CHAPTER 7 Cellular Respiration Two examples of catabolic

CHAPTER 7 Cellular Respiration
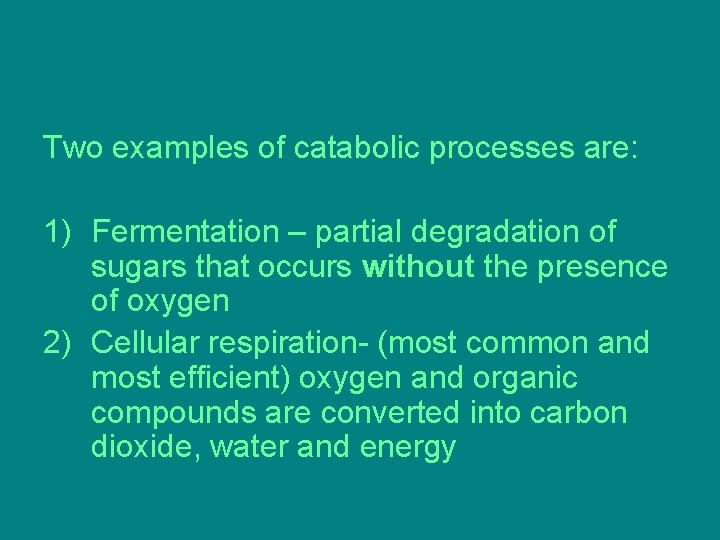
Two examples of catabolic processes are: 1) Fermentation – partial degradation of sugars that occurs without the presence of oxygen 2) Cellular respiration- (most common and most efficient) oxygen and organic compounds are converted into carbon dioxide, water and energy

• Carbs, fats and protein can all be converted into fuel for the cell, but the basic pathway is for the conversion of glucose • Breakdown of glucose is exergonic – releasing 686 kcal per mole

Redox Reactions • In many reactions there is a transfer of one or more electrons from one reactant to another • Oxidation- the loss of an electron from one substance • Reduction- the addition of electrons to another substance

• Reducing agent – electron donor • Oxidizing agent – electron acceptor • Not all redox reactions completely transfer electrons, some change the degree of sharing in covalent bonds

cell respiration is a redox reaction C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + Energy - Glucose is oxidized into carbon dioxide - Oxygen is reduced into water - Electrons lose potential energy along the way so energy is released!
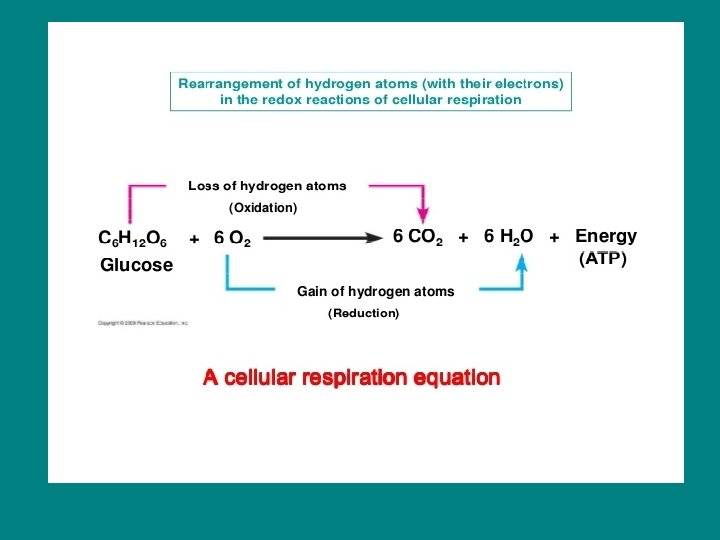

• Glucose and other organic fuels are broken down in a series of steps • Each step is catalyzed by an enzyme • Electrons travel with protons as H atoms • H atoms taken from glucose are not transferred directly to oxygen • First they are passed to coenzyme NAD+ • NAD+ is an electron acceptor (oxidizing agent) during respiration
• When it is reduced NAD+ becomes NADH • NAD+ is versatile and functions in a number of different steps of cell resp • Electrons lose very little potential energy when transferred from food to NAD+ • Each NADH molecule represents stored energy that can be used to make ATP once electrons complete their “fall” down the energy gradient from NADH to O 2
ETC • Cell respiration uses an electron transport chain to break the fall of electrons to oxygen into several energy releasing steps instead of one explosive reaction • The ETC consists mostly of protein molecules embedded in the inner membrane of a mitochondrion

• Electrons removed from food are shuttled by NADH to the “top” (higher energy) end of the chain • At the “bottom” is oxygen (lower energy) • Oxygen is the final electron acceptor in the ETC • Oxygen accepts the electron (H atom) and forms water

• Electron transfer from NADH to Oxygen is exergonic • Instead of this energy (53 kcal/mole) being released and wasted in a single step, electrons cascade down the chain from one carrier molecule to the next • Each step loses a small amount of energy until it reaches Oxygen at the bottom
• Each carrier molecule is more electronegative than its “uphill neighbor” • Electrons from food move down an energy gradient in the ETC to a more stable oxygen atom Basic cell resp route: Food NADH ETC Oxygen
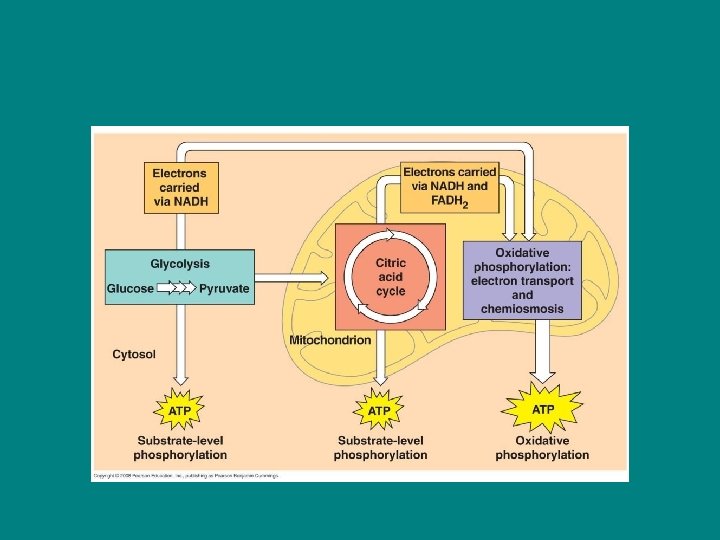
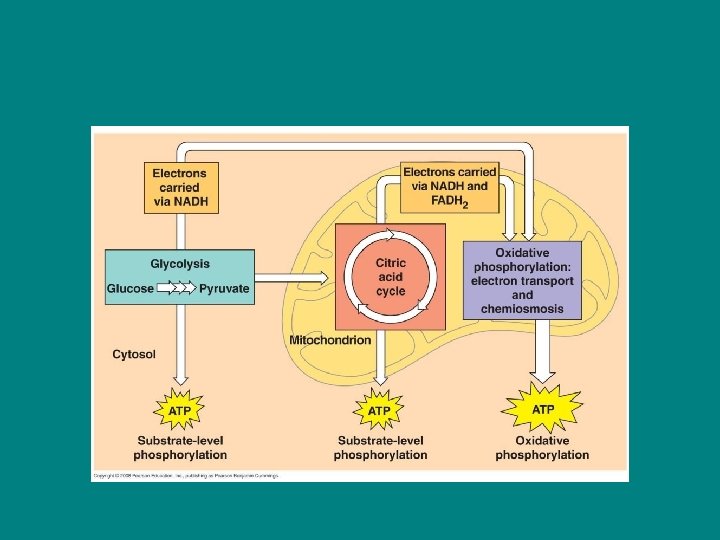
Preview of the stages of cell resp 1) Glycolysis 2) Citric Acid Cycle 3) Oxidative Phosphorylation (ETC and chemiosmosis)


Glycolysis First step: Glycolysis • Technically not part of cell resp because it does not require oxygen but is usually included b/c it is an essential first step • Catabolic • Occurs in the cytosol • Breaks one glucose into two molecules of pyruvate

Citric Acid Cycle • • • Second Step: Citric Acid Cycle Catabolic follows right after glycolysis Requires oxygen Takes place in the mitochondrial matrix Completes the breakdown of glucose by oxidizing a derivative of pyruvate into CO 2

• Some of the reactions of the first 2 steps of cell resp are redox reactions • Transferring some electrons to form NADH

Oxidative phosphorylation Third Step: Oxidative Phosphorylation • Consists of the ETC and chemiosmosis • Occurs right after citric acid cycle • Occurs in inner mitochondrial membrane • The energy released at the end of the ETC is stored in a form the mitochondrion can use to make ATP
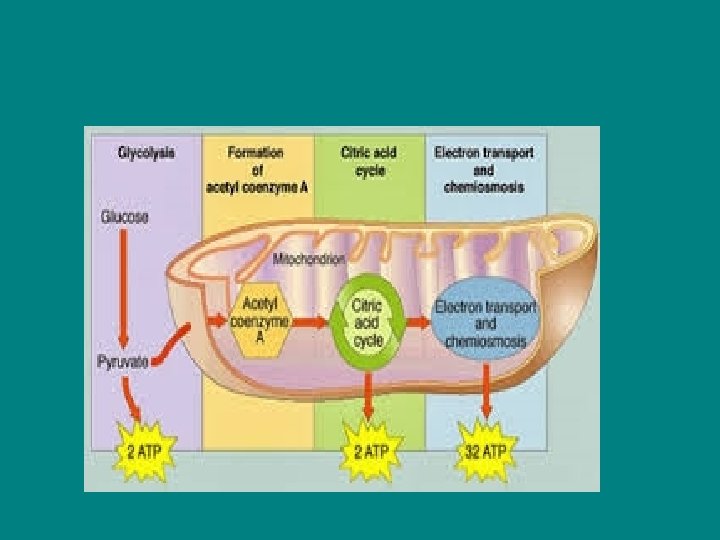
• 90% of ATP generation of cell resp comes from oxidative phosphorylation • (a little ATP is formed during the first 2 steps through a process called substrate level phosphorylation)

• For each molecule of glucose, the cell can make UP TO 38 molecuels of ATP • Each ATP molecule has 7. 3 kcal/mole of free energy • Cell respiration takes the 686 kcal/mole of energy in a glucose molecule and packages it in usable amounts (ATP)

Glycolysis (more detail) • Splits glucose (6 carbon sugar) into two 3 carbon sugars • The smaller sugars are oxidized and rearranged into 2 molecules of pyruvate • NET energy yield from glycolysis is 2 ATP and 2 NADH per glucose molecule • (4 ATP are formed, but 2 ATP had to be invested so only 2 ATP are NET)

• Glycolysis occurs whether or not oxygen is present • No carbon dioxide is released • If Oxygen is present, glycolysis will be followed by the citric acid cycle and then oxidative phosphorylation

Citric Acid Cycle AKA Krebs Cycle (more detail) • Glycolysis releases only a little of the energy stored in glucose • Most of the energy remains in the 2 molecules of pyruvate • If molecular oxygen is present, the pyruvate enters the mitochondrion and undergoes the citric acid cycle • Pyruvate enters mitochondrion through active transport

• As soon as pyruvate enters the mitochondrion, it is converted to acetyl coenzyme A (acetly co. A) • This is an intermediate step between glycolysis and citric acid cycle

• Citric acid cycle has eight steps, each catalyzed by a different enzyme • For each “turn” of the cycle, 2 carbons enter in the form of an acetyl group • At the end, two completely oxidized C’s leave as carbon dioxide molecules • Acetyl Co. A joins the cycle by combining with oxaloacetate to form citrate

• The next 7 steps decompose citrate back to oxaloacetate • For each acetyl group that enters the cycle: 3 NAD+ are reduced to NADH and one FAD is reduced to FADH 2 • Keep in mind that this is per acetyl group (pyruvate molecule) and each glucose breaks into 2 pyruvates, so the citric acid cycle turns TWICE for each glucose

• Citric acid cycle yields 2 ATP directly, 6 NADH and 2 FADH 2 per glucose molecule!!!! • The energy held in the 2 coenzymes (NADH and FADH 2 ) will be harvested, but they must go through oxidative phosphorylation first

Oxidative phosphorylation (ETC and chemiosmosis) (more detail) • Up until this point in glycolysis and citric acid cycle- only 4 net ATP have been produced and all from substrate level phosphorylation • Oxidative phosphorylation is where most of the ATP is made • oxid phos- uses energy released by the ETC to power ATP synthesis
ETC • Collection of molecules embedded in the inner membrane of the mitochondria • The foldings (cristae) of this membrane increase surface area and allow for thousands of copies of the ETC in each mitochondrion

• Along the ETC electron carriers alternate between reduced and oxidized states as they accept and donate electrons • Many electron carriers are cytochromes – proteins with a prosthetic group called a heme group (has an iron atom that accepts and donates electrons) • A number of different cytochromes are found in the ETC

• Each cytochrome has a slightly different electron carrying heme group • The last cytochrome of the chain is cyt a 3 which passes its electrons to oxygen (very electronegative) • Each oxygen also picks up a pair of hydrogen atoms (from the aqueous solution) and this is how water is formed!

• Sources of electrons for the ETC are NADH and FADH 2 • FADH 2 enters the ETC at a lower energy level (complex II instead of complex I) than NADH • This means that more energy for ATP synthesis is yielded from NADH going down the ETC

• The ETC does not make ATP directly, this occurs during the next step of chemiosmosis
Chemiosmosis (energy coupling) • ATP synthase- protein complex found in the inner mitochondrial membrane • ATP synthase – the enzyme that actually makes ATP from ADP and inorganic phosphate (Pi) • ATP synthase is like a reverse ion pump • Uses the energy of an existing ion gradient to power ATP synthesis

• This phosphorylation is powered by a proton (hydrogen ion) gradient • The power source for ATP synthase is a difference in the concentration of H+ on opposite sides of the inner mitochondrial membrane • You can also think of it as a difference in p. H because technically p. H measures the amount of H+ in solution
• Chemiosmosis- the process in which energy stored in the form of a H+ gradient across a membrane is used to drive cellular work such as the synthesis of ATP • The H+ gradient is created by the ETC • The ETC is an energy converter that uses exergonic flow of electrons to pump H+ across the membrane, from the mitochondrial matrix to the intermembrane space

• This gradient makes the H+ want to diffuse back down its gradient but the only places freely permeable to H+ are the ATP synthase complexes • To get back out, the H+ must pass through a channel in ATP synthase • The ATP synthase can then use the exergonic flow of the H+ to drive the phosphorylation of ADP to ATP
• So…. the energy stored in the H+ gradient across a membrane couples the redox reactions of the ETC to ATP synthesis • This H+ gradient is called a proton-motive force- it shows the capacity of the gradient to perform work
• In mitochondria the energy for gradient formation comes from exergonic redox reactions and the work performed is ATP synthesis • Chemiosmosis also occurs in chloroplasts and bacterial cells with different variations
Flow of most energy during resp Glucose NADH ETC proton gradient ATP
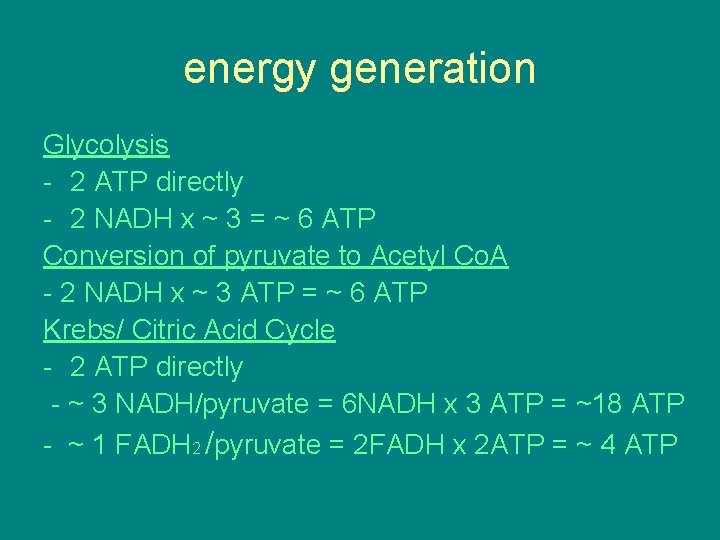
energy generation Glycolysis - 2 ATP directly - 2 NADH x ~ 3 = ~ 6 ATP Conversion of pyruvate to Acetyl Co. A - 2 NADH x ~ 3 ATP = ~ 6 ATP Krebs/ Citric Acid Cycle - 2 ATP directly - ~ 3 NADH/pyruvate = 6 NADH x 3 ATP = ~18 ATP - ~ 1 FADH 2 /pyruvate = 2 FADH x 2 ATP = ~ 4 ATP

• All energy is not directly harvested as ATP, that means the energy in NADH and FADH needs to go through oxid phos to harvest that energy as ATP. • TOTAL ATP YIELD ~ 36 -38
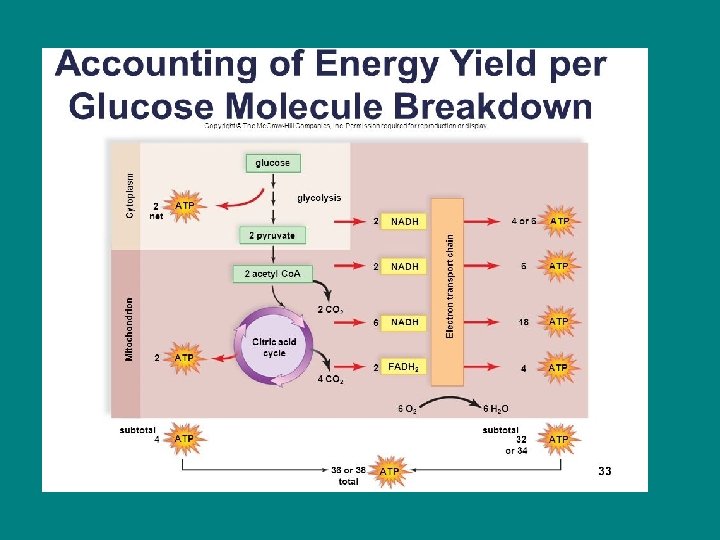

How efficient is respiration? • Resp stores 7. 3 kcal/mole per ATP • 7. 3 X 38 / 686 = 0. 4 • 40% of the energy in glucose is stored as energy in ATP • Rest is lost as heat
FERMENTATION • Glycolysis still occurs, does not need oxygen • This is because the oxidizing agent of glycolysis is NAD+ not oxygen • Glycolysis will produce 2 net ATP whether aerobic or anaerobic • Fermentation yields 2 ATP

• Fermentation is anaerobic catabolism of organic nutrients • Fermentation occurs after glycolysis when no oxygen is present • This produces ATP through substrate level phosphorylation • This occurs as long as there is enough NAD+ to accept electrons
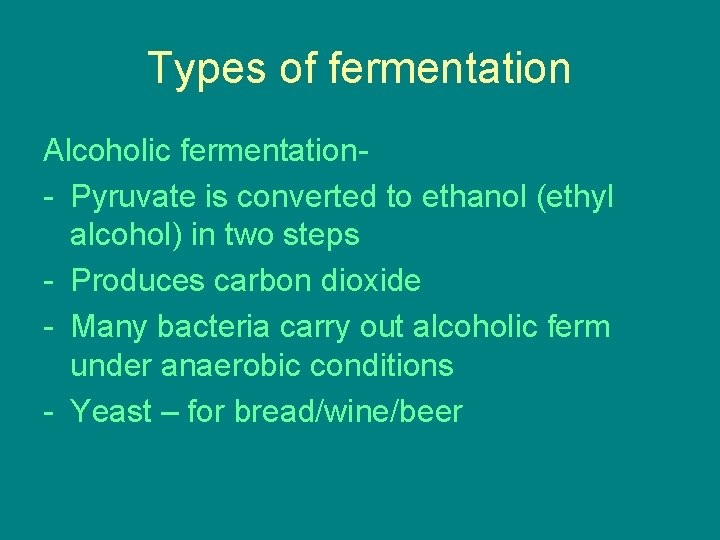
Types of fermentation Alcoholic fermentation- Pyruvate is converted to ethanol (ethyl alcohol) in two steps - Produces carbon dioxide - Many bacteria carry out alcoholic ferm under anaerobic conditions - Yeast – for bread/wine/beer

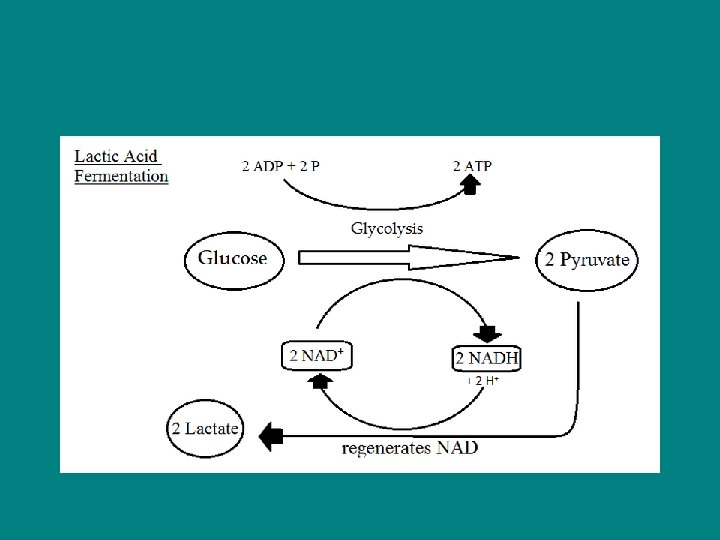
Lactic Acid Fermentation – - Lactate (ionized lactic acid) formed - No carbon dioxide released - Used by bacteria and fungi to make cheese and yogurt - In human muscle cells when become fatigued
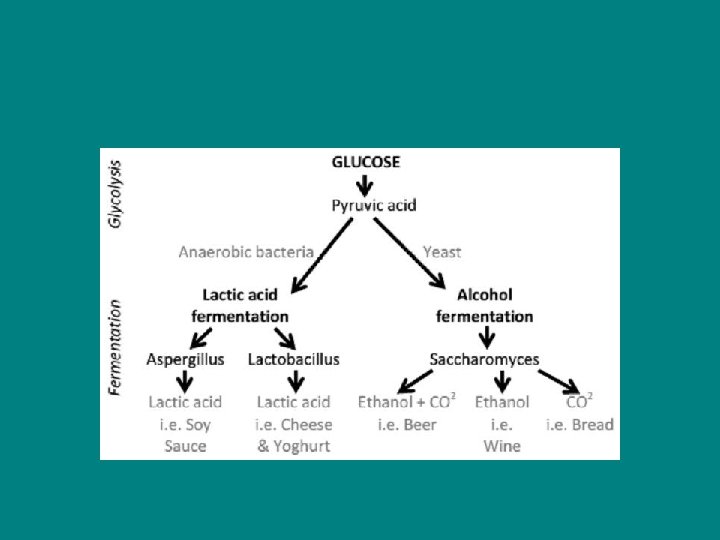
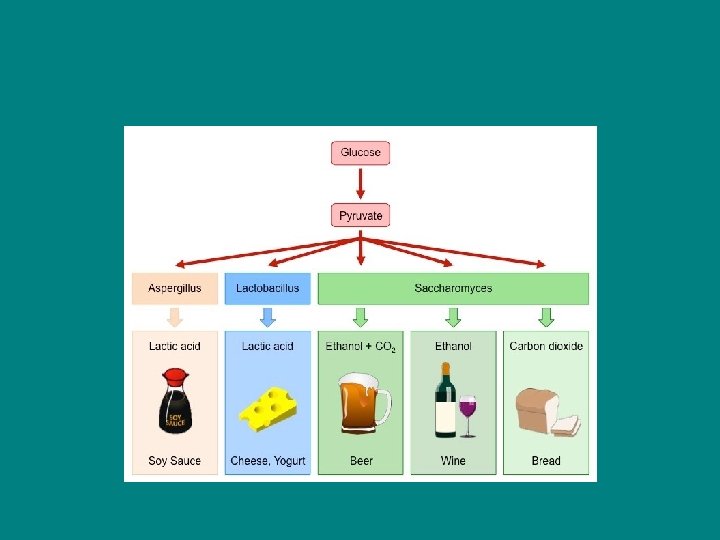

• Facultative anaerobes – - Organisms that perform resp when oxygen is present - Can perform fermentation when oxygen is not present - This allows them to stay alive during periods of reduced oxygen

• In humans, fermentation can occur in our muscles to keep us alive and going during short periods of oxygen deprivation • As soon as oxygen becomes available, the citric acid cycle will resume and resp will start up again
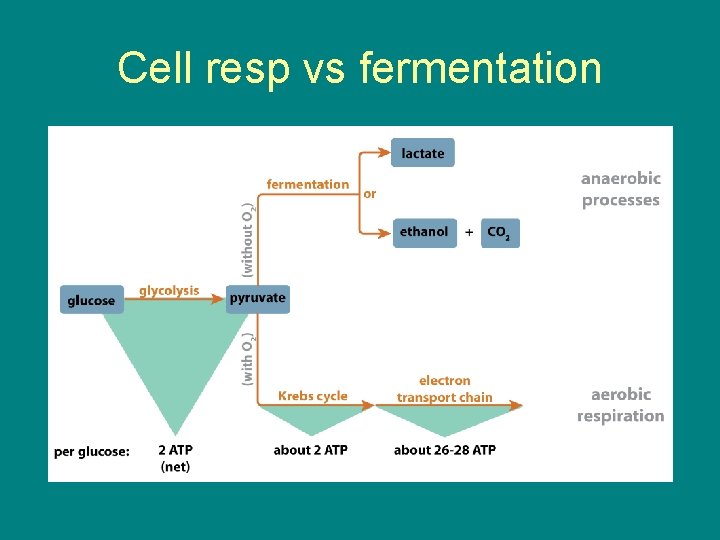
Cell resp vs fermentation

What happens when it’s not glucose? • Most of our diet is in the form of fats, proteins, di and polysaccharides • All of these can be used by resp to make ATP

carbs • Di and polysaccharides – are digested into glucose and other monomers that can enter into resp

proteins - first digested into amino acids - most AA are used by the cells to make new proteins - The left over AA are converted by enzymes into a form that can enter resp - This is done by deamination (removing the amino group) - The nitrogenous amino group is then excreted in the form of ammonia, urea or other waste products

fats - Can harvest energy stored in fat either from food or from storage cells in the body - First digested into glycerol and fatty acids - Glycerol is converted into: glyceraldehyde-3 -phosphate - This an intermediate to glycolysis - The fatty acids (which hold the most energy) are broken down through beta oxidation into a form that can enter krebs
• Glycolysis and the krebs cycle allow us to convert molecules into different molecules depending on what is needed

Regulation of metabolism through feedback mechanisms • Cell does not waste energy making more of a substance than it needs • Feedback inhibition- the end product of metabolic pathway inhibits the enzyme that catalyzes an early step of the pathway
- Slides: 64