Chapter 7 1 The Mole and Molar Conversions














- Slides: 29

Chapter 7. 1: The Mole and Molar Conversions o Objectives: o (1) To define the mole. o (2) To perform molar conversions.


Do you know the following? o 1 pair = o 1 dozen = o 1 score = o 1 gross = o 1 ream = o 1 mole (mol) =

o 1 pair = 2 o 1 dozen = 12 o 1 score = 20 o 1 gross = 144 o 1 ream = 500 o 1 mole (mol) = 6. 02 x 1023

The MOLE o. A mole of a substance is 6. 02 x 1023 representative particles of that substance. o. This number is called Avogadro’s number. o. The symbol for mole is mol.

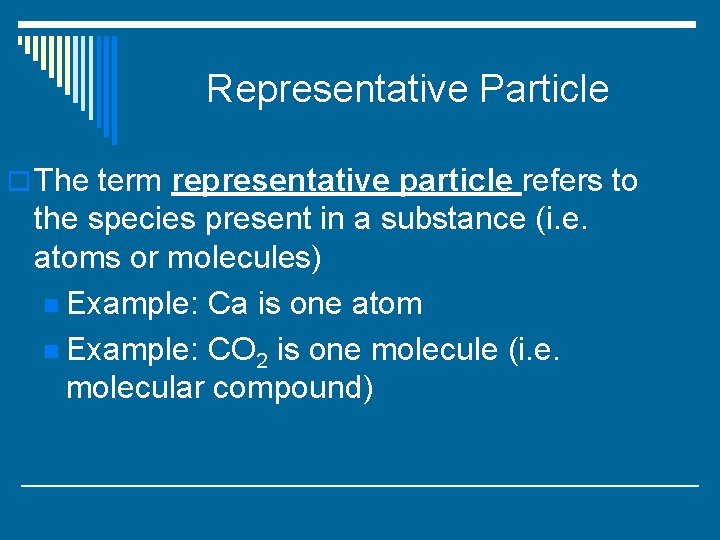
Representative Particle o The term representative particle refers to the species present in a substance (i. e. atoms or molecules) n Example: Ca is one atom n Example: CO 2 is one molecule (i. e. molecular compound)

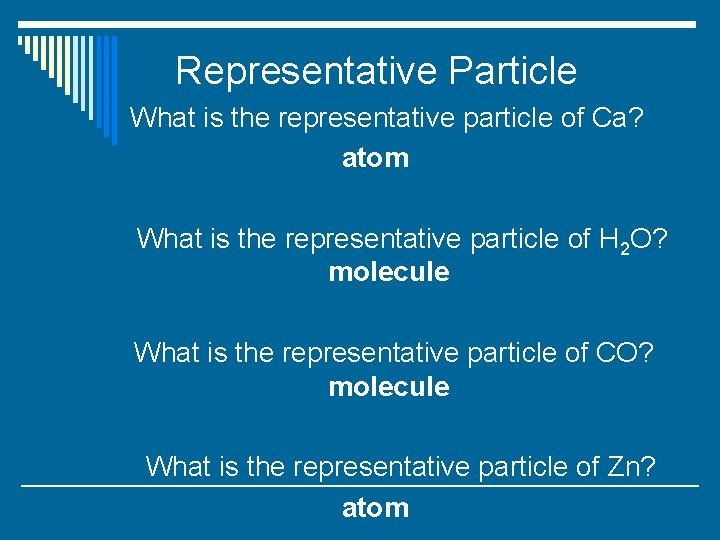
Representative Particle o What is the representative particle of Ca? o What is the representative particle of H 2 O? o What is the representative particle of CO? o What is the representative particle of Zn?

Representative Particle What is the representative particle of Ca? atom What is the representative particle of H 2 O? molecule What is the representative particle of CO? molecule What is the representative particle of Zn? atom

The Concept of the Mole o One mole of donuts contains 6. 02 x 1023 donuts o One mole of H 2 O contains 6. 02 x 1023 o o molecules One mole of nails contains 6. 02 x 1023 nails One mole of Fe contains 6. 02 x 1023 atoms One mole of dogs contains 6. 02 x 1023 dogs One mole of electrons contains 6. 02 x 1023 electrons Get the idea?

Just How Big is a Mole? Mole Facts o 6. 02 X 1023 Watermelon Seeds: Would be found inside a melon slightly larger than the moon. o 6. 02 X 1023 Donut Holes: Would cover the earth and be 5 miles (8 km) deep. o 6. 02 X 1023 Pennies: Would make at least 7 stacks that would reach the moon. o 6. 02 X 1023 Grains of Sand: Would be more than all of the sand on Miami Beach.

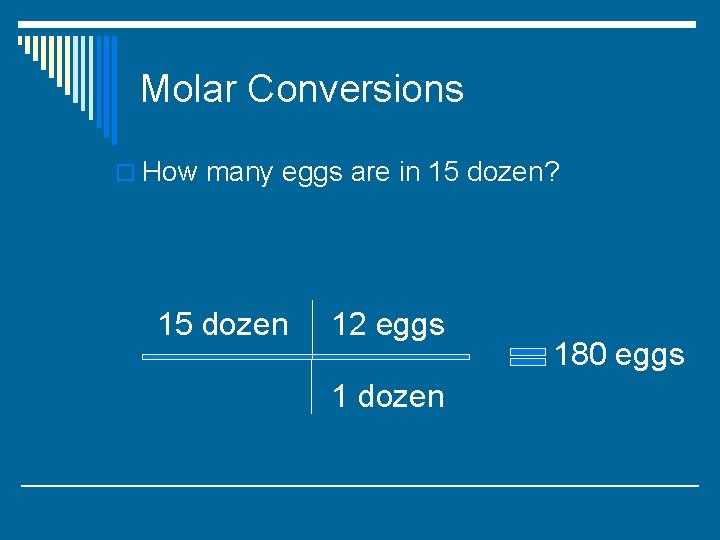
Molar Conversions o How many eggs are in 15 dozen?

Molar Conversions o How many eggs are in 15 dozen? 15 dozen 12 eggs 1 dozen 180 eggs

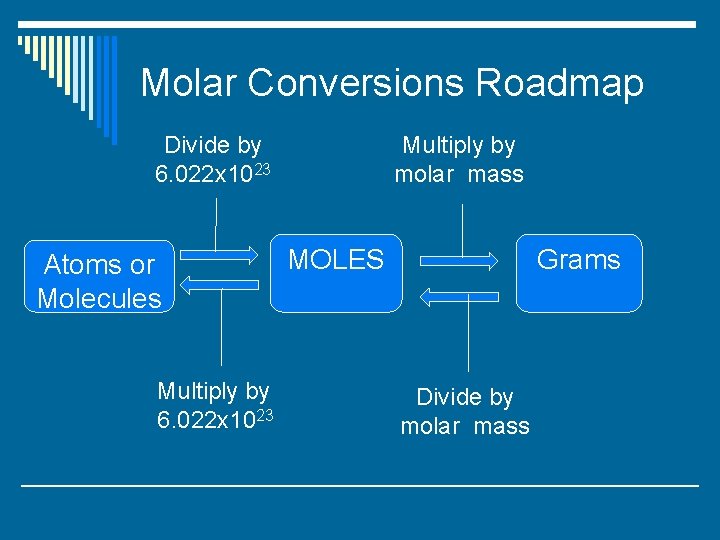
Molar Conversions Roadmap Divide by 6. 022 x 1023 Atoms or Molecules Multiply by 6. 022 x 1023 Multiply by molar mass MOLES Grams Divide by molar mass

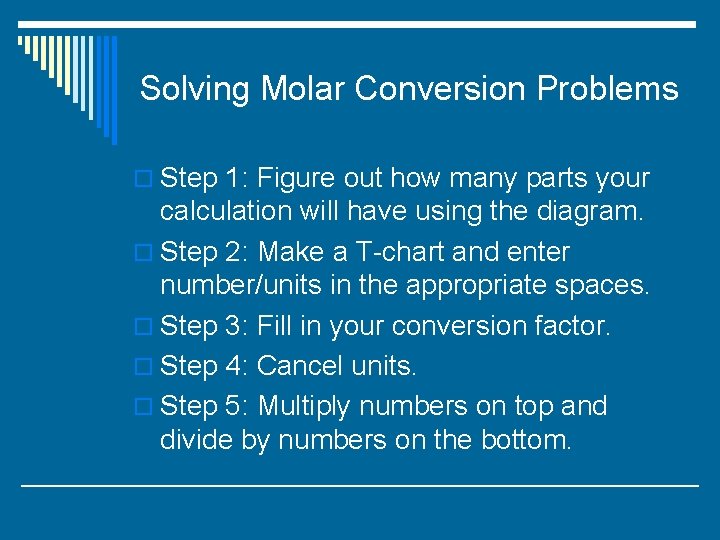
Solving Molar Conversion Problems o Step 1: Figure out how many parts your calculation will have using the diagram. o Step 2: Make a T-chart and enter number/units in the appropriate spaces. o Step 3: Fill in your conversion factor. o Step 4: Cancel units. o Step 5: Multiply numbers on top and divide by numbers on the bottom.

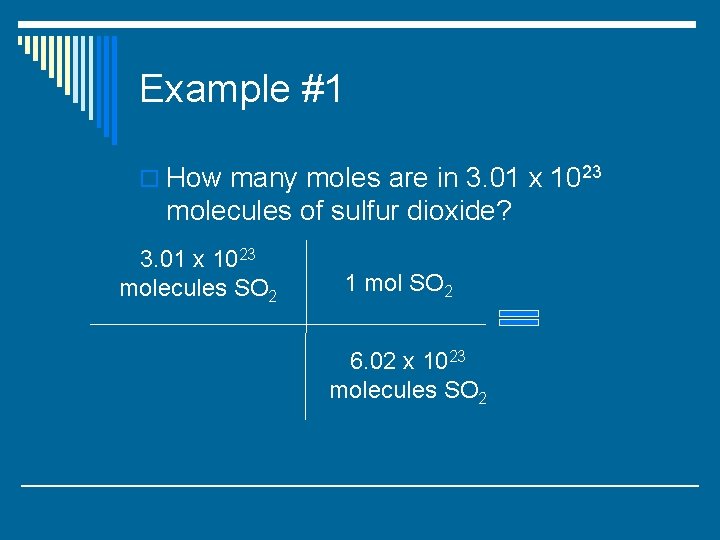
Example #1 o How many moles are in 3. 01 x 1023 molecules of sulfur dioxide?

Example #1 o How many moles are in 3. 01 x 1023 molecules of sulfur dioxide? 3. 01 x 1023 molecules SO 2 1 mol SO 2 6. 02 x 1023 molecules SO 2

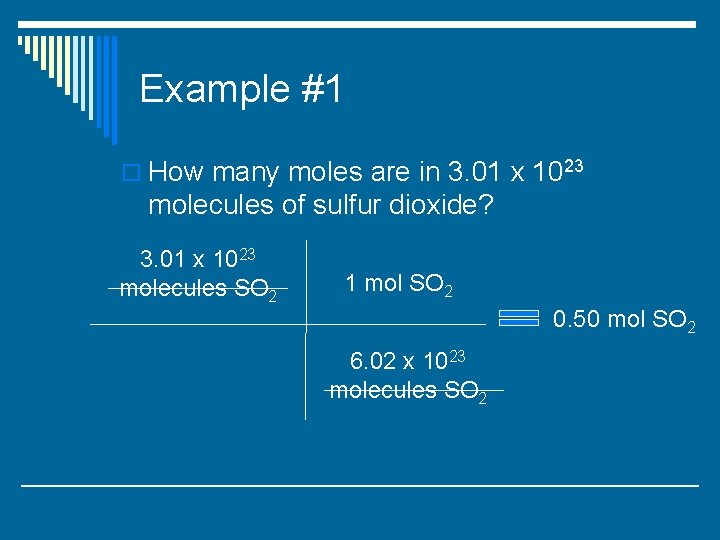
Example #1 o How many moles are in 3. 01 x 1023 molecules of sulfur dioxide? 3. 01 x 1023 molecules SO 2 1 mol SO 2 0. 50 mol SO 2 6. 02 x 1023 molecules SO 2

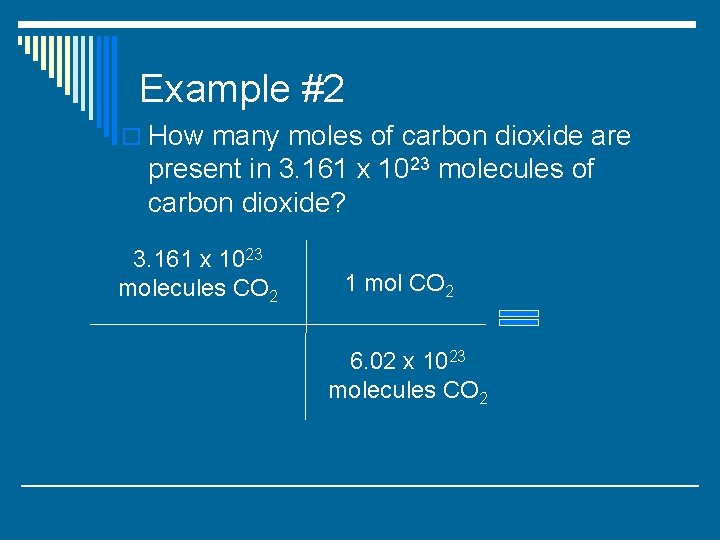
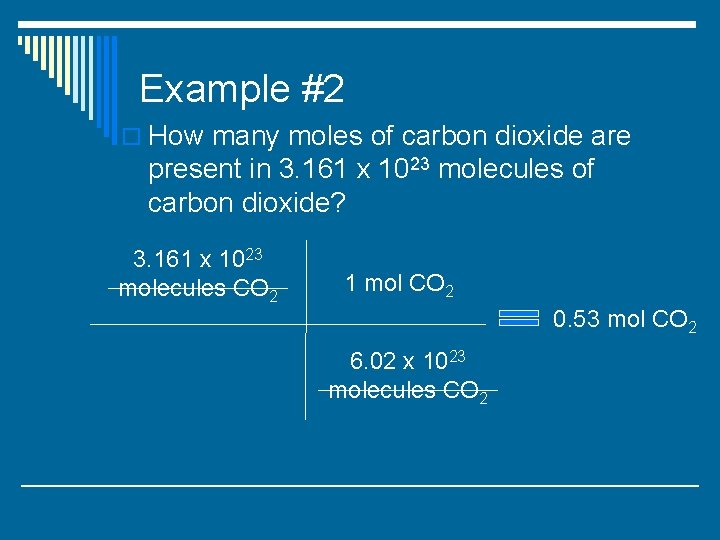
Example #2 o How many moles of carbon dioxide are present in 3. 161 x 1023 molecules of carbon dioxide?

Example #2 o How many moles of carbon dioxide are present in 3. 161 x 1023 molecules of carbon dioxide? 3. 161 x 1023 molecules CO 2 1 mol CO 2 6. 02 x 1023 molecules CO 2

Example #2 o How many moles of carbon dioxide are present in 3. 161 x 1023 molecules of carbon dioxide? 3. 161 x 1023 molecules CO 2 1 mol CO 2 0. 53 mol CO 2 6. 02 x 1023 molecules CO 2

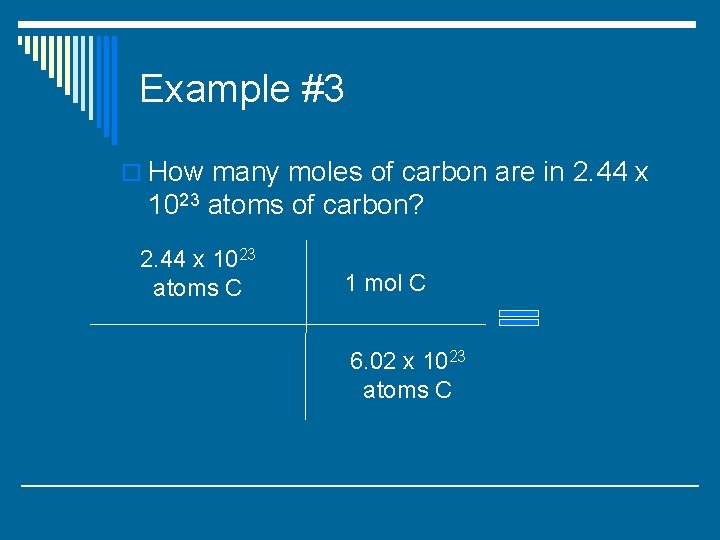
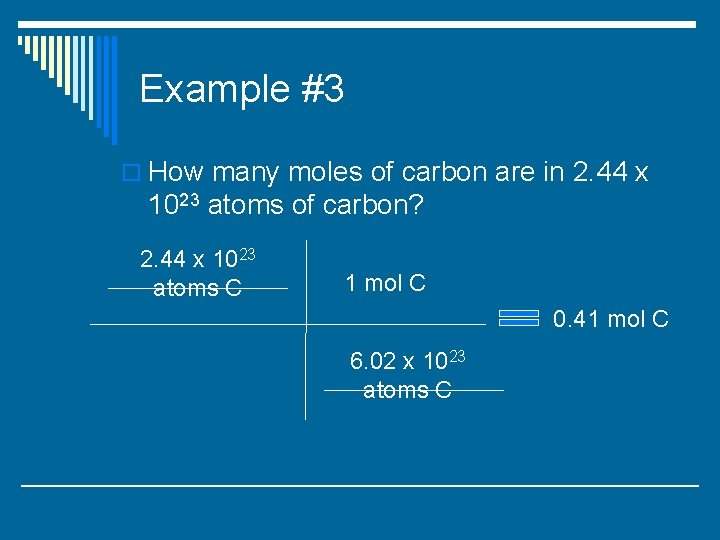
Example #3 o How many moles of carbon are in 2. 44 x 1023 atoms of carbon?

Example #3 o How many moles of carbon are in 2. 44 x 1023 atoms of carbon? 2. 44 x 1023 atoms C 1 mol C 6. 02 x 1023 atoms C

Example #3 o How many moles of carbon are in 2. 44 x 1023 atoms of carbon? 2. 44 x 1023 atoms C 1 mol C 0. 41 mol C 6. 02 x 1023 atoms C

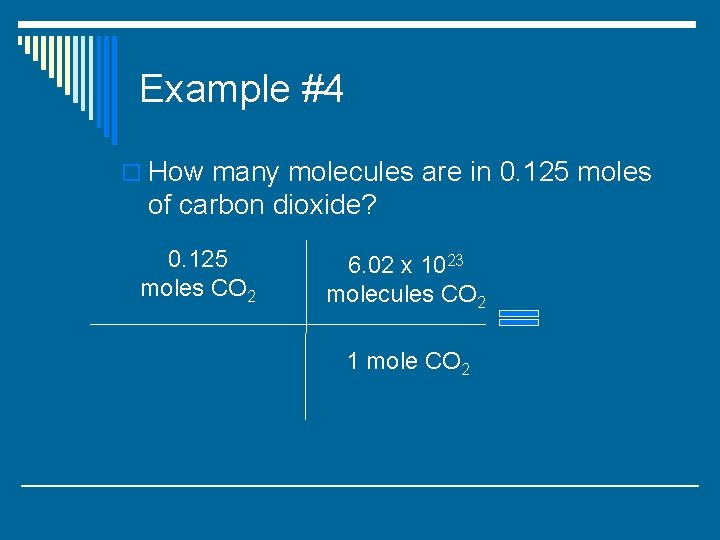
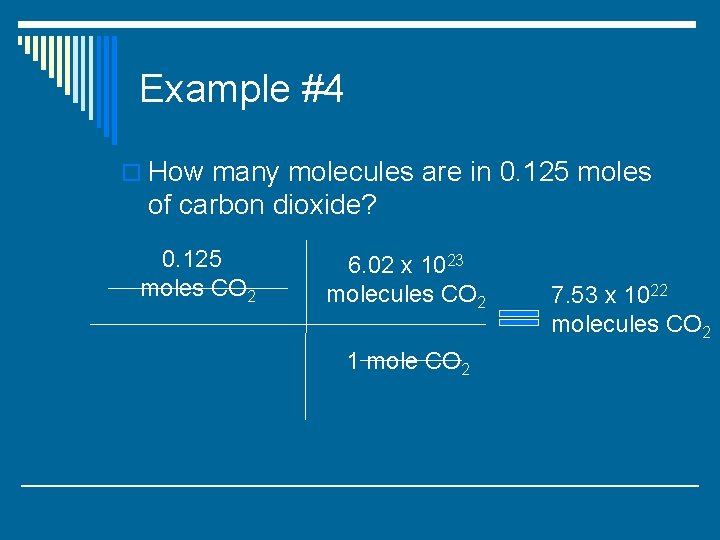
Example #4 o How many molecules are in 0. 125 moles of carbon dioxide?

Example #4 o How many molecules are in 0. 125 moles of carbon dioxide? 0. 125 moles CO 2 6. 02 x 1023 molecules CO 2 1 mole CO 2

Example #4 o How many molecules are in 0. 125 moles of carbon dioxide? 0. 125 moles CO 2 6. 02 x 1023 molecules CO 2 1 mole CO 2 7. 53 x 1022 molecules CO 2

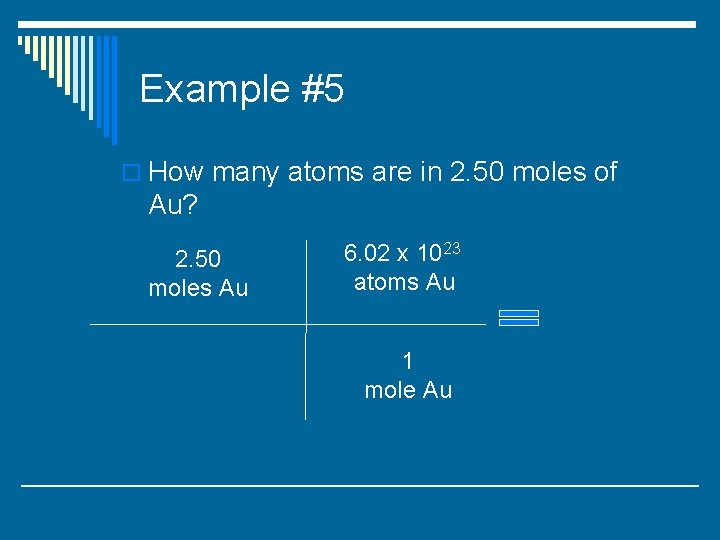
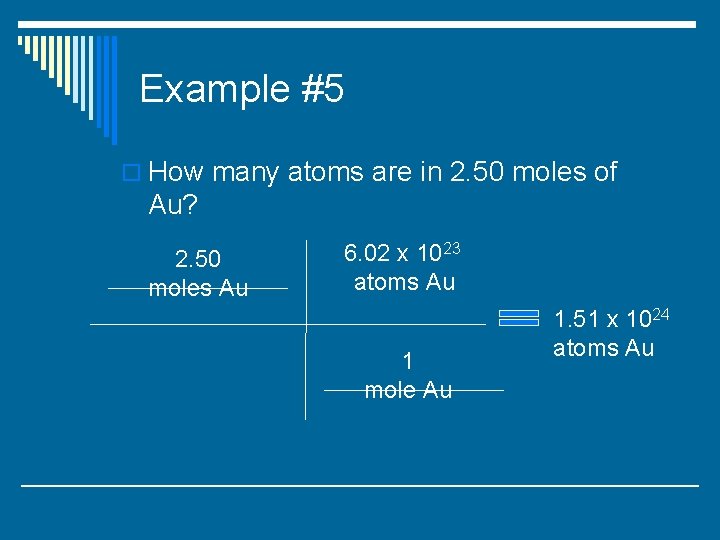
Example #5 o How many atoms are in 2. 50 moles of Au?

Example #5 o How many atoms are in 2. 50 moles of Au? 2. 50 moles Au 6. 02 x 1023 atoms Au 1 mole Au

Example #5 o How many atoms are in 2. 50 moles of Au? 2. 50 moles Au 6. 02 x 1023 atoms Au 1 mole Au 1. 51 x 1024 atoms Au