Chapter 7 Carbon and Organic Compounds Timberlake Lecture

Chapter 7 Carbon and Organic Compounds Timberlake Lecture. PLUS 1

Organic Chemistry § Organic originally meant chemicals that came from organisms § 1828 German chemist Friedrich Wohler synthesized urea in a lab § Today, organic chemistry is the chemistry of virtually all compounds containing the element carbon Timberlake Lecture. PLUS 2

Organic Compounds Contain carbon Have covalent bonds Have low melting points Have low boiling points Burn in air (oxygen) Are soluble in nonpolar solvents Timberlake Lecture. PLUS Form large molecules 3

Organic Chemistry and Hydrocarbons § Over a million organic compounds, with a dazzling array of properties § Why so many? Carbon’s unique bonding ability! § Let’s start with the simplest of the organic compounds: Hydrocarbons Timberlake Lecture. PLUS 4

Hydrocarbons § Hydrocarbons contain only two elements: hydrogen and carbon § simplest hydrocarbons called alkanes, which contain only single covalent bonds § methane (CH 4) with one carbon is the simplest alkane; also known as swamp gas; main component of natural gas. Timberlake Lecture. PLUS 5
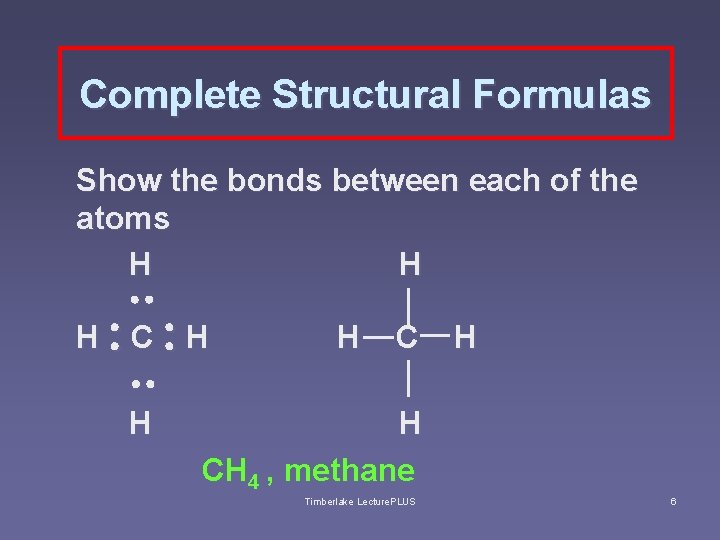
Complete Structural Formulas Show the bonds between each of the atoms H H H CH 4 , methane Timberlake Lecture. PLUS 6

alkanes Carbon has 4 valence electrons, thus forms 4 covalent bonds § not only with other elements, but also forms bonds WITH ITSELF. § Ethane (C 2 H 6) is the simplest alkane with a carbon to carbon bond Timberlake Lecture. PLUS 7
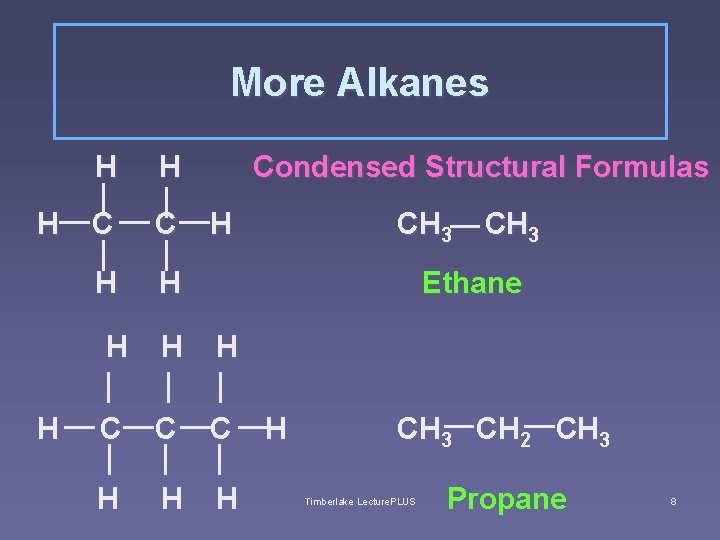
More Alkanes H H C C H H Condensed Structural Formulas H CH 3 Ethane H H H C C C H H CH 3 CH 2 CH 3 Timberlake Lecture. PLUS Propane 8

Straight-Chain Alkanes § Straight-chain alkanes contain any number of carbon atoms, one after the other, in a chain - meaning one linked to the next C-C-C-C Timberlake Lecture. PLUS etc. 9

Straight-Chain Alkanes Many alkanes are used for fuels: methane, propane, butane, octane § As the number of carbons increases, so does the boiling and melting pt. § The first 4 are gases; #5 -15 are liquids; higher alkanes are solids Timberlake Lecture. PLUS 10

Alkanes ØSince the electrons are shared equally, the molecule is nonpolar – thus, not attracted to water – oil (a hydrocarbon) not soluble in H 2 O – “like dissolves like” Timberlake Lecture. PLUS 11

Naming Straight-Chain Alkanes v. Names recommended by IUPAC - the International Union of Pure and Applied Chemistry – end with -ane, the root part of the name is a prefix for the # of carbons Table 7 -3, page 244 Timberlake Lecture. PLUS 12
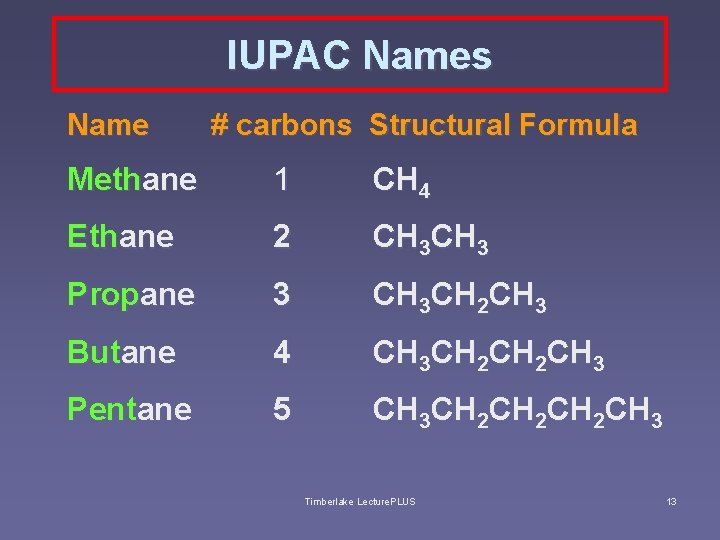
IUPAC Names Name # carbons Structural Formula Methane 1 CH 4 Ethane 2 CH 3 Propane 3 CH 3 CH 2 CH 3 Butane 4 CH 3 CH 2 CH 3 Pentane 5 CH 3 CH 2 CH 2 CH 3 Timberlake Lecture. PLUS 13
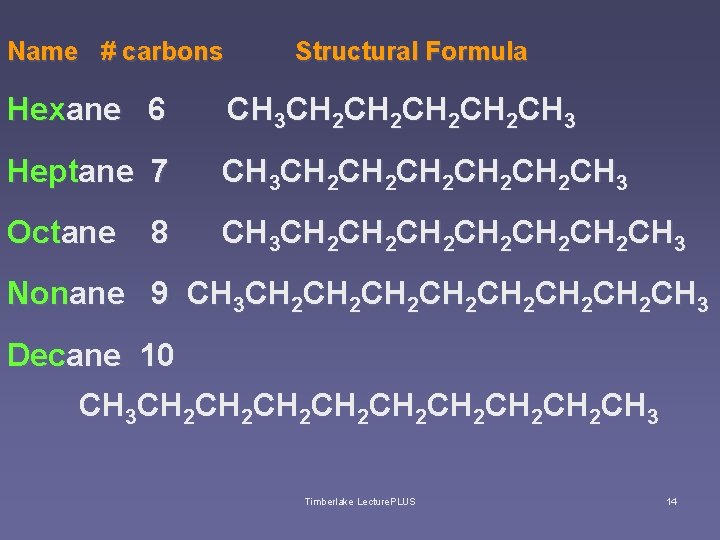
Name # carbons Structural Formula Hexane 6 CH 3 CH 2 CH 2 CH 3 Heptane 7 CH 3 CH 2 CH 2 CH 2 CH 3 Octane CH 3 CH 2 CH 2 CH 2 CH 3 8 Nonane 9 CH 3 CH 2 CH 2 CH 3 Decane 10 CH 3 CH 2 CH 2 CH 3 Timberlake Lecture. PLUS 14

Straight-Chain Alkanes § Homologous series- a group of compounds that have a constant increment of change § In alkanes, it is: -CH 2 - Timberlake Lecture. PLUS 15
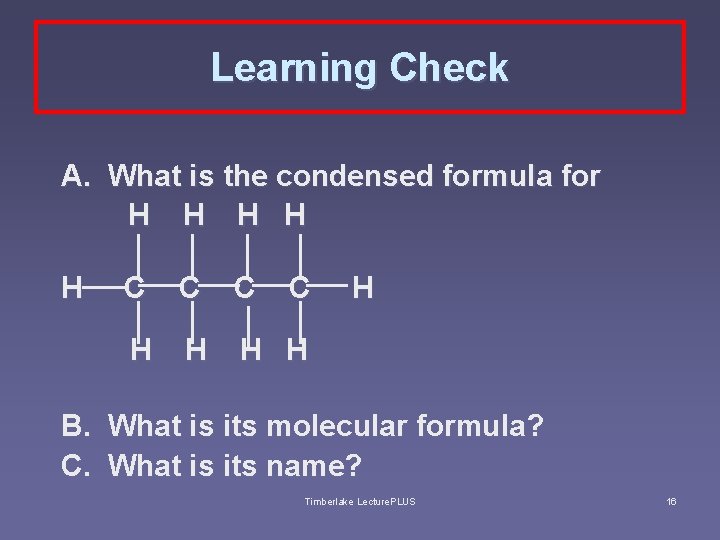
Learning Check A. What is the condensed formula for H H H B. C. C C H H H What is its molecular formula? What is its name? Timberlake Lecture. PLUS 16
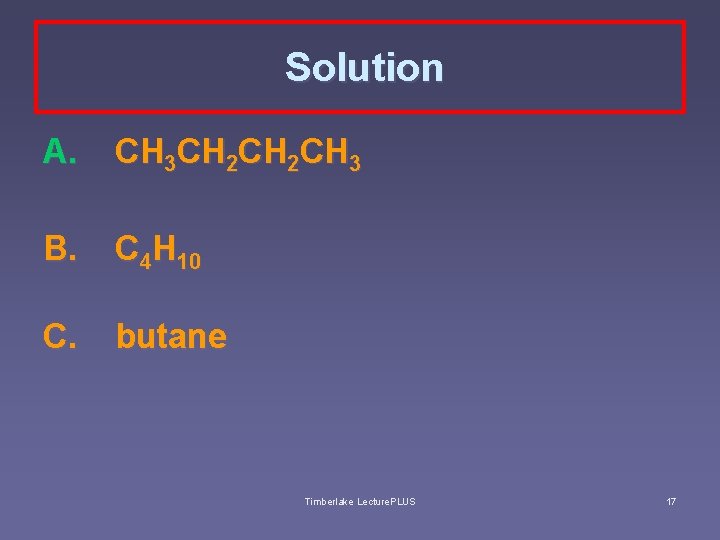
Solution A. CH 3 CH 2 CH 3 B. C 4 H 10 C. butane Timberlake Lecture. PLUS 17
- Slides: 17