (Ch. 8) ◦ ________________________: the electrons belong to the molecule as a whole and are in molecular orbitals instead of belonging to each atom (Ch. 9)
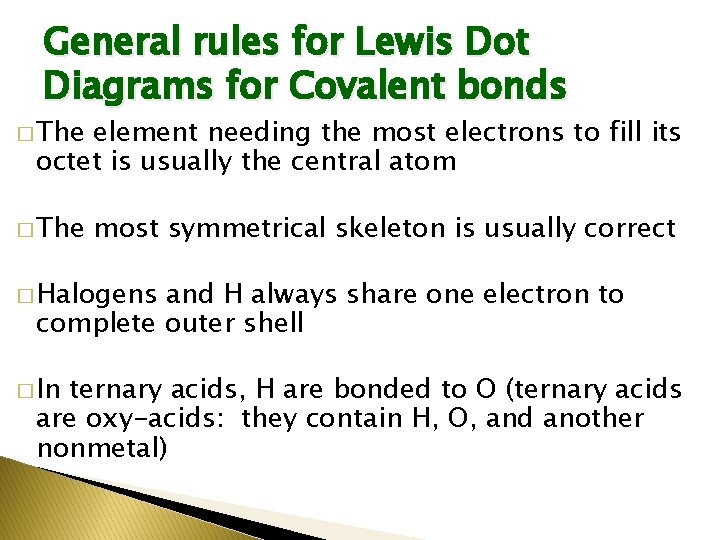
General rules for Lewis Dot Diagrams for Covalent bonds � The element needing the most electrons to fill its octet is usually the central atom � The most symmetrical skeleton is usually correct � Halogens and H always share one electron to complete outer shell � In ternary acids, H are bonded to O (ternary acids are oxy-acids: they contain H, O, and another nonmetal)
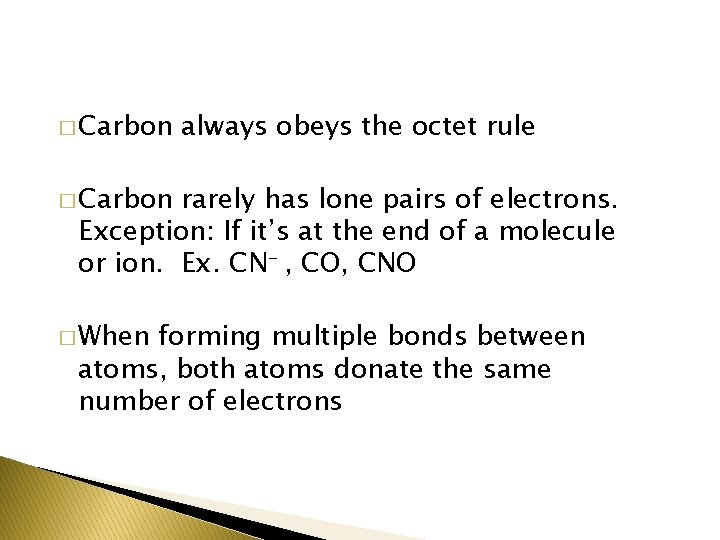
� Carbon always obeys the octet rule � Carbon rarely has lone pairs of electrons. Exception: If it’s at the end of a molecule or ion. Ex. CN- , CO, CNO � When forming multiple bonds between atoms, both atoms donate the same number of electrons
� Oxygen atoms normally bond to other nonmetals, not to each other � Oxygen can do several things depending on the mlcl. ◦ Single bond by sharing an electron ◦ Single bond by accepting 2 electrons from another atom and not sharing at all ◦ Double bonds by sharing 2 of its electrons
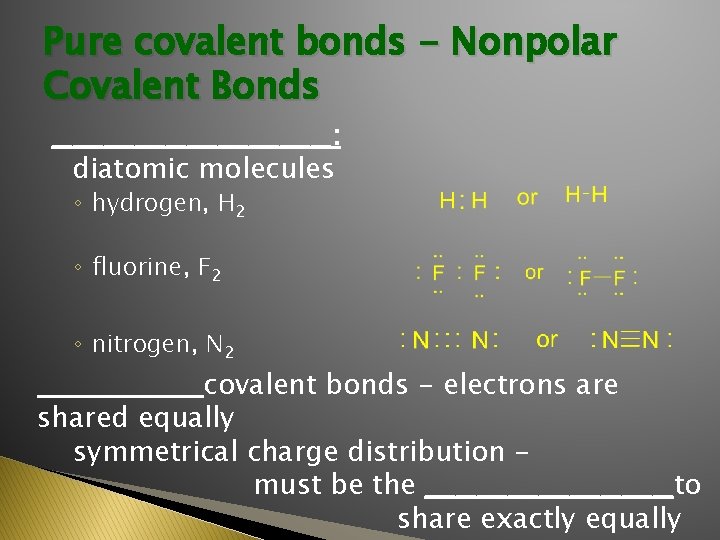
Pure covalent bonds - Nonpolar Covalent Bonds ______________: diatomic molecules ◦ hydrogen, H 2 ◦ fluorine, F 2 ◦ nitrogen, N 2 ________covalent bonds - electrons are shared equally symmetrical charge distribution must be the ____________to share exactly equally
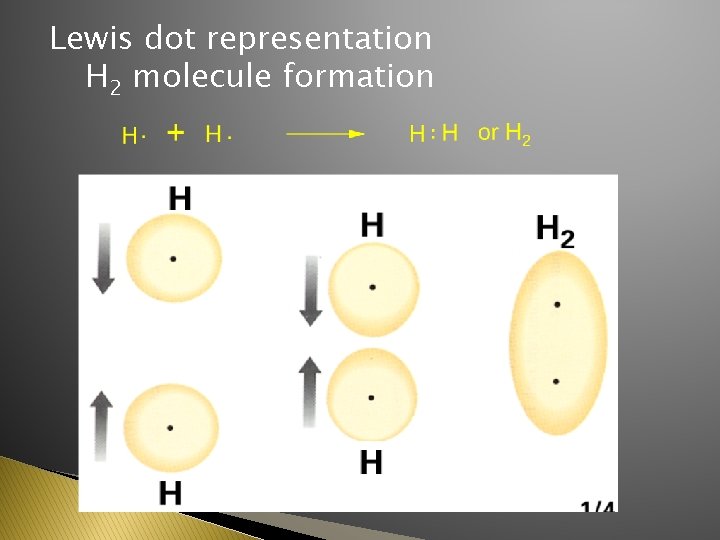
Lewis dot representation H 2 molecule formation
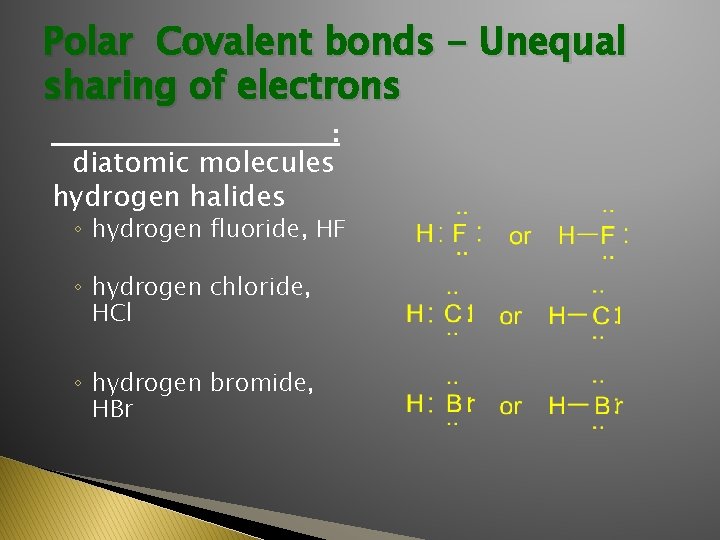
Polar Covalent bonds - Unequal sharing of electrons ______________: diatomic molecules hydrogen halides ◦ hydrogen fluoride, HF ◦ hydrogen chloride, HCl ◦ hydrogen bromide, HBr
________________bonds - unequally shared electrons • _______________ charge distribution • different ________________ Some bonds are ________, Ex. HF
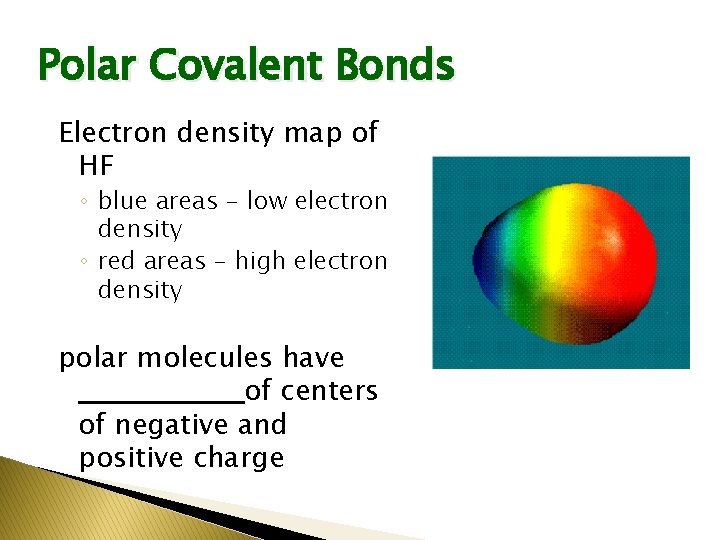
Polar Covalent Bonds Electron density map of HF ◦ blue areas - low electron density ◦ red areas - high electron density polar molecules have ________of centers of negative and positive charge

Some bonds are only _____________, ex. HI
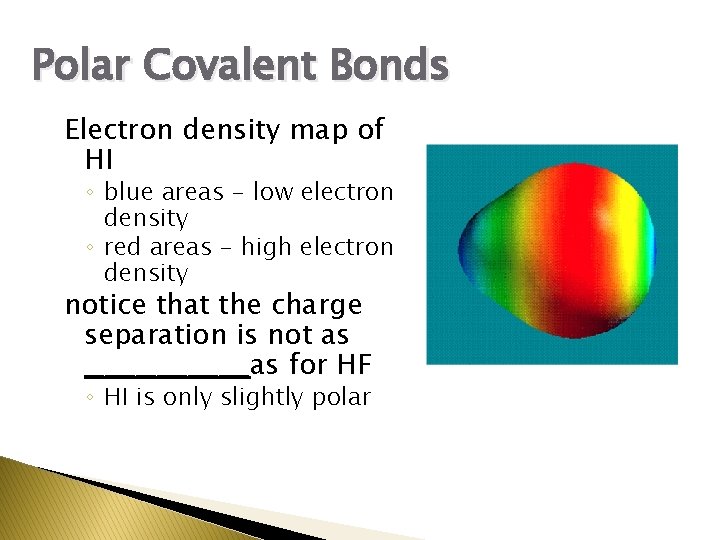
Polar Covalent Bonds Electron density map of HI ◦ blue areas - low electron density ◦ red areas - high electron density notice that the charge separation is not as ________as for HF ◦ HI is only slightly polar
The Octet Rule ________________elements achieve noble gas configurations in most of their compounds. Lewis dot formulas are based on the ________. �H needs two electrons to have Helium's noble gas configuration, everything else wants 8
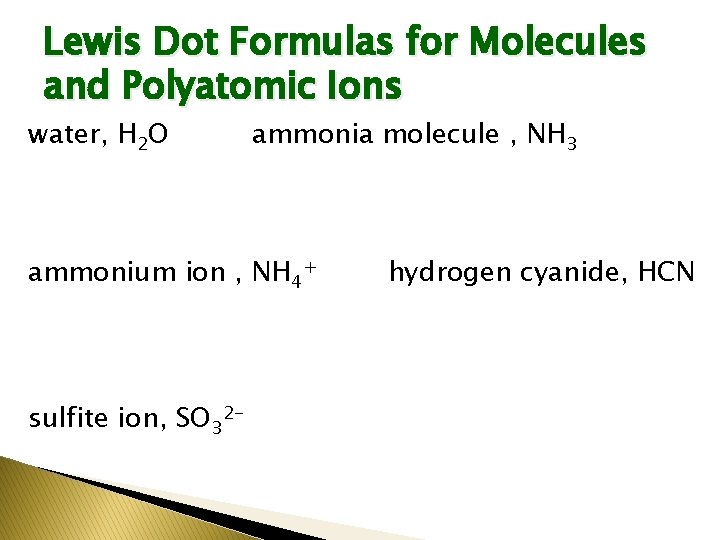
Lewis Dot Formulas for Molecules and Polyatomic Ions water, H 2 O ammonia molecule , NH 3 ammonium ion , NH 4+ sulfite ion, SO 32 - hydrogen cyanide, HCN
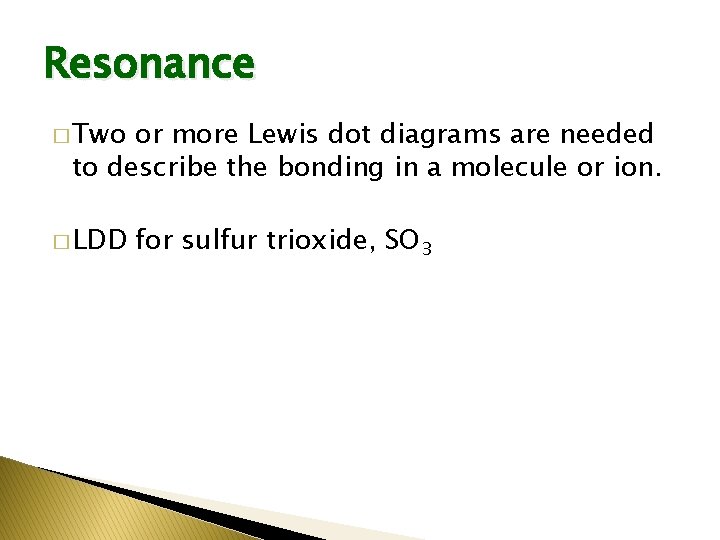
Resonance � Two or more Lewis dot diagrams are needed to describe the bonding in a molecule or ion. � LDD for sulfur trioxide, SO 3

Resonance three possible structures for SO 3 invoke resonance ◦ Double-headed arrows are used to indicate resonance formulas.
Resonance flaw in our representations of molecules no single or double bonds in SO 3 all bonds are the same length best picture
Formal Charges � The concept of formal charges helps us choose the correct Lewis structure for a molecule. If a ________________has a high formal charge it’s not a very good one. � Formal Or charge = group # - e- you can assign to that atom F. C. = (valence e- ) – (# of bonds + # of unshared e- ) pg 289
Sigma and Pi bonds ________________ (σ) : result of head-on (end to end overlap, there is a free rotation around σ bonds. ________(π) : result of side-on overlap of p orbitals. There is no free rotation around a π bond. The side –on overlap locks the molecule into place. All ________bonds are sigma bonds: 1σ bond All ________bonds: 1 σ bond, 1 π bond All ________bonds: 1 σ bond, 2 π bonds
Limitations of the Octet Rule for Lewis Formulas ¹ º species in which the central element must have a share of more or less than 8 valence electrons to accommodate all substituents compounds of the d- and f-transition metals In cases where the octet rule does not apply, the elements attached to the central atom nearly always attain noble gas configurations. ◦ ________________________

Limitations of the Octet Rule for Lewis Formulas � Write LDD for BBr 3 � Write LDD for As. F 5 � Write LDD for Xe. F 4

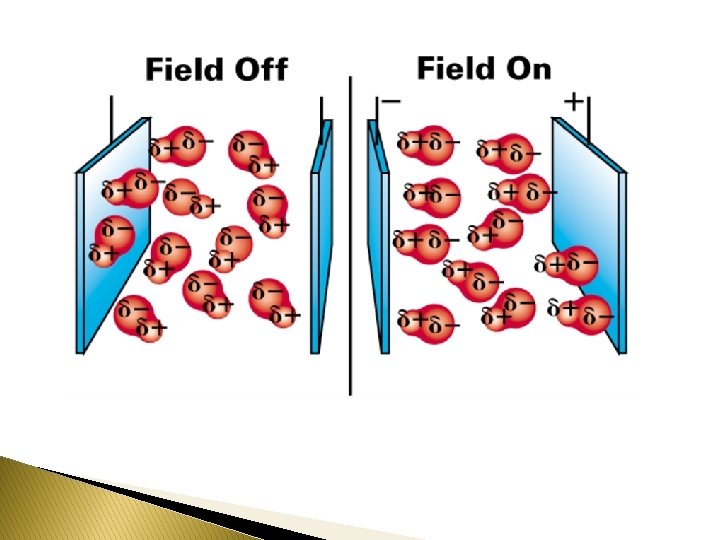
As we all know, in the wintertime we are more likely to get shocked when we walk across carpet and touch the door knob. Here’s another experiment to perform. Turn on a water faucet until you have a continuous but small stream of water coming from the faucet. Brush your hair vigorously then hold the brush near the stream of water. You will notice that the stream bends towards the brush. Why does the water bend? On a “infomercial” it claimed that placing a small horseshoe magnet over the fuel intake line to your car’s carburetor would increase fuel mileage by 50%. The reason given for the mileage increase was that “the magnet aligned the molecules causing them to burn more efficiently. ” Will this work? Should you buy this product?
- Slides: 49